
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vikram Singh Rawat | + 2294 word(s) | 2294 | 2021-09-18 08:08:02 | | | |
| 2 | Catherine Yang | Meta information modification | 2294 | 2021-09-28 06:26:37 | | |
Video Upload Options
Nucleotide-binding oligomerization domain NOD-like receptors (NLRs) are conserved cytosolic pattern recognition receptors (PRRs) that track the intracellular milieu for the existence of infection, disease-causing microbes, as well as metabolic distresses. The NLRP3 inflammasome agglomerates are consequent to sensing a wide spectrum of pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs). Certain members of the NLR family have been documented to lump into multimolecular conglomerates called inflammasomes, which are inherently linked to stimulation of the cysteine protease caspase-1.
1. Introduction
Nucleotide-binding oligomerization domain NOD-like receptors (NLRs) are preserved pattern recognition receptors located in cell cytosol that track the intracellular milieu for the presence of infection, disease-causing microbes, as well as metabolic distresses. In humans, the NLR family is made up of 23 cytosolic proteins, and some 34 nlr murine genes have been determined. The usual segment structure of the NLR family members comprises of an amino-terminal effector part made up of a protein–protein interaction region, like the caspase-recruitment domain (CARD), pyrin domain (PYD), or Baculovirus inhibitor of apoptosis protein repeat (BIR) segment, a centrally positioned NOD domain and leucine-rich repeats associated with danger sensing at the carboxyl-terminal. Alterations at the N-terminal domain are employed for the subsequent assortment of NLR protein members. The biggest assortment incorporates the N-terminal PYD and has been christened the NLRPs. Certain members of the NLR family have been known to agglomerate into multimolecular structures called inflammasomes, which are inherently linked to the stimulation of the cysteine protease caspase-1. Following activation, caspase-1 severs the proinflammatory cytokines interleukin (IL)-1β and IL-18 into their biologically active entities, leading to the commencement of caspase-1-associated pyroptosis. IL-1β is an archetypic inflammatory cytokine implicated in multiple types of inflammatory maladies. Approaches to impede IL-1β ’s actions are possible, and their therapeutic effects have been demonstrated; nevertheless, such strategies are associated with certain constraints. For instance, treatments that focus on systemically negating IL-1β (i.e., anakinra, rilonacept, and canakinumab) have been reported to subsequently result in an escalated peril of infections and are hence deemed improper for oral use. NLRP3 is the most common inflammasome sensor probed for its association in a multitude of conditions, such as sterile inflammation, infections, as well as uncommon genetic autoimmune syndromes ( Table 1 ).
Table 1. Some inflammatory diseases with their targets (involving inflammasome signaling components) for possible therapeutic intervention.
| Disease | Targets in Inflammasome Signaling Cascade | Therapeutic Molecule |
|---|---|---|
| Acute Myocardial Infarction | NLRP3 | Colchicine |
| Type 2 Diabetes Mellitus | NLRP3(indirect action) | Metformin, Glyburide |
| IL-1β | Rilonacept | |
| Rheumatoid Arthritis | IL-1 Receptor | Anakinra |
| Caspase-1 | Pralnacasan(VX-740) | |
| P2X7 | AZD9056, CE-224535, GSK 1482169 | |
| Muckle–Wells Syndrome | Caspase-1 | Emricasan(VX-765) |
| IL-1β | Canakinumab | |
| Gout | IL-1β | Rilonacept |
| Xanthine Oxidase(XOD) | Allopurinol | |
| Systemic Lupus Erythematosus | NFκB (IKKβ kinase activity)/NLRP3 ATPase | Bay 11-7082 |
| Cryopyrin-Associated Periodic Syndromes(CAPS) | IL-1β | Rilonacept |
| Inflammatory Bowel Disease(IBD) | IL-18 | GSK1070806 |
| Familial Cold Autoinflammatory Syndrome(FCAS) | IL-1β | Canakinumab |
| Cancer | Caspase-1/NF-κB (IKKβ kinase activity)/NLRP3 ATPase | Parthenolide |
| B-cell Non-Hodgkin’s Lymphoma | IL-18 | GSK1070806 |
Given the therapeutic promise of an NLRP3 inhibitor, the concerted escalated venture of the scientific fraternity in the yesteryears towards the development of small molecules focusing on NLRP3 is quite predictable. However, incomplete comprehension of the steps leading to the NLRP3 inflammasome agglomeration and also insufficient understanding of the sensor crystal structure compound the odds of developing such inhibitor agents. Despite the fact that certain NLRP3 inflammasome antagonists have been developed and studied in preclinical protocols as well as cell-based assays, an NLRP3-specific inhibitor with therapeutic intent for humans has yet to be licensed. This review is intended to showcase the current developments with regard to promising NLRP3 inhibitors for clinical applications ( Table 2 ).
Table 2. Targets of some known NLRP3 Inhibitors.
| Inhibitor | Target(s) | Documented Mechanism(s) | References |
|---|---|---|---|
| Sulfonylureas | |||
| Glyburide | NLRP3(indirect action) | Abrogation of ASC agglomeration acting downstream of P2X7; Suppression of KATP channels |
[1] |
| MCC950 | NLRP3 | NLRP3 inflammasome activation involves a role of its ATPase domain. MCC950 is known to directly target and restrain this ATP-hydrolysis motif in both canonical as well as non-canonical NLRP3 inflammasomes | [2][3] |
| Glitazones | |||
| CY-09 | NLRP3 | Effective and direct suppressor of NLRP3 inflammasome with remarkable capability to impede NLRP3 inflammasome activation in vivo in murine models and ex vivo in human cells; blocks NLRP3 ATPase actions | [4] |
| Substituted 2-pyrazolin-5-ones | |||
| Edaravone | NLRP3 | Scavenge reactive oxygen species(ROS) thereby impeding NLRP3-evoked IL-1β processing and release; also known to suppress IL-1β, caspase 1 and NF-kB-reliant NLRP3 inflammation signaling | [5][6] |
| Arsenic compounds | |||
| Arsenic trioxide(As2O3) | NLRP3 | As2O3 suppresses NLRP3 inflammasome stimulation and consequent IL-1β and IL-18 release | [7][8] |
| Alkaloid | |||
| Colchicine | NLRP3 | Efficaciously attenuates the expression levels of IL-1β, IL-6 and IL-18 by abrogating NLRP3 inflammasome activation cascade | [9][10] |
| Biguanide | |||
| Metformin | NLRP3 | Adenosine monophosphate-activated protein kinase(AMPK) is known to modulate NLRP3 inflammasome stimulation; decreases the expression of NLRP3 as well as kindling of the NLRP3 inflammasome signaling pathway | [11][12] |
| GLP-1 analogs | |||
| Liraglutide | NLRP3(hepatic) | Repression of the hepatic NLRP3 inflammasome | [13] |
| Statins | |||
| Atorvastatin | NLRP3 | Conspicuously decrements levels of NLRP3, caspase-1, and IL-1β; also, the NF-κB suppressor attenuate levels of inflammatory cytokines in inflammatory cells. The stimulation of the NF-κB signaling cascade is engaged in NLRP3 inflammasome activity modulation | [14][15] |
| SGLT-2 Inhibitors(Dapagliflozin, Empagliflozin)-P2Y12 Antagonist(Ticagrelor) | |||
| Dapagliflozin | NLRP3 | Extenuates inflammation-evoked renal damage and glomerulosclerosis in diabetic kidneys by ameliorating NLRP3 inflammasome stimulation; AMPK activation | [16] |
| Empagliflozin | NLRP3 | Impedes kindling of NLRP3 inflammasome and decrements downstream inflammatory signaling in the diabetic kidneys | [17] |
| Ticagrelor | NLRP3 | Repress NLRP3 inflammasome stimulation; AMPK activation | [18] |
| Xanthine oxidase(XOD) enzyme inhibitor | |||
| Allopurinol | NLRP3, XOD | Represses xanthine oxidase(XOD) action and subsequently attenuates generation of uric acid (UA) and reactive oxygen species (ROS), which are known to kindle the NLRP3 pathway | [19][20] |
| Vinylsulfones | |||
| BAY11-7082 | NLRP3, IKK, E2/3 enzymes, PTPs | Leads to cysteine alkylation of NLRP3 inflammasome ATPase domains; represses NLRP3 ATPase actions | [4][21] |
| Beta-Nitrostyrenes | |||
| MNS | NLRP3 | Leads to cysteine alteration of NLRP3 inflammasome ATPase domains; represses NLRP3 inflammasome actions | [22] |
| Acrylate Derivatives | |||
| INF39 | NLRP3 | Abrogates NLRP3 inflammasome ATPase actions; represses priming | [23] |
| Acylhydrazone | |||
| EMD638683 | NLRP3 | Suppression of NLRP3 and IL-1β expression | [24] |
| Benzimidazoles | |||
| FC11A-2 | NLRP3(indirect effect) | Hampers pro-caspase-1 autocleavage; impedes IL-1beta/18 secretion | [25][26] |
| Sulfonylnitriles | |||
| Dapansutrile(OLT1177) | NLRP3 | Abrogates NLRP3 inflammasome ATPase actions; suppresses NLRP3 inflammasome stimulation | [27][28] |
| Benzoxathiole Derivatives | |||
| BOT-4-one | NLRP3 | Akin to various covalent modulators that repress NLRP3, this agent blunts its ATPase activity; inhibits priming | [29][30][31] |
| Tryptophan Derivative | |||
| Tranilast | NLRP3 | Interacts with NACHT segment of NLRP3 to abrogate NLRP3-NLRP3 and NLRP3-ASC association | [4][32] |
| Natural Products | |||
| BHB | NLRP3(indirectly) | Abrogation of outward movement of K+ with consequent decrement in ASC agglomeration and IL-1beta/18 release | [33] |
| Parthenolide | NLRP1 & 3, Caspase- 1, NF-kB, IKKB kinase activity | Alkyl modification of cysteine moieties present in ATPase segments of NLRP3 and caspase-1; abrogates NLRP3 ATPase actions | [34] |
| Oridonin | NLRP3 | Selectively represses NLRP3 inflammasome stimulation; associates with cysteine 279 residue of NLRP3 and abrogates NLRP3-NEK7 association | [35] |
| Caspase Inhibitors | |||
| Pralnacasan(VX-740) | Caspase-1 | Covalent alteration of catalytic cysteine moiety in caspase-1 active site with consequent abrogation of caspase-1 effects and splitting of pro-IL-1Beta/18 | [36][37][38] |
| Emricasan(VX-765) | Caspase-1 | Covalent alteration of catalytic cysteine moiety in caspase-1 active site with consequent abrogation of caspase-1 effects and splitting of pro-IL-1Beta/18 | [36][37][38] |
2. NLRP3 Inflammasome Agglomeration
The NLRP3 inflammasome agglomerates are consequent to responding to a wide spectrum of pathogen-related molecular arrangements and damage-related molecular motifs. There is usually little cellular concentration of NLRP3, and in order to attain the critical threshold needed to spark caspase-1 activation, the canonical NLRP3 inflammasome stimulation hinges upon two crucial phases.
The first step, termed priming, leads to activation of nuclear factor kappa light chain enhancer of activated B cells (NF-kB) and various transcription factors following the involvement of pattern recognition receptors (PRRs). This engenders the expression of NLRP3 as well as pro-IL-1β. The second step consists of signal (or trigger) detection that in turn governs the triggering mechanism of NLRP3 and the subsequent generation of the inflammasome. The NLRP3 NACHT region, which possesses ATPase property, is essential for the agglomeration of the NLRP3 inflammasome. Following stimulation and self-oligomerization of NLRP3, the PYD–PYD cooperation among NLRP3 and the inflammasome adaptor protein apoptosis-associated speck-like protein having a CARD (ASC) leads to the generation of speck-like entities, which serves as a scaffold for the engagement by contiguity of procaspase-1 via CARD–CARD interaction. Following autocatalytic enzyme cleavage and the subsequent generation of active caspase-1 (p10 and p20), the transformation of pro-IL-1β and pro-IL-18 into their biologically active forms occurs and gasdermin D–moderated pyroptotic cell lethality ensues. Since a wide array of signals finally culminates in NLRP3 inflammasome generation, it is believed that NLRP3 can detect downstream developments arising from the archetype trigger that produces disruption of cellular homeostasis, with subsequent inflammasome agglomeration ( Figure 1 ). This disruption consists of alterations of ion flux (K + , Cl − , and Ca 2+ ), reactive oxygen species (ROS) generation, and lysosomal injury.
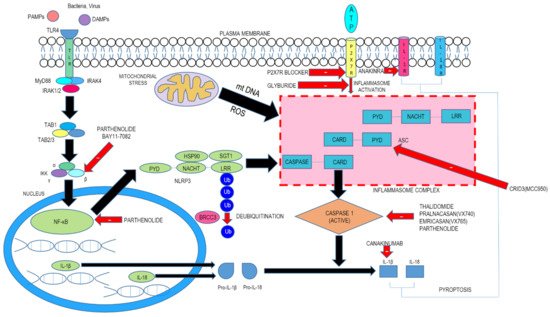
3. Inhibitors of NLRP3 Inflammasome-Driven In Vivo Disease Models
NLRP3 inflammasome brings about the sterile inflammatory reaction provoked by tissue injury and influences the causative pathology of myocardial ischemia–reperfusion injury [39][40][41]. Abrogating inflammasome stimulation by genetic method conspicuously attenuates infarct progress and myocardial fibrosis and malfunction [39][40].
In those afflicted with type 2 diabetes, enhanced NLRP3 inflammasome stimulation and processing of IL-1β were notably repressed by treatment with the antidiabetic drug metformin via AMPK stimulation [42].
The anticancer properties of BOT-4-one are mediated through the blockade of janus kinase 3 (JAK3)/signal transducer and activator of transcription 3 (STAT3) signaling [43] and immunomodulatory action via suppression of IKKb [44]. Upon testing for NF-kB-unrelated effects, BOT4-one was found to act akin to other alkylating agents like Bay 11-7082 and MNS, decrementing IL-1β release following canonical and noncanonical NLRP3 inflammasome stimulation, but with enhanced potency [29].
Scant information is available regarding the interaction of kinases with NLRP3; some other inflammasomes have been even less researched. Only if we can comprehend and dissect the kinase signaling networks encompassing these processes, their entire promise can be utilized, and pertinent kinase inhibitors may be ushered or repurposed into therapeutics as anti-inflammatory agents.
4. Conclusions
The intensive role of the inflammasome in masterminding innate immune responses (arising out of microbial infections and non-infectious diseases) has been proven beyond doubt by the occurrence of several heritable and acquired maladies which stem out of dysregulated NLRP3 inflammasome activation and the effectiveness of antagonists of IL-1β or its receptor for intervention in many of these disease conditions. NLRP3-induced pyroptosis and IL-1β/18 secretion are associated with numerous maladies. The degree to which NLRP3 inflammasome stimulation confers towards pyroptosis is still unclear, but NLRP3 activation does lead to pyroptosis, which consequently can inflict severe damage to crucial body organs [45]. Currently, NLRP3-linked afflictions may be ameliorated by agents which could abrogate IL-1β, such as counteracting IL-1β antibody canakinumab, recombinant IL-1 receptor antagonist anakinra, and the soluble camouflage IL-1 receptor, rilonacept. These biological molecules have been employed to manage cryopyrin-associated periodic syndromes (CAPS) as well as various maladies related to IL-1β [46]. Apart from NLRP-3 generated IL-1β, other cytokines like IL-18 may also assist with the development of the NLRP3-related afflictions [47][48]. Other inflammasomes or inflammasome-independent pathways can also lead to IL-1β production; hence, IL-1β inhibitors can only lead to non-intentional immunosuppressive actions. Thus, pharmacological inhibitors focusing on NLRP3 inflammasome inhibition would be a preferred alternative for combating NLRP3-mediated maladies. NLRP3-mediated pyroptosis has been documented by a plethora of contemporary studies as a crucial system adding to the NLRP3 inflammasome linked disease processes [49][50]. Documentation surfacing has noted gasdermin D (GSDMD) as a controlling protein culpable for pyroptosis [51][52], making it an enticing therapeutic target for ameliorating NLRP3-evoked pyroptosis associated disorders. Since knowledge of the structure and components of the NLRP3 inflammasome has become available, forthcoming studies should harness this lead and effect progress of development of direct NLRP3 inflammasome inhibitors endowed with increased exactitude and potency. Moreover, nanobodies (Nbs) are lately being scrutinized comprehensively as therapeutics owing to their high precision, stability, and low propensity to induce side effects [53][54]. It can be foreseen that Nbs may also be assessed for inhibiting NLRP3 inflammasome activation. Significant strides have been undertaken to unravel the NLRP3 inflammasome structure, the processes leading to its activation, and its role in the initiation and evolution of various disorders. Moreover, a multitude of small molecules as NLRP3 inflammasome inhibitors have been documented in the research literature, and a few of these leads have highlighted admirable therapeutic prowess. However, none of them has been approved by Food and Drug Administration (FDA) or other drug regulatory agencies. Contemporary research must continuously concentrate on the development of specific, small-molecule NLRP3 inflammasome activation inhibitors with enhanced pharmacokinetic characteristics, the ability to permeate efficiently across the blood–brain barrier and cell membranes, and it must be affordable too. Undoubtedly, the ongoing contemporary characterization of clinical inflammasome activators and blockers will spur interest in acquiring deeper insights into inflammasome-driven autoinflammatory mechanisms from a futuristic point of view. This is bound to widen therapeutic modalities for patients suffering from NLRP3-mediated metabolic and neurodegenerative diseases as well as certain cancers, where there is a tremendous unmet therapeutic need [55]. Finally, an appreciation of the molecular biology in connection with inflammasome priming and activation facilitates the prediction that an array of nutraceuticals could possibly confer salutary clinical promise for impeding inflammasome activity—antioxidants such as phycocyanobilin, phase 2 inducers, melatonin, and N-acetylcysteine, the AMPK activator berberine, glucosamine, zinc, and various nutraceuticals which ramp up production of hydrogen sulfide. Complex nutraceuticals or functional foods consisting of several of such compounds may find value in the obviation and modulation of a great variety of medical disorders [56].
References
- Perregaux, D.G.; McNiff, P.; Laliberte, R.; Hawryluk, N.; Peurano, H.; Stam, E.; Eggler, J.; Griffiths, R.; Dombroski, M.A.; Gabel, C.A. Identification and characterization of a novel class of interleukin-1 post-translational processing inhibitors. J. Pharmacol. Exp. Ther. 2001, 299, 187–197.
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Munoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255.
- Coll, R.C.; Hill, J.R.; Day, C.J.; Zamoshnikova, A.; Boucher, D.; Massey, N.L.; Chitty, J.L.; Fraser, J.A.; Jennings, M.P.; Robertson, A.A.; et al. MCC950 directly targets the NLRP3 ATP-hydrolysis motif for inflammasome inhibition. Nat. Chem. Biol. 2019, 15, 556–559.
- Jiang, H.; He, H.; Chen, Y.; Huang, W.; Cheng, J.; Ye, J.; Wang, A.; Tao, J.; Wang, C.; Liu, Q.; et al. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J. Exp. Med. 2017, 214, 3219–3238.
- Wang, H.M.; Zhang, T.; Huang, J.K.; Xiang, J.Y.; Chen, J.J.; Fu, J.L.; Zhao, Y.W. Edaravone attenuates the proinflammatory response in amyloid-beta-treated microglia by inhibiting NLRP3 inflammasome-mediated IL-1beta secretion. Cell Physiol. Biochem. 2017, 43, 1113–1125.
- Miao, H.; Jiang, Y.; Geng, J.; Zhang, B.; Zhu, G.; Tang, J. Edaravone administration confers neuroprotection after experimental intracerebral hemorrhage in rats via NLRP3 suppression. J. Stroke Cerebrovasc. Dis. 2020, 29, 104468.
- Ahn, H.; Kim, J.; Kang, S.G.; Yoon, S.I.; Ko, H.J.; Kim, P.H.; Hong, E.J.; An, B.S.; Lee, E.; Lee, G.S. Mercury and arsenic attenuate canonical and non-canonical NLRP3 inflammasome activation. Sci. Rep. 2018, 8, 13659.
- Lo, Y.H.; Huang, Y.W.; Wu, Y.H.; Tsai, C.S.; Lin, Y.C.; Mo, S.T.; Kuo, W.C.; Chuang, Y.T.; Jiang, S.T.; Shih, H.M.; et al. Selective inhibition of the NLRP3 inflammasome by targeting to promyelocytic leukemia protein in mouse and human. Blood 2013, 121, 3185–3194.
- Martinez, G.J.; Robertson, S.; Barraclough, J.; Xia, Q.; Mallat, Z.; Bursill, C.; Celermajer, D.S.; Patel, S. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J. Am. Heart Assoc. 2015, 4, e002128.
- Otani, K.; Watanabe, T.; Shimada, S.; Takeda, S.; Itani, S.; Higashimori, A.; Nadatani, Y.; Nagami, Y.; Tanaka, F.; Kamata, N.; et al. Colchicine prevents NSAID-induced small intestinal injury by inhibiting activation of the NLRP3 inflammasome. Sci. Rep. 2016, 6, 32587.
- Zhang, L.; Lu, L.; Zhong, X.; Yue, Y.; Hong, Y.; Li, Y.; Li, Y. Metformin reduced NLRP3 inflammasome activity in Ox-LDL stimulated macrophages through adenosine monophosphate activated protein kinase and protein phosphatase 2A. Eur. J. Pharmacol. 2019, 852, 99–106.
- Tan, Y.; Chen, J.; Jiang, Y.; Chen, X.; Li, J.; Chen, B.; Gao, J. The anti-periodontitis action of metformin via targeting NLRP3 inflammasome. Arch. Oral Biol. 2020, 114, 104692.
- Zhu, W.; Feng, P.P.; He, K.; Li, S.W.; Gong, J.P. Liraglutide protects non-alcoholic fatty liver disease via inhibiting NLRP3 inflammasome activation in a mouse model induced by high-fat diet. Biochem. Biophys. Res. Commun. 2018, 505, 523–529.
- Kong, F.; Ye, B.; Lin, L.; Cai, X.; Huang, W.; Huang, Z. Atorvastatin suppresses NLRP3 inflammasome activation via TLR4/MyD88/NF-κB signaling in PMA-stimulated THP1 monocytes. Biomed. Pharmacother. 2016, 82, 167–172.
- Peng, S.; Xu, L.W.; Che, X.Y.; Xiao, Q.Q.; Pu, J.; Shao, Q.; He, B. Atorvastatin inhibits inflammatory response, attenuates lipid deposition, and improves the stability of vulnerable atherosclerotic plaques by modulating autophagy. Front. Pharmacol. 2018, 9, 438.
- Birnbaum, Y.; Bajaj, M.; Yang, H.C.; Ye, Y. Combined SGLT2 and DPP4 inhibition reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic nephropathy in mice with type 2 diabetes. Cardiovasc. Drugs Ther. 2018, 32, 135–145.
- Benetti, E.; Mastrocola, R.; Vitarelli, G.; Cutrin, J.C.; Nigro, D.; Chiazza, F.; Mayoux, E.; Collino, M.; Fantozzi, R. Empagliflozin protects against diet- Induced NLRP-3 Inflammasome activation and lipid accumulation. J. Pharmacol. Exp. Therapeut. 2016, 359, 45–53.
- Chen, H.; Tran, D.; Yang, H.C.; Nylander, S.; Birnbaum, Y.; Ye, Y. Dapagliflozin and ticagrelor have additive effects on the attenuation of the activation of the NLRP3 inflammasome and the progression of diabetic cardiomyopathy: An AMPK–mTOR interplay. Cardiovasc. Drugs Ther. 2020, 34, 443–461.
- Foresto-Neto, O.; Ávila, V.F.; Arias, S.C.; Zambom, F.F.; Rempel, L.C.; Faustino, V.D.; Machado, F.G.; Malheiros, D.M.; Abensur, H.; Camara, N.O.; et al. NLRP3 inflammasome inhibition ameliorates tubulointerstitial injury in the remnant kidney model. Lab. Investig. 2018, 98, 773–782.
- Schlesinger, N.; Brunetti, L. Beyond urate lowering: Analgesic and anti-inflammatory properties of allopurinol. Semin. Arthritis Rheum. 2019, 50, 444–450.
- Juliana, C.; Fernandes-Alnemri, T.; Wu, J.; Datta, P.; Solorzano, L.; Yu, J.W.; Meng, R.; Quong, A.A.; Latz, E.; Scott, C.P.; et al. Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J. Biol. Chem. 2010, 285, 9792–9802.
- He, Y.; Varadarajan, S.; Munoz-Planillo, R.; Burberry, A.; Nakamura, Y.; Nunez, G. 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J. Biol. Chem. 2014, 289, 1142–1150.
- Cocco, M.; Garella, D.; Di Stilo, A.; Borretto, E.; Stevanato, L.; Giorgis, M.; Marini, E.; Fantozzi, R.; Miglio, G.; Bertinaria, M. Electrophilic warhead-based design of compounds preventing NLRP3 inflammasome-dependent pyroptosis. J. Med. Chem. 2014, 57, 10366–10382.
- Gan, W.; Ren, J.; Li, T.; Lv, S.; Li, C.; Liu, Z.; Yang, M. The SGK1 inhibitor EMD638683, prevents Angiotensin II-induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochim. Biophys. Acta 2017, 1864, 1–10.
- Liu, W.; Guo, W.; Wu, J.; Luo, Q.; Tao, F.; Gu, Y.; Shen, Y.; Li, J.; Tan, R.; Xu, Q.; et al. A novel benzo imidazole derivate prevents the development of dextran sulfate sodium-induced murine experimental colitis via inhibition of NLRP3 inflammasome. Biochem. Pharmacol. 2013, 85, 1504–1512.
- Pan, L.; Hang, N.; Zhang, C.; Chen, Y.; Li, S.; Sun, Y.; Li, Z.; Meng, X. Synthesis and biological evaluation of novel benzimidazole derivatives and analogs targeting the NLRP3 inflammasome. Molecules 2017, 22, 213.
- Marchetti, C.; Swartzwelter, B.; Gamboni, F.; Neff, C.P.; Richter, K.; Azam, T.; Carta, S.; Tengesdal, I.; Nemkov, T.; D’Alessandro, A.; et al. OLT1177, a beta-sulfonyl nitrile compound, safe in humans, inhibits the NLRP3 inflammasome and reverses the metabolic cost of inflammation. Proc. Natl. Acad. Sci. USA 2018, 115, E1530–E1539.
- Hoffman, H.M.; Mueller, J.L.; Broide, D.H.; Wanderer, A.A.; Kolodner, R.D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001, 29, 301–305.
- Shim, D.-W.; Shin, W.Y.; Yu, S.H.; Kim, B.H.; Ye, S.K.; Koppula, S.; Won, H.S.; Kang, T.B.; Lee, K.H. BOT-4-one attenuates NLRP3 inflammasome activation: NLRP3 alkylation leading to the regulation of its ATPase activity and ubiquitination. Sci. Rep. 2017, 7, 1–12.
- Misawa, T.; Takahama, M.; Kozaki, T.; Lee, H.; Zou, J.; Saitoh, T.; Akira, S. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013, 14, 454–460.
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414.
- Huang, Y.; Jiang, H.; Chen, Y.; Wang, X.; Yang, Y.; Tao, J.; Deng, X.; Liang, G.; Zhang, H.; Jiang, W.; et al. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. EMBO Mol. Med. 2018, 10, e8689.
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269.
- Han, S.C.; Cai, W.X.; Yang, X.K.; Jia, Y.H.; Zheng, Z.; Wang, H.T.; Li, J.; Li, Y.; Gao, J.X.; Fan, L.; et al. ROS-mediated NLRP3 inflammasome activity is essential for burn-induced acute lung injury. Mediat. Inflamm. 2015, 2015, 720457.
- He, H.; Jiang, H.; Chen, Y.; Ye, J.; Wang, A.; Wang, C.; Liu, Q.; Liang, G.; Deng, X.; Jiang, W.; et al. Oridonin is a covalent NLRP3 inhibitor with strong antiinflammasome activity. Nat. Commun. 2018, 9, 2550.
- MacKenzie, S.H.; Schipper, J.L.; Clark, A.C. The potential for caspases in drug discovery. Curr. Opin. Drug Discov. Dev. 2010, 13, 568–576.
- Lee, H.; Shin, E.A.; Lee, J.H.; Ahn, D.; Kim, C.G.; Kim, J.H.; Kim, S.H. Caspase inhibitors: A review of recently patented compounds (2013–2015). Expert Opin. Ther. Pat. 2017, 28, 47–59.
- Shi, J.J.; Zhao, Y.; Wang, Y.P.; Gao, W.Q.; Ding, J.J.; Li, P.; Hu, L.Y.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192.
- Toldo, S.; Marchetti, C.; Mauro, A.G.; Chojnacki, J.; Mezzaroma, E.; Carbone, S.; Zhang, S.; Van Tassell, B.; Salloum, F.N.; Abbate, A. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int. J. Cardiol. 2016, 209, 215–220.
- Kawaguchi, M.; Takahashi, M.; Hata, T.; Kashima, Y.; Usui, F.; Morimoto, H.; Izawa, A.; Takahashi, Y.; Masumoto, J.; Koyama, J.; et al. Inflammasome Activation of Cardiac Fibroblasts Is Essential for Myocardial Ischemia/Reperfusion Injury. Circulation 2011, 123, 594–604.
- Sandanger, O.; Ranheim, T.; Vinge, L.E.; Bliksoen, M.; Alfsnes, K.; Finsen, A.V.; Dahl, C.P.; Askevold, E.T.; Florholmen, G.; Christensen, G.; et al. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2013, 99, 164–174.
- Lee, H.M.; Kim, J.J.; Kim, H.J.; Shong, M.; Ku, B.J.; Jo, E.K. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 2013, 62, 194–204.
- Kim, B.-H.; Min, Y.S.; Choi, J.S.; Baeg, G.H.; Kim, Y.; Shin, J.W.; Kim, T.Y.; Ye, S.K. Benzoxathiol derivative BOT-4-one suppresses L540 lymphoma cell survival and proliferation via inhibition of JAK3/STAT3 signaling. Exp. Mol. Med. 2011, 43, 313–321.
- Lee, H.G.; Cho, N.C.; Jeong, A.J.; Li, Y.C.; Rhie, S.J.; Choi, J.S.; Lee, K.H.; Kim, Y.; Kim, Y.N.; Kim, M.H.; et al. Immunomodulatory activities of the benzoxathiole derivative BOT-4-One ameliorate pathogenic skin inflammation in mice. J. Investig. Dermatol. 2016, 136, 107–116.
- Jiang, D.; Chen, S.; Sun, R.; Zhang, X.; Wang, D. The NLRP3 inflammasome: Role in metabolic disorders and regulation by metabolic pathways. Cancer Lett. 2018, 419, 8–19.
- Dinarello, C.A.; van der Meer, J.W. Treating inflammation by blocking interleukin-1 in humans. Semin. Immunol. 2013, 25, 469–484.
- Nowarski, R.; Jackson, R.; Gagliani, N.; de Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 2015, 163, 1444–1456.
- Lu, B.; Nakamura, T.; Inouye, K.; Li, J.; Tang, Y.; Lundbäck, P.; Valdes-Ferrer, S.I.; Olofsson, P.S.; Kalb, T.; Roth, J.; et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature 2012, 488, 670–674.
- Kanneganti, A.; Malireddi, R.S.; Saavedra, P.H.; Walle, L.V.; Van Gorp, H.; Kambara, H.; Tillman, H.; Vogel, P.; Luo, H.R.; Xavier, R.J.; et al. GSDMD is critical for autoinflammatory pathology in a mouse model of Familial Mediterranean Fever. J. Exp. Med. 2018, 215, 1519–1529.
- Xiao, J.; Wang, C.; Yao, J.-C.; Alippe, Y.; Xu, C.; Kress, D.; Civitelli, R.; Abu-Amer, Y.; Kanneganti, T.D.; Link, D.C.; et al. Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 2018, 16, e3000047.
- Gaidt, M.M.; Hornung, V. Pore formation by GSDMD is the effector mechanism of pyroptosis. EMBO J. 2016, 35, 2167–2169.
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158.
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding featuRes. for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713.
- Konning, D.; Zielonka, S.; Grzeschik, J.; Empting, M.; Valldorf, B.; Krah, S.; Schröter, C.; Sellmann, C.; Hock, B.; Kolmar, H. Camelid and shark single domain antibodies: Structural featuRes. and therapeutic potential. Curr. Opin. Struct. Biol. 2017, 45, 10–16.
- Chauhan, D.; Vande Walle, L.; Lamkanfi, M. Therapeutic modulation of inflammasome pathways. Immunol. Rev. 2020, 297, 123–138.
- McCarty, M.F.; Iloki Assanga, S.B.; Lewis Luján, L.; O’Keefe, J.H.; DiNicolantonio, J.J. Nutraceutical Strategies for Suppressing NLRP3 Inflammasome Activation: Pertinence to the Management of COVID-19 and Beyond. Nutrients 2021, 13, 47.





