Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Madhan Jeyaraman | + 2594 word(s) | 2594 | 2021-09-26 05:16:29 | | | |
| 2 | Peter Tang | Meta information modification | 2594 | 2021-09-29 08:29:03 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Jeyaraman, M. Dental Derived Mesenchymal Stromal Cells. Encyclopedia. Available online: https://encyclopedia.pub/entry/14676 (accessed on 07 February 2026).
Jeyaraman M. Dental Derived Mesenchymal Stromal Cells. Encyclopedia. Available at: https://encyclopedia.pub/entry/14676. Accessed February 07, 2026.
Jeyaraman, Madhan. "Dental Derived Mesenchymal Stromal Cells" Encyclopedia, https://encyclopedia.pub/entry/14676 (accessed February 07, 2026).
Jeyaraman, M. (2021, September 28). Dental Derived Mesenchymal Stromal Cells. In Encyclopedia. https://encyclopedia.pub/entry/14676
Jeyaraman, Madhan. "Dental Derived Mesenchymal Stromal Cells." Encyclopedia. Web. 28 September, 2021.
Copy Citation
Dental-derived MSCs possess similar phenotypes and genotypes like other sources of MSCs along with specific markers such as dentin matrix acidic phosphoprotein (DMP) -1, dentin sialophosphoprotein (DSPP), alkaline phosphatase (ALP), osteopontin (OPN), bone sialoprotein (BSP), and STRO-1. Concerning chondrogenicity, there is literature with marginal use of dental-derived MSCs.
dental pulp
mesenchymal stromal cell
chondrogenicity
1. Introduction
Cartilage is an avascular and aneural structure with poorly cellularized connective tissue [1][2]. Cartilage tissue facilitates mechanical load transmission with a low frictional coefficient resulting in cartilage injury that has an inherent limited healing potential [3]. The recent idea of “Orthobiologics” led to the exploration of stem cells and regenerative medicine in treating musculoskeletal disorders [4]. Orthobiologics provide administration of osteoinductive and osteoconductive micromolecules to enhance regeneration of degenerated tissues, tendons, bones, and cartilages [4].
Tissue Engineering (TE) is defined as in-vitro or in-vivo regeneration of tissues for repairing and replacing the diseased tissue or organ to enhance and restore the tissue function and maintain tissue homeostasis and improve the biomechanical strength of the tissues [5][6][7]. Cartilage tissue engineering provides a new strategy by transplanting chondrogenic cells along with biocompatible 3D scaffolds and micromolecules to produce engineered cartilage tissue [6][8]. Chondrogenic cells are derived from mesenchymal stromal cells from various sources, namely, bone marrow [9], adipose tissue [10], placenta [11], amniotic fluid [12], Wharton jelly [13], umbilical cord [14], synovium [15], hair follicles [16], dental pulp [17], and gingiva [18]. The tissue engineering triad comprises mesenchymal stromal cells, scaffolds, and biomolecules such as growth factors and cytokines [8][19].
Mesenchymal stromal cells (MSCs) form an integral part of regenerative medicine for cartilage regeneration. MSCs are multipotent stem cells with clonigenicity, plasticity, self-renewal, and differentiation [20][21]. MSC differentiate into trilineage namely osteogenesis, chondrogenesis, and adipogenesis [22][23][24]. Chondrogenesis is mediated by various mediators such as TGF-β1 and -β3; BMP-2, -4, and -7; IGF-1; and GDF-5 [25][26]. Dental structures provide a variety of stem cells with ease of isolation, non-invasiveness, and availability [17][27][28]. Stem cells of dental origin have similar properties of multipotency, diverse differentiative potential, anti-inflammation, immunomodulation, immune privilege like BM-MSCs, AD-MSCs, and Sy-MSCs [29][30]. This article throws light on MSCs of dental origin in chondrogenesis and cartilage regeneration in osteoarthritic knees.
2. MSCs of Dental Origin
Stem cells of dental origin form a good therapeutic paradigm in regenerating tissues, bones, and cartilage. MSCs of dental origin include (a) dental pulp MSCs (DP-MSCs) [31][32], (b) stem cells from human exfoliated deciduous teeth (SHEDs) [33][34], (c) periodontal ligament stem cells (PDLSCs) [35][36], (d) dental follicle precursor cells (DFPCs) [37][38], (e) stem cells from apical papilla (SCAPs) [39][40], and (f) gingival derived MSCs (G-MSCs) [41][42]. Among all these sources of dental stem cells, researchers pay attention to DP-MSCs and SHEDs because of ease in their accessibility. The various sources of MSCs of dental origin are shown in Figure 1.
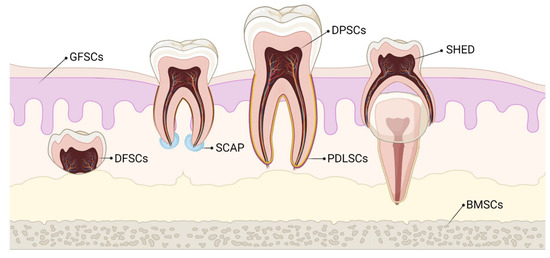
Figure 1. Various sources of stem cells of dental origin. BMSCs–Bone Marrow Stem Cells; DFSCs–Dental Follicle Stem Cells; DPSCs–Dental Pulp Stem Cells; GFSCs–Gingival Fibroblastic Stem Cells; PDLSCs–Periodontal Ligament Stem Cells; SCAP–Stem Cells of Apical Papilla; SHED–Stem cells of Human Exfoliated Deciduous teeth.
3. Characterization of Dental-Derived MSCs (D-MSCs)
DP-MSCs and SHEDs possess similar immunophenotype like BM-MSCs, AD-MSCs, and Sy-MSCs [43][44][45]. DP-MSCs and SHEDs possess cell markers of MSCs (CD-13 [alanyl aminopeptidase], -44, -73 [ecto-5′-nucleotidase], -90, -105 [endoglin], -146, and -166, STRO-1) [46], osteogenic markers (BMP-2, OCN, OPN, osteonectin, and COL-1) [47], adipogenic markers (LPL and PPAR-γ) [48], chondrogenic markers (SOX-9 and COL-2) [45][47], myogenic markers (myosin, myogenin, and SMA-α) [49], neurogenic markers (nestin, GFAP, MAP-2, and β3 tubulin) [50][51][52], and pluripotency markers (OCT-4, SOX-2, Nanog, and IGF-1R) [53][54]. They demonstrate negative staining for hematopoietic markers (CD-14, -19, -34, -45, and HLA-DR) [44][55].
The specific markers for D-MSCs are markers of odontoblast differentiation [dental matrix protein-1 (DMP-1) and dentine sialophosphoprotein (DSPP)] [56], markers of extracellular matrix [alkaline phosphatase (ALP)] [57], makers of osteogenic differentiation [osteopontin (OPN)] [58], markers of mineralized tissue differentiation [bone sialoprotein (BSP)] [59], and markers of differentiating potential of D-MSCs [STRO-1] [60].
Biodentine, a bioactive dentine substitute, is capable of inducing DP-MSCs differentiation of odontoblasts. Luo et al. demonstrated odontoblast differentiation of DP-MSCs by increased expression of ALP, OCN, DSPP, DMP1, and BSP [61]. Optimal mechanical compression increased the expression of DSPP, BMP-7, and Wnt10a genes for odontoblast differentiation by DP-MSCs [62]. BBX gene expression induces the differentiation of odontoblasts by DP-MSCs [63]. DNA methylation and PTEN expression were increased in DP-MSCs, which are responsible for lineage differentiation and reduced oncogenesis when compared with BM-MSCs [64].
4. Harvesting and Delivery Methods of D-MSCs
Various regenerative medicine experts followed different methods to extract and harvest stromal cells from dental pulp.
Raoof et al. used three different methods to isolate DP-MSCs, namely, (a) digestion of dental pulp tissue with collagenase and placement of isolated trypsinized cells in petri dishes, (b) explantation of undigested dental pulp pieces to culture plates, and (c) explantation of trypsinized dental pulp tissues to petri dishes for outgrowth [65]. These tissues are plated to MEM medium supplemented with 20% fetal bovine serum at 37 °C with a 5% CO2 incubator. A total of 60% cellular confluency was achieved within days of culture and checked for pluripotency markers by RT/PCR analysis [65].
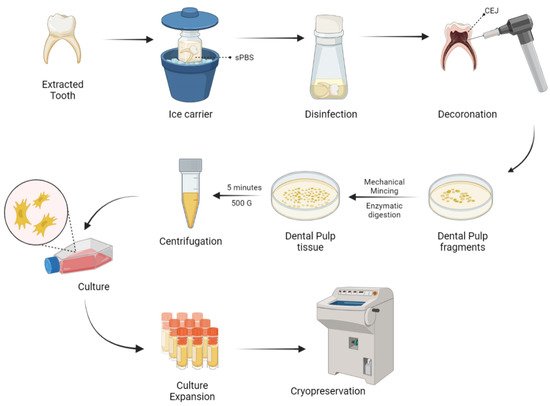
Naz et al. expanded DP-MSCs and SHEDs via the explant culture method after extirpation of dental pulp tissues from deciduous teeth [66]. As a result of culture expansion, MSCs exhibit fibroblast-like cells with long cytoplasmic processes. DP-MSCs and SHEDs characterization was done and cryopreserved for future use as shown in Figure 2.

Figure 2. Harvesting and delivery method of DP-MSCs. sPBS—sterile phosphate buffer saline; CEJ—cementum enamel junction.
No significant change was observed in the differentiating capabilities and immunophenotypic properties of cryopreserved and non-cryopreserved DP-MSCs isolated from dental pulp, but there were significant differences in the morphology and proliferative potential of cryopreserved DP-MSCs than non-cryopreserved DP-MSCs [67].
The survival rates of DP-MSCs in DMSO free medium by static magnetic cryopreservation increased by 2 to 2.5 fold when the cells were exposed to 0.4 or 0.8-T static magnetic fields [68]. Gioventù et al. demonstrated that cryopreserved teeth by laser piercing have maintained cellular viability [69].
To isolate a pure population of DP-MSCs, the identification of cell surface markers like LNGFR and THY-1 are significant [70][71]. The assessment of the number of colonies showed that LNGFRLow+THY-1High+ cells in the dental pulp have a significantly higher colony-forming potential than LNGFR+THY-1+ cells in the bone marrow [72][73][74].
5. Chondrogenicity of Dental-Derived MSCs
Though dental-derived stem cells possess higher osteogenic potential, they are being explored very marginally for chondrogenicity. DP-MSCs act as a promising source for cartilage tissue engineering and regeneration. DP-MSCs possess a strong potential to differentiate into hyaline and fibrocartilage [75]. Sophia et al. demonstrated that hyaline cartilage contains few chondrocytes in their extracellular matrix rich in GAGs and type 2 collagen [2], whereas Allen et al. stated that fibrocartilage contains fibroblastic cells with small amounts of GAGs and type 1 collagen [76].
Longoni et al. expanded DP-MSCs from seven molar teeth and induced chondrogenesis in a 3D pellet culture system [77]. These culture-expanded DP-MSCs display GAGs, aggrecan, and type 2 collagen after three weeks. The assessment of culture-expanded cells revealed fibroblastic cells with long cytoplasmic processes with a predominance of type 1 collagen to state the formation of fibrocartilage. They concluded that DP-MSCs regenerate fibrocartilage in joints, rather than hyaline cartilage.
DP-MSCs provide a rapid ex-vivo expansion and chondrogenic differentiation potential and hence provide a favorable cell type for treating cartilage disorders. Khajeh et al. demonstrated a significant role of hypoxia mimicking agent and cobalt chloride on chondrogenesis with DP-MSCs [78]. Cobalt chloride exposure to DP-MSCs increases the cellular pellet mass in culture, cellular morphology and integrity, ECM deposition, and cellular organizations. There were elevated levels of GAGs and type 2 collagen expression [79]. Cobalt chloride enhances the stemness of DP-MSCs where flow cytometry reveals the increased expression of STRO-1+ cells [80].
SHEDs lose stemness and compromise the therapeutic effects when cultured and expanded in vitro for the long term. Hypoxia is a major factor in the maintenance of stemness of MSCs [81][82]. SHEDs, when co-cultured with cobalt chloride, increased the hypoxia-inducible factor-1α in a dose-dependent manner, resulted in increased expression of STRO-1+ cells and stem/stromal cell markers such as OCT4, NANOG, SOX2, and c-Myc and decreased osteogenic differentiation by reducing ALP levels [79]. Hypoxia suppresses chondrogenic hypertrophy in agarose or alginate-chondroitin sulfate-platelet lysate hydrogel and 3D pellet culture system in cartilage tissue engineering [83][84]. At the protein level, the inhibitors of chondrocyte hypertrophy are PTHrP, TGF-β3, BMP-4, -7, and -13, GG86/2, Dorsomorphin, and FK506, whereas, at the gene level, Nkx3.2, SOX-9, Smad6, HDAC4, ChM1, sFlt-1, and C-1-1 are responsible for inhibiting chondrocyte hypertrophy during culture [85][86][87][88][89]. The chondrogenesis due to hypoxia is mediated through SOX-9 gene transcription or p38 MAPK gene activation [90]. Hypoxia promotes chondrogenic differentiation and cartilage extracellular matrix synthesis and suppresses terminal chondrocyte differentiation and hence the hypoxia phenomenon preserves chondrocyte phenotype and function during chondrogenesis [91].
Hsu et al. cultured human gingival fibroblast (HGF) cells on chitosan membrane to observe in-vitro chondrogenesis. On culture, increased spheroid formation resulted, which indicates the stemness of HGF. Spheroid formation by HGF was supported by Rho/Rho-associated kinases and the connexion 43 pathway. Hence, they concluded that culturing HGF on chitosan membrane induces spheroid formation, which further induces chondrogenesis by the ROCK pathway [92].
Ferre et al. demonstrated osteogenesis and chondrogenesis by human gingival stem cells in vitro in 3D floating micromass pellet cultures in a specified medium. Osteogenic cells exhibited the increased expression of Runx-2, ALP, presence of osteoid-like mass, and osterix expression whereas chondrogenic cells exhibited increased expression of type 2 collagen, GAGs, and SOX-9 transcription gene [93].
SOX-9, the master gene for chondrogenesis, helps in the proliferation of chondrocytes, but not in chondrocyte hypertrophy [94]. SOX-9 gene was expressed in human gingival stem cells at the basal level without chondrogenic stimulation [95]. Such basal expression of SOX-9 explains efficient chondrogenesis. SOX-9 gene knock-out mice were unable to regenerate normal cartilage despite MSC condensation [96][97][98][99]. The chondrogenesis by SOX-9 is due to
- (1) chondrocyte expression of SOX-9 until growth plate hypertrophy and in articular cartilage throughout life in adults,
- (2) to secure lineage specificity towards chondrogenesis in fetal and postnatal growth plates,
- (3) to maintain adult cartilage homeostasis,
- (4) and to repress non-chondrogenic lineages in gene level [50][51][52].
Ferre et al. demonstrated the differentiation of type 10 collagen secreting hypertrophic chondrocytes and fibroblast-like synoviocytes by human gingival stem cells. Under hypoxia and hypoxic mimicking environment, G-MSCs express high levels of VEGF-α, which promote vasculogenesis for regenerative therapies [93].
NOTCH ligand signaling plays a major role in the chondrogenic differentiation of cells [100]. NOTCH-2 modulates the activities of NOTCH-3 and -1, hence influence the growth and development, and homeostasis of chondrocytes and articular cartilage [101][102][103]. NOTCH-3 represses the proliferation of terminally differentiated chondrocytes within the cartilaginous tissues [104]. In a 3D-cultured chondrogenesis, there is a downregulation of NOTCH ligands and receptors [105][106]. While MSC undergoes terminal chondrocyte differentiation, NOTCH-3 receptors were upregulated and were highly expressed [100][107].
6. Engineered Chondrogenesis by D-MSCs
Due to the intrinsic limited potential of cartilage tissue to heal, cartilage tissue engineering gave a robust breakthrough in the field of regenerative and translational medicine. The field of tissue engineering provides the biological substitute of limited available tissues to restore and maintain the naïve homeostasis and to improve the biomechanical strength and function of the tissues. The integral components of tissue engineering are stem/stromal cells, scaffolds, and bio-micromolecules [108][109][110]. The successfully engineered tissue relies on tissue ergonomics that include harvest and expansion of appropriate cells, the addition of optimum levels of growth factors and cytokines, and provision of 3D scaffold and extracellular matrices until the healing gets completed.
Growth factors: The cardinal growth factors responsible for cartilage engineering are TGF-β1 and -β3, BMP-2 and -7, IGF-1, and bFGF. TGF-β1 induces and maintains chondrogenesis of MSC through chondrostimulatory signaling by p38, ERK-1, JNK, N-cadherin expression, and suppresses the IL-1 catabolism [111]. TGF-β1 controls Wnt-mediated signaling and β-catenin TCF pathway in early MSC chondrogenesis [112]. TGF-β3, when co-cultured with bovine MSCs in a chitosan scaffold, stimulates the growth of hyaline cartilage and integrates into the host cartilage [113]. BMPs are involved in Hedgehog and TGF- β signaling in regulating chondrogenesis both in vitro and in vivo [114][115][116]. BMP-2 inhibits IL-1 effects and enhances cartilage cell production by inducing chondrogenic factors, whereas BMP-7 enhances ECM production [117]. IGF-1 downregulates MMP-13 by upregulating collagen 2 expression and GAG synthesis [118]. IGF-1 induces in vitro and in vivo chondrogenesis in a dose- and time-dependent manner [119]. The evidence stated that bFGF-18 in a concentration-dependent manner stimulates and enhances chondrogenesis in the osteochondral lesion of rabbit knees [120][121].
Bioactive molecules: Kartogenin (KGN) enhances MSC chondrogenesis by upregulation of CBFβ-RUNX1 transcription [122]. Evidence states that kartogenin promotes tendon and meniscus regeneration [123][124]. KGN inhibits pain stimulus, attenuates chondral degeneration and inflammation, supplements the biomechanical strength of repaired bones and tendons in-vivo animals, and robust chondrogenic differentiation of DP-MSCs [125]. Simvastatin, a hypolipidaemic molecule, enhances positive effects on synovium and cartilage tissues, thereby reducing inflammation, degeneration and halts arthritis progression [126]. A higher concentration of statins decreased the production of nitric oxide in chondrocytes and cartilage explants [127].
Bioactive scaffolds: Scaffolds, an integral part of tissue engineering, are of natural [collagen, fibrin, hyaluronan, alginate, agarose, and chitosan] and synthetic [polylactic acid (PLA), polyglycolic acid (PGA), and copolymer polylactic-co-glycolic acid (PLGA)] polymers. The ideal scaffold should be biocompatible, optimum porosity, biodegradable, elastic natured, mechanical strength, easy fabrication, non-toxic, long-term effectiveness, and support cell attachment and proliferation [128][129]. Platelet-rich plasma seeded with agarose enhance cartilage and tendon regeneration [130]. MSCs cocultured with collagen or agarose enhance chondrocyte differentiation along with increased production of ECM and GAGs [131]. Alginate, an injectable scaffold, is used in regenerating focal chondral defects and in autologous chondrocyte implantation [132][133]. DP-MSCs accelerate chondrogenesis when cultured with growth factors and alginate beads [134]. The synergistic effects of chitosan and hyaluronic acid hydrogel enhance the healing of cartilage defects in rabbits [135]. Synthetic polymer scaffolds are used in the repair of osteochondral defects in rabbits [136] and meniscal lesions in dogs [137].
Chondrocytes release factor XIIIA, whose upregulation leads to hypertrophic chondrocyte differentiation in OA chondrocytes. In the murine OA cartilage model, there is an interplay between FXIIIA and α1 subunit of α1β1 integrin and tissue glutaminase 2 (TG2) mobilization, which leads to remodeling of the cartilage matrix. In absence of TG2, FXIIIA fails to undergo chondrocyte hypertrophy [138]. The conjunction of plasma membrane-bound TG2 and FXIIIA with a raised expression of FXIIIA upregulates the p38 MAP kinase signaling pathway in chondrocytes of OA cartilage in situ [139]. In turn, p38 signaling significantly increases SOX-9, which inhibits both in vitro and in vivo chondrocyte maturation to hypertrophy by DP-MSC-induced chondrogenesis [140].
Cordycepin is a potent antioxidant molecule with anti-tumorigenic and anti-inflammatory properties [141]. During MSC-induced chondrogenesis, cordycepin upregulates type 2 collagen, SOX-9, and TGF-β1 and -β3 expression, whereas downregulates type 10 collagen and Runx-2 [100]. Cordycepin has the potential to construct engineered cartilage by the inhibition of chondrocyte hypertrophy through PI3K/Bapx1 and the westin signaling pathway [142]. Hence, cordycepin plays a major role in cartilage and chondrocyte metabolism.
DP-MSCs loaded onto nanostructured PEG-GELMA-HA hydrogel form 3D spheroids, which further differentiate into chondrocytes in vitro [143]. Scaffold-assisted chondrogenesis upregulates procollagen type 2 and 10, aggrecan, alkaline phosphatase, and SOX-9 genes and downregulates Nanog, Slug, Snail, and Twist genes [144][145]. IHC analysis exhibit type 2 collagen deposition in DP-MSCs co-cultured with PEG-GELMA-HA hydrogel scaffold. These findings direct the usage of DP-MSCs to construct engineered cartilage in focal cartilage and osteochondral defects.
Expanded chondrocytes from MSCs co-cultured with PGA-fibrin scaffolds revealed considerable expression of type 1 and 2 collagen and further resulted in the formation of hyaline cartilage. Upon optimal addition of platelet-rich plasma to cartilage tissue, the formation of hyaline cartilage was robust with higher expression of collagen type 2 [146].
Loading of human dedifferentiated chondrocytes into collagen sponge, in the presence of hypoxia and BMP-2, resulted in chondrogenesis, which is transfected onto siRNAs targeting collagen type 1 and HtrA1 serine protease, which are raised in OA cartilage. Such a mechanism led to the improvement of chondrocyte phenotype differentiation. Transplantation of in vitro cultured cells into nude mouse model in vivo resulted in neochondrogenesis with hyaline matrix formation [147].
References
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69.
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage. Sports Health 2009, 1, 461–468.
- Baugé, C.; Boumédiene, K. Use of adult stem cells for cartilage tissue engineering: Current status and future developments. Stem Cells Int. 2015, 2015, e438026.
- Ramesh, R.; Jeyaraman, M.; Chaudhari, K.; Dhamsania, H.J.; Prajwal, G.S. Mesenchymal stem cells—A boon to orthopedics. Open J. Regen. Med. 2018, 7, 19–27.
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72.
- Kessler, M.W.; Grande, D.A. Tissue engineering and cartilage. Organogenesis 2008, 4, 28–32.
- Almouemen, N.; Kelly, H.M.; O′Leary, C. Tissue engineering: Understanding the role of biomaterials and biophysical forces on cell functionality through computational and structural biotechnology analytical methods. Comput. Struct. Biotechnol. J. 2019, 17, 591–598.
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144.
- Mehrabani, D.; Mojtahed Jaberi, F.; Zakerinia, M.; Hadianfard, M.J.; Jalli, R.; Tanideh, N.; Zare, S. The healing effect of bone marrow-derived stem cells in knee osteoarthritis: A case report. World J. Plast. Surg. 2016, 5, 168–174.
- Pak, J.; Lee, J.H.; Pak, N.; Pak, Y.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Cartilage regeneration in humans with adipose tissue-derived stem cells and adipose stromal vascular fraction cells: Updated status. Int. J. Mol. Sci. 2018, 19, 2146.
- Mohamed, N.S.; Wilkie, W.A.; Remily, E.A.; Delanois, R.E. Can human placental extract help patients with osteoarthritis? Ann. Transl. Med. 2020, 8.
- Huddleston, H.P.; Cohn, M.R.; Haunschild, E.D.; Wong, S.E.; Farr, J.; Yanke, A.B. Amniotic product treatments: Clinical and basic science evidence. Curr. Rev. Musculoskelet. Med. 2020, 13, 148–154.
- Cheng, J.-H.; Wang, C.-J.; Chou, W.-Y.; Hsu, S.-L.; Chen, J.-H.; Hsu, T.-C. Comparison efficacy of ESWT and Wharton′s jelly mesenchymal stem cell in early osteoarthritis of rat knee. Am. J. Transl. Res. 2019, 11, 586–598.
- Liang, H.; Suo, H.; Wang, Z.; Feng, W. Progress in the treatment of osteoarthritis with umbilical cord stem cells. Hum. Cell 2020, 33, 470–475.
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 381.
- Bahney, C.S.; Miclau, T. Therapeutic potential of stem cells in orthopedics. Indian J. Orthop. 2012, 46, 4–9.
- Bansal, R.; Jain, A. Current overview on dental stem cells applications in regenerative dentistry. J. Nat. Sci. Biol. Med. 2015, 6, 29–34.
- Xudong, G.; Zhengguo, C. Gingiva-derived mesenchymal stem cells and their potential applications in oral and maxillofacial diseases. Curr. Stem Cell Res. Ther. 2019, 15, 43–53.
- Scheller, E.L.; Krebsbach, P.H.; Kohn, D.H. Tissue engineering: State of the art in oral rehabilitation. J. Oral Rehabil. 2009, 36, 368–389.
- Giai Via, A.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012, 2, 154–162.
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125.
- Robert, A.W.; Marcon, B.H.; Dallagiovanna, B.; Shigunov, P. Adipogenesis, osteogenesis, and chondrogenesis of human mesenchymal stem/stromal cells: A comparative transcriptome approach. Front. Cell Dev. Biol. 2020, 8, 561.
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells 2019, 37, 855–864.
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal stem cells current clinical applications: A systematic review. Arch. Med Res. 2021, 52, 93–101.
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285.
- Goldring, M.B. Chondrogenesis, joint formation, and cartilage metabolism. Arthritis Res. Ther. 2012, 14, A5.
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic potential of dental stem cells. J. Tissue Eng. 2017, 8, 2041731417702531.
- Aly, L.A.A. Stem cells: Sources, and regenerative therapies in dental research and practice. World J. Stem Cells 2015, 7, 1047–1053.
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191.
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062.
- Rajendran, R.; Gopal, S.; Masood, H.; Vivek, P.; Deb, K. Regenerative potential of dental pulp mesenchymal stem cells harvested from high caries patient′s teeth. J. Stem Cells 2013, 8, 25–41.
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal stem cells derived from dental pulp: A review. Stem Cells Int. 2016, 2016, 4709572.
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812.
- Ko, C.-S.; Chen, J.-H.; Su, W.-T. Stem cells from human exfoliated deciduous teeth: A concise review. Curr. Stem Cell Res. Ther. 2020, 15, 61–76.
- Zhao, Z.; Liu, J.; Weir, M.D.; Zhang, N.; Zhang, L.; Xie, X.; Zhang, C.; Zhang, K.; Bai, Y.; Xu, H.H.K. Human periodontal ligament stem cells on calcium phosphate scaffold delivering platelet lysate to enhance bone regeneration. RSC Adv. 2019, 9, 41161–41172.
- Song, I.S.; Han, Y.S.; Lee, J.-H.; Um, S.; Kim, H.Y.; Seo, B.M. Periodontal ligament stem cells for periodontal regeneration. Curr. Oral Health Rep. 2015, 2, 236–244.
- Zhang, J.; Ding, H.; Liu, X.; Sheng, Y.; Liu, X.; Jiang, C. Dental follicle stem cells: Tissue engineering and immunomodulation. Stem Cells Dev. 2019, 28, 986–994.
- Honda, M.J.; Imaizumi, M.; Tsuchiya, S.; Morsczeck, C. Dental follicle stem cells and tissue engineering. J. Oral Sci. 2010, 52, 541–552.
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem cells from the apical papilla: A promising source for stem cell-based therapy. BioMed Res. Int. 2019, 2019, e6104738.
- Nada, O.A.; El Backly, R.M. Stem Cells from the Apical Papilla (SCAP) as a tool for endogenous tissue regeneration. Front. Bioeng. Biotechnol. 2018, 6, 103.
- Grawish, M.E. Gingival-derived mesenchymal stem cells: An endless resource for regenerative dentistry. World J. Stem Cells 2018, 10, 116–118.
- Venkatesh, D.; Kumar, K.P.M.; Alur, J.B. Gingival mesenchymal stem cells. J. Oral Maxillofac. Pathol. 2017, 21, 296–298.
- Kawashima, N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch. Oral Biol. 2012, 57, 1439–1458.
- Karaöz, E.; Doğan, B.N.; Aksoy, A.; Gacar, G.; Akyüz, S.; Ayhan, S.; Genç, Z.S.; Yürüker, S.; Duruksu, G.; Demircan, P.C.; et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem. Cell Biol. 2010, 133, 95–112.
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535.
- Yamada, Y.; Fujimoto, A.; Ito, A.; Yoshimi, R.; Ueda, M. Cluster analysis and gene expression profiles: A cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials 2006, 27, 3766–3781.
- Patel, M.; Smith, A.J.; Sloan, A.J.; Smith, G.; Cooper, P.R. Phenotype and behaviour of dental pulp cells during expansion culture. Arch. Oral Biol. 2009, 54, 898–908.
- Bakopoulou, A.; Apatzidou, D.; Aggelidou, E.; Gousopoulou, E.; Leyhausen, G.; Volk, J.; Kritis, A.; Koidis, P.; Geurtsen, W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res. Ther. 2017, 8, 247.
- Spath, L.; Rotilio, V.; Alessandrini, M.; Gambara, G.; De Angelis, L.; Mancini, M.; Mitsiadis, T.A.; Vivarelli, E.; Naro, F.; Filippini, A.; et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J. Cell Mol. Med. 2010, 14, 1635–1644.
- Feng, X.; Xing, J.; Feng, G.; Sang, A.; Shen, B.; Xu, Y.; Jiang, J.; Liu, S.; Tan, W.; Gu, Z.; et al. Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/β-catenin signaling. Cell Mol. Neurobiol. 2013, 33, 1023–1031.
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90.
- Király, M.; Porcsalmy, B.; Pataki, A.; Kádár, K.; Jelitai, M.; Molnár, B.; Hermann, P.; Gera, I.; Grimm, W.-D.; Ganss, B.; et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem. Int. 2009, 55, 323–332.
- Vishwanath, V.R.; Nadig, R.R.; Nadig, R.; Prasanna, J.S.; Karthik, J.; Pai, V.S. Differentiation of isolated and characterized human dental pulp stem cells and stem cells from human exfoliated deciduous teeth: An in vitro study. J. Conserv. Dent. 2013, 16, 423–428.
- Kaukua, N.; Chen, M.; Guarnieri, P.; Dahl, M.; Lim, M.L.; Yucel-Lindberg, T.; Sundström, E.; Adameyko, I.; Mao, J.J.; Fried, K. Molecular differences between stromal cell populations from deciduous and permanent human teeth. Stem Cell Res. Ther. 2015, 6, 59.
- Akpinar, G.; Kasap, M.; Aksoy, A.; Duruksu, G.; Gacar, G.; Karaoz, E. Phenotypic and proteomic characteristics of human dental pulp derived mesenchymal stem cells from a natal, an exfoliated deciduous, and an impacted third molar tooth. Stem Cells Int. 2014, 2014, 457059.
- Tziafas, D.; Kodonas, K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J. Endod. 2010, 36, 781–789.
- Liu, Q.; Cen, L.; Yin, S.; Chen, L.; Liu, G.; Chang, J.; Cui, L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and β-TCP ceramics. Biomaterials 2008, 29, 4792–4799.
- Jiang, X.; Zhao, J.; Wang, S.; Sun, X.; Zhang, X.; Chen, J.; Kaplan, D.L.; Zhang, Z. Mandibular repair in rats with premineralized silk scaffolds and BMP-2-modified bMSCs. Biomaterials 2009, 30, 4522–4532.
- Lee, J.-S.; Lee, J.-M.; Im, G.-I. Electroporation-mediated transfer of Runx2 and Osterix genes to enhance osteogenesis of adipose stem cells. Biomaterials 2011, 32, 760–768.
- Yu, J.; He, H.; Tang, C.; Zhang, G.; Li, Y.; Wang, R.; Shi, J.; Jin, Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010, 11, 32.
- Luo, Z.; Kohli, M.R.; Yu, Q.; Kim, S.; Qu, T.; He, W. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase II pathways. J. Endod. 2014, 40, 937–942.
- Miyashita, S.; Ahmed, N.E.M.B.; Murakami, M.; Iohara, K.; Yamamoto, T.; Horibe, H.; Kurita, K.; Takano-Yamamoto, T.; Nakashima, M. Mechanical forces induce odontoblastic differentiation of mesenchymal stem cells on three-dimensional biomimetic scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 434–446.
- Choi, Y.-A.; Seol, M.-Y.; Shin, H.-I.; Park, E.K. Bobby sox homology regulates odontoblast differentiation of human dental pulp stem cells/progenitors. Cell Commun. Signal 2014, 12, 35.
- Shen, W.-C.; Lai, Y.-C.; Li, L.-H.; Liao, K.; Lai, H.-C.; Kao, S.-Y.; Wang, J.; Chuong, C.-M.; Hung, S.-C. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat. Commun. 2019, 10, 2226.
- Raoof, M.; Yaghoobi, M.M.; Derakhshani, A.; Kamal-abadi, A.M.; Ebrahimi, B.; Abbasnejad, M.; Shokouhinejad, N. A modified efficient method for dental pulp stem cell isolation. Dent. Res. J. 2014, 11, 244–250.
- Naz, S.; Khan, F.R.; Zohra, R.R.; Lakhundi, S.S.; Khan, M.S.; Mohammed, N.; Ahmad, T. Isolation and culture of dental pulp stem cells from permanent and deciduous teeth. Pak. J. Med. Sci. 2019, 35, 997–1002.
- Lindemann, D.; Werle, S.B.; Steffens, D.; Garcia-Godoy, F.; Pranke, P.; Casagrande, L. Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch. Oral Biol. 2014, 59, 970–976.
- Lin, S.-L.; Chang, W.-J.; Lin, C.-Y.; Hsieh, S.-C.; Lee, S.-Y.; Fan, K.-H.; Lin, C.-T.; Huang, H.-M. Static magnetic field increases survival rate of dental pulp stem cells during DMSO-free cryopreservation. Electromagn. Biol. Med. 2015, 34, 302–308.
- Gioventù, S.; Andriolo, G.; Bonino, F.; Frasca, S.; Lazzari, L.; Montelatici, E.; Santoro, F.; Rebulla, P. A novel method for banking dental pulp stem cells. Transfus. Apher. Sci. 2012, 47, 199–206.
- Human Dental Follicle Cells Express Embryonic, Mesenchymal and Neural Stem Cells Markers—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27764680/ (accessed on 28 March 2021).
- Rezai-Rad, M.; Bova, J.F.; Orooji, M.; Pepping, J.; Qureshi, A.; Del Piero, F.; Hayes, D.; Yao, S. Evaluation of bone regeneration potential of dental follicle stem cells for treatment of craniofacial defects. Cytotherapy 2015, 17, 1572–1581.
- Mabuchi, Y.; Morikawa, S.; Harada, S.; Niibe, K.; Suzuki, S.; Renault-Mihara, F.; Houlihan, D.D.; Akazawa, C.; Okano, H.; Matsuzaki, Y. LNGFR+THY-1+VCAM-1hi+ cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Rep. 2013, 1, 152–165.
- Bühring, H.-J.; Battula, V.L.; Treml, S.; Schewe, B.; Kanz, L.; Vogel, W. Novel markers for the prospective isolation of human MSC. Ann. N. Y. Acad. Sci. 2007, 1106, 262–271.
- Yasui, T.; Mabuchi, Y.; Toriumi, H.; Ebine, T.; Niibe, K.; Houlihan, D.D.; Morikawa, S.; Onizawa, K.; Kawana, H.; Akazawa, C.; et al. Purified human dental pulp stem cells promote osteogenic regeneration. J. Dent. Res. 2016, 95, 206–214.
- Baghaban Eslaminejad, M.; Malakooty Poor, E. Mesenchymal stem cells as a potent cell source for articular cartilage regeneration. World J. Stem Cells 2014, 6, 344–354.
- Allen, K.D.; Athanasiou, K.A. Tissue engineering of the TMJ disc: A review. Tissue Eng. 2006, 12, 1183–1196.
- Longoni, A.; Utomo, L.; van Hooijdonk, I.E.; Bittermann, G.K.; Vetter, V.C.; Kruijt Spanjer, E.C.; Ross, J.; Rosenberg, A.J.; Gawlitta, D. The chondrogenic differentiation potential of dental pulp stem cells. Eur. Cell Mater. 2020, 39, 121–135.
- Khajeh, S.; Razban, V.; Talaei-Khozani, T.; Soleimani, M.; Asadi-Golshan, R.; Dehghani, F.; Ramezani, A.; Mostafavi-Pour, Z. Enhanced chondrogenic differentiation of dental pulp-derived mesenchymal stem cells in 3D pellet culture system: Effect of mimicking hypoxia. Biologia 2018, 73, 715–726.
- Chen, Y.; Zhao, Q.; Yang, X.; Yu, X.; Yu, D.; Zhao, W. Effects of cobalt chloride on the stem cell marker expression and osteogenic differentiation of stem cells from human exfoliated deciduous teeth. Cell Stress Chaperones 2019, 24, 527–538.
- Laksana, K.; Sooampon, S.; Pavasant, P.; Sriarj, W. Cobalt chloride enhances the stemness of human dental pulp cells. J. Endod. 2017, 43, 760–765.
- Ejtehadifar, M.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Dehdilani, N.; Abbasi, P.; Molaeipour, Z.; Saleh, M. The effect of hypoxia on mesenchymal stem cell biology. Adv. Pharm. Bull. 2015, 5, 141–149.
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci. World J. 2013, 2013, e632972.
- Jahangir, S.; Eglin, D.; Pötter, N.; Khozaei Ravari, M.; Stoddart, M.J.; Samadikuchaksaraei, A.; Alini, M.; Baghaban Eslaminejad, M.; Safa, M. Inhibition of hypertrophy and improving chondrocyte differentiation by MMP-13 inhibitor small molecule encapsulated in alginate-chondroitin sulfate-platelet lysate hydrogel. Stem Cell Res. Ther. 2020, 11, 436.
- Chen, S.; Fu, P.; Cong, R.; Wu, H.; Pei, M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015, 2, 76–95.
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Kujat, R.; Prantl, L.; Nerlich, M.; Tuan, R.S.; Angele, P. Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. Int. Orthop. 2013, 37, 945–951.
- Bertram, H.; Boeuf, S.; Wachters, J.; Boehmer, S.; Heisel, C.; Hofmann, M.W.; Piecha, D.; Richter, W. Matrix metalloprotease inhibitors suppress initiation and progression of chondrogenic differentiation of mesenchymal stromal cells in vitro. Stem Cells Dev. 2009, 18, 881–892.
- Shintani, N.; Siebenrock, K.A.; Hunziker, E.B. TGF-ß1 enhances the BMP-2-Induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PLoS ONE 2013, 8, e53086.
- Pei, M.; Chen, D.; Li, J.; Wei, L. Histone deacetylase 4 promotes TGF-beta1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation 2009, 78, 260–268.
- Lengner, C.J.; Hassan, M.Q.; Serra, R.W.; Lepper, C.; van Wijnen, A.J.; Stein, J.L.; Lian, J.B.; Stein, G.S. Nkx3.2-mediated repression of Runx2 promotes chondrogenic differentiation. J. Biol. Chem. 2005, 280, 15872–15879.
- Hirao, M.; Tamai, N.; Tsumaki, N.; Yoshikawa, H.; Myoui, A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J. Biol. Chem. 2006, 281, 31079–31092.
- Lee, H.-H.; Chang, C.-C.; Shieh, M.-J.; Wang, J.-P.; Chen, Y.-T.; Young, T.-H.; Hung, S.-C. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci. Rep. 2013, 3, 2683.
- Hsu, S.; Huang, G.-S.; Lin, S.Y.F.; Feng, F.; Ho, T.-T.; Liao, Y.-C. Enhanced chondrogenic differentiation potential of human gingival fibroblasts by spheroid formation on chitosan membranes. Tissue Eng. Part A 2012, 18, 67–79.
- Ferré, F.C.; Larjava, H.; Loison-Robert, L.-S.; Berbar, T.; Owen, G.R.; Berdal, A.; Chérifi, H.; Gogly, B.; Häkkinen, L.; Fournier, B.P.J. Formation of cartilage and synovial tissue by human gingival stem cells. Stem Cells Dev. 2014, 23, 2895–2907.
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14.
- Jo, A.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.L.; Mok, J.; Lee, M.J.; et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014, 1, 149–161.
- Zhang, X.; Wu, S.; Naccarato, T.; Prakash-Damani, M.; Chou, Y.; Chu, C.-Q.; Zhu, Y. Regeneration of hyaline-like cartilage in situ with SOX9 stimulation of bone marrow-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0180138.
- Jiang, X.; Huang, X.; Jiang, T.; Zheng, L.; Zhao, J.; Zhang, X. The role of Sox9 in collagen hydrogel-mediated chondrogenic differentiation of adult mesenchymal stem cells (MSCs). Biomater. Sci. 2018, 6, 1556–1568.
- Akiyama, H. Control of chondrogenesis by the transcription factor Sox9. Mod. Rheumatol. 2008, 18, 213–219.
- Ng, L.-J.; Wheatley, S.; Muscat, G.E.O.; Conway-Campbell, J.; Bowles, J.; Wright, E.; Bell, D.M.; Tam, P.P.L.; Cheah, K.S.E.; Koopman, P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 1997, 183, 108–121.
- Hardingham, T.E.; Oldershaw, R.A.; Tew, S.R. Cartilage, SOX9 and notch signals in chondrogenesis. J. Anat. 2006, 209, 469–480.
- Oldershaw, R.A.; Hardingham, T.E. Notch signaling during chondrogenesis of human bone marrow stem cells. Bone 2010, 46, 286–293.
- Karlsson, C.; Lindahl, A. Notch signaling in chondrogenesis. Int. Rev. Cell Mol. Biol. 2009, 275, 65–88.
- Mead, T.J.; Yutzey, K.E. Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc. Natl. Acad. Sci. USA 2009, 106, 14420–14425.
- Sassi, N.; Laadhar, L.; Driss, M.; Kallel-Sellami, M.; Sellami, S.; Makni, S. The role of the Notch pathway in healthy and osteoarthritic articular cartilage: From experimental models to ex vivo studies. Arthritis Res. Ther. 2011, 13, 208.
- Green, J.D.; Tollemar, V.; Dougherty, M.; Yan, Z.; Yin, L.; Ye, J.; Collier, Z.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. Multifaceted signaling regulators of chondrogenesis: Implications in cartilage regeneration and tissue engineering. Genes Dis. 2015, 2, 307–327.
- Yi, S.W.; Kim, H.J.; Oh, H.J.; Shin, H.; Lee, J.S.; Park, J.S.; Park, K.-H. Gene expression profiling of chondrogenic differentiation by dexamethasone-conjugated polyethyleneimine with SOX trio genes in stem cells. Stem Cell Res. Ther. 2018, 9, 341.
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016, 92, 41–51.
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379.
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. StemJournal 2019, 1, 1–25.
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, e2495848.
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019, 8, 1118.
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003, 278, 41227–41236.
- Tang, Q.O.; Shakib, K.; Heliotis, M.; Tsiridis, E.; Mantalaris, A.; Ripamonti, U.; Tsiridis, E. TGF-beta3: A potential biological therapy for enhancing chondrogenesis. Expert Opin. Biol. Ther. 2009, 9, 689–701.
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009.
- Guo, X.; Wang, X.-F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009, 19, 71–88.
- Keller, B.; Yang, T.; Chen, Y.; Munivez, E.; Bertin, T.; Zabel, B.; Lee, B. Interaction of TGFβ and BMP signaling pathways during chondrogenesis. PLoS ONE 2011, 6, e16421.
- Thielen, N.G.M.; van der Kraan, P.M.; van Caam, A.P.M. TGFβ/BMP signaling pathway in cartilage homeostasis. Cells 2019, 8, 969.
- Zhang, M.; Zhou, Q.; Liang, Q.-Q.; Li, C.-G.; Holz, J.D.; Tang, D.; Sheu, T.-J.; Li, T.-F.; Shi, Q.; Wang, Y.-J. IGF-1 regulation of type II collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways. Osteoarthr. Cartil. 2009, 17, 100–106.
- Li, Y.; Wang, Y.; Chubinskaya, S.; Schoeberl, B.; Florine, E.; Kopesky, P.; Grodzinsky, A.J. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: Relevance to post traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 266–274.
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715.
- Zhang, Z.; Li, L.; Yang, W.; Cao, Y.; Shi, Y.; Li, X.; Zhang, Q. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthr. Cartil. 2017, 25, 309–320.
- Chen, C.-Y.; Li, C.; Ke, C.-J.; Sun, J.-S.; Lin, F.-H. Kartogenin enhances chondrogenic differentiation of MSCs in 3D Tri-copolymer scaffolds and the self-designed bioreactor system. Biomolecules 2021, 11, 115.
- Liu, F.; Xu, H.; Huang, H. A novel kartogenin-platelet-rich plasma gel enhances chondrogenesis of bone marrow mesenchymal stem cells in vitro and promotes wounded meniscus healing in vivo. Stem Cell Res. Ther. 2019, 10, 201.
- Huang, H.; Xu, H.; Zhao, J. A novel approach for meniscal regeneration using kartogenin-treated autologous tendon graft. Am. J. Sports Med. 2017, 45, 3289–3297.
- Kwon, J.Y.; Lee, S.H.; Na, H.-S.; Jung, K.; Choi, J.; Cho, K.H.; Lee, C.-Y.; Kim, S.J.; Park, S.-H.; Shin, D.-Y.; et al. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci. Rep. 2018, 8, 13832.
- Leung, B.P.; Sattar, N.; Crilly, A.; Prach, M.; McCarey, D.W.; Payne, H.; Madhok, R.; Campbell, C.; Gracie, J.A.; Liew, F.Y.; et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J. Immunol. 2003, 170, 1524–1530.
- Conaghan, P.G. The effects of statins on osteoarthritis structural progression: Another glimpse of the Holy Grail? Ann. Rheum. Dis. 2012, 71, 633–634.
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271.
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502.
- Yin, Z.; Yang, X.; Jiang, Y.; Xing, L.; Xu, Y.; Lu, Y.; Ding, P.; Ma, J.; Xu, Y.; Gui, J. Platelet-rich plasma combined with agarose as a bioactive scaffold to enhance cartilage repair: An in vitro study. J. Biomater. Appl. 2014, 28, 1039–1050.
- Hubka, K.M.; Dahlin, R.L.; Meretoja, V.V.; Kasper, F.K.; Mikos, A.G. Enhancing chondrogenic phenotype for cartilage tissue engineering: Monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng. Part B Rev. 2014, 20, 641–654.
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 2019, 6, 129–140.
- Iwasa, J.; Engebretsen, L.; Shima, Y.; Ochi, M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol. Arthrosc. 2009, 17, 561–577.
- Dashtdar, H.; Murali, M.R.; Selvaratnam, L.; Balaji Raghavendran, H.; Suhaeb, A.M.; Ahmad, T.S.; Kamarul, T. Ultra-structural changes and expression of chondrogenic and hypertrophic genes during chondrogenic differentiation of mesenchymal stromal cells in alginate beads. PeerJ 2016, 4.
- Mohan, N.; Mohanan, P.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Biol. Macromol. 2017, 104, 1936–1945.
- Nagura, I.; Fujioka, H.; Kokubu, T.; Makino, T.; Sumi, Y.; Kurosaka, M. Repair of osteochondral defects with a new porous synthetic polymer scaffold. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 258–264.
- Warnock, J.J.; Baker, L.; Ballard, G.A.; Ott, J. In vitro synthesis of tensioned synoviocyte bioscaffolds for meniscal fibrocartilage tissue engineering. BMC Vet. Res. 2013, 9, 242.
- Johnson, K.A.; Rose, D.M.; Terkeltaub, R.A. Factor XIIIA mobilizes transglutaminase 2 to induce chondrocyte hypertrophic differentiation. J. Cell Sci. 2008, 121, 2256–2264.
- Adamczyk, M. Transglutaminase 2 in cartilage homoeostasis: Novel links with inflammatory osteoarthritis. Amino Acids 2017, 49, 625–633.
- Li, J.; Dong, S. The signaling pathways involved in chondrocyte differentiation and hypertrophic differentiation. Stem Cells Int. 2016, 2016, e2470351.
- Soltani, M.; Malek, R.A.; Elmarzugi, N.A.; Mahomoodally, M.F.; Uy, D.; Leng, O.M.; El-Enshasy, H.A. Cordycepin: A biotherapeutic molecule from medicinal mushroom. Biol. Macrofungi 2019, 319–349.
- Cao, Z.; Dou, C.; Li, J.; Tang, X.; Xiang, J.; Zhao, C.; Zhu, L.; Bai, Y.; Xiang, Q.; Dong, S. Cordycepin inhibits chondrocyte hypertrophy of mesenchymal stem cells through PI3K/Bapx1 and Notch signaling pathway. BMB Rep. 2016, 49, 548–553.
- Nemeth, C.L.; Janebodin, K.; Yuan, A.E.; Dennis, J.E.; Reyes, M.; Kim, D.-H. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng. Part A 2014, 20, 2817–2829.
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; de Llano, J.J.M.; Carda, C. In vivo articular cartilage regeneration using human dental pulp stem cells cultured in an alginate scaffold: A preliminary study. Stem Cells Int. 2017, 2017, e8309256.
- Jacek, P.; Szustak, M.; Kubiak, K.; Gendaszewska-Darmach, E.; Ludwicka, K.; Bielecki, S. Scaffolds for chondrogenic cells cultivation prepared from bacterial cellulose with relaxed fibers structure induced genetically. Nanomaterials 2018, 8, 66.
- Kreuz, P.C.; Gentili, C.; Samans, B.; Martinelli, D.; Krüger, J.P.; Mittelmeier, W.; Endres, M.; Cancedda, R.; Kaps, C. Scaffold-assisted cartilage tissue engineering using infant chondrocytes from human hip cartilage. Osteoarthr. Cartil. 2013, 21, 1997–2005.
- Ollitrault, D.; Legendre, F.; Drougard, C.; Briand, M.; Benateau, H.; Goux, D.; Chajra, H.; Poulain, L.; Hartmann, D.; Vivien, D.; et al. BMP-2, hypoxia, and COL1A1/HtrA1 siRNAs favor neo-cartilage hyaline matrix formation in chondrocytes. Tissue Eng. Part C Methods 2015, 21, 133–147.
More
Information
Subjects:
Orthopedics
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
732
Revisions:
2 times
(View History)
Update Date:
29 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




