
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Magdalena Druszczyńska | + 2242 word(s) | 2242 | 2021-08-11 08:13:59 | | | |
| 2 | Lindsay Dong | Meta information modification | 2242 | 2021-09-28 08:00:51 | | |
Video Upload Options
Although the therapeutic effect of mycobacteria as antitumor agents has been known for decades, recent epidemiological and experimental studies have revealed that mycobacterium-related chronic inflammation may be a possible mechanism of cancer pathogenesis. Mycobacterium tuberculosis and non-tuberculous Mycobacterium avium complex infections have been implicated as potentially contributing to the etiology of lung cancer, whereas Mycobacterium ulcerans has been correlated with skin carcinogenesis. The risk of tumor development with chronic mycobacterial infections is thought to be a result of many host effector mechanisms acting at different stages of oncogenesis.
1. Introduction
2. Association of M.tb Infection with Malignancy Development
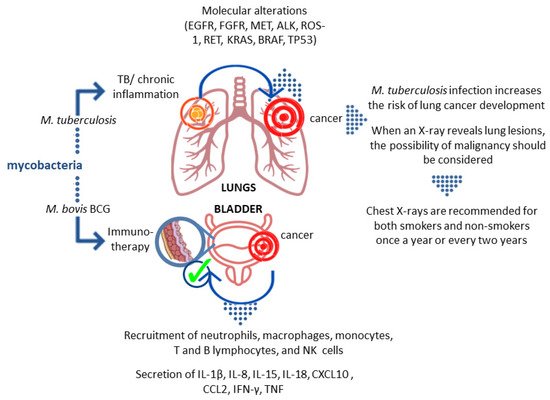
It has been documented that pulmonary TB caused by the intracellular pathogen M.tb increases the risk and mortality of lung cancer [1][2][3][4][5]. In a cohort study adjusted for comorbidities by Yu et al., the adjusted hazard ratio (aHR) for lung cancer in TB patients was 3.32 (95% CI: 2.704.09) [2]. It was estimated that lung cancer was 11 times more common in TB patients than in non-TB patients, and the association between lung cancer and prior TB (RR 3.43; 95% CI: 1.936.11) was confirmed in a systematic review by Liang et al. [6]. The most recent cohort study involving 20,252 participants in South Korea showed that when compared to the control group, the hazard ratio of lung cancer among individuals with old pulmonary TB was 3.24 (95% CI, 1.875.62). Furthermore, in patients with old pulmonary TB, the hazard ratios of lung cancer for never-smokers, ex-smokers, and current smokers were 3.52 (95% CI, 1.1710.63), 2.16 (95% CI, 0.895.24), and 3.71 (95% CI, 1.499.22), respectively, compared to the control group. This suggests that individuals with old pulmonary TB are at increased risk of developing lung cancer when compared to the general population without pulmonary TB [7]. On the other hand, cancer patients are known to have a higher incidence of TB. The aHR for TB was 1.67 (95% CI:1.421.96) in a retrospective cohort analysis of cancer patients [8].
The association between TB and lung cancer is not fully understood. Lung cancer can develop independently and decrease local immunity, leading to latent TB reactivation or new exogenous infection. Prolonged inflammatory response in TB with substantial remodeling of lung tissue may function as a cause of cancer. Chronic M.tb infection has also been linked to cell dysplasia and squamous cell lung cancer (SCC). By generating DNA damage, Mycobacterium-infected macrophages may play a role in TB-induced carcinogenesis. These findings support a causal relationship between TB and malignant transformation [9]. The main rationale for considering a strong association between TB and lung cancer is that carcinoma can develop from TB scars (scar carcinoma), occur by epithelium metaplasia of tuberculous cavities, develop in old TB lesions, and reactivate the old focus of TB [10]. TB-related chronic inflammation and fibrosis can cause genetic mutations and changes. Lung parenchyma tissue is involved in both TB and lung cancer. Furthermore, continuous cough in lung cancer, morphological vascular variations, lymphocytosis processes, and the production of certain immune mediators such as interleukins are factors that have led to the hypothesis that TB plays a role in lung cancer [1][6][11][12][13].
Another factor that might be considered to play a critical role in the malignancy development in the context of M.tb infection can be immunodeficiency defined as an inherited or acquired disorder with defects in the function of the immune system [14]. Genetic disorders underlying immunodeficiency may predispose to oncogenesis due to impaired immune surveillance of the tumor and abnormalities related to the course of infection and/or inflammation [15].
3. Mycobacteria as Causative Agents of Cancer
The success of M.tb as a persistent pathogen is largely attributed to its ability to survive in the host tissues. It has acquired various strategies to reside and multiply within macrophages, frontline host immune defense cells, avoiding an acquired immune response or subverting its consequences [16][17]. By suppressing macrophage maturation and lysosomal acidification as well as inhibiting oxidative stress, apoptosis, and autophagy, M.tb is capable of remaining in the host for a long time. The hallmark of chronic tuberculous inflammation is the formation of granulomas comprising aggregates of immune cells, including macrophages, giant cells, and foamy macrophages in the center, surrounded by the lymphocyte-rich marginal zone [18]. The efficient maintenance of granulomas during M.tb infection, requiring the activity of a wide variety of immunocompetent cells, may generate a microenvironment predisposing to malignant transformation [19][20][21]. Many hypotheses based on in vitro and in vivo experiments try to explain the contribution of M.tb-induced inflammatory events to lung cancer development [22][23]. It is generally accepted that the process of tumorigenesis includes several steps: an initiation stage, involving DNA damage; the promotion stage, involving cell proliferation and the fixation of mutations from premutational lesions; and the tumor progression step, involving expansion of the mutant cells and subsequent tumor growth [24][25]. M.tb-associated cancer may arise as a consequence of chronic inflammatory changes that lead to metaplasia of epithelium in the lung caverns, in calcified lymph nodes, and old scars in the bronchi [10]. The most common forms of cancers are cavern carcinoma, carcinoma of the drainage bronchus, and peripheral lung scar cancer [26][27]. The initiation of the tumor development process is a result of attracting macrophages and other cells to the sites of M.tb infection [23][28][29]. A wide range of toxic agents, such as reactive oxygen intermediates, tissue-destructive proteases, as well as prostaglandins, leukotrienes, and cytokines, produced by activated macrophages and other leukocytes, elicit a profound inflammatory reaction leading to tissue damage and genomic alterations [23][28][29][30][31][32][33]. Activation of the proinflammatory pathway mediated by nuclear factor NF-κβ in macrophages and epithelial cells is accompanied by an increase in the proliferation rate of cells with damaged DNA, which in combination with increased angiogenesis stimulated by cyclooxygenase-2 products leads to the initiation of lung tumorigenesis [24][34]. Moreover, repairing the tissue damaged by M.tb-induced inflammatory reactions can lead to the fibrosis and scarring of the lung tissue, which is also linked to an increased risk of lung cancer. Lung tissues infected for years with M.tb undergo multiple processes of inflammation and tissue repair, which generates a favorable environment for tumorigenesis and increases the risk of lung cancer development [22][23][34]. Moreover, M.tb can induce the release of inflammatory mediators, e.g., tumor necrosis factor (TNF)-α and interleukin (IL)-1, IL-2, and IL-12, which can be viewed as cancer promotors [33].
M.tb can affect other organs as well as the lungs. Five cases of primary liver malignancy co-existing with isolated hepatic TB have been reported so far [35]. It is suggested that mycobacteria-induced reactive forms of oxygen species can damage cell DNA and lead to the development of cancer. Moreover, purified protein derivative (PPD) of M.tb can upregulate the expression of vascular endothelial growth factor in lymphocytes, which has significant angiogenic and mitogenic properties [35]. On the other hand, cancer treatment can also contribute to the development of TB [36].
4. Mycobacteria as Therapeutic Agents
Although bacteria are mostly considered pathogens, it was already suspected over 150 years ago that they might be useful in the treatment of cancer. This hypothesis was put forward independently by two German doctors, W. Bush and F. Fehleisen, when they noticed regression of neoplastic tumors in hospitalized patients accidentally infected with Streptococcus pyogenes [37]. The use of microbes in cancer therapy dates back to the 19th century when Dr. William Coley (1862–1936) developed a mixture of bacterial microbes and, for the first time in the history of modern medicine, successfully treated certain types of cancer, which led him to become a pioneer of immunotherapy [38]. Knowing about the spontaneous regression of sarcomas in patients with severe bacterial infection, he undertook the development of an experimental therapy to administer Streptococcus pyogenes to a patient with inoperable bone sarcoma. The results were extremely promising as the patient was diagnosed with complete tumor regression [39].
The first microorganism that was widely used in the fight against cancer was Mycobacterium bovis (M. bovis) BCG. Studies carried out in the late 1970s showed that intravesical administration of these bacteria reduces the risk of recurrence of non-muscle invasive bladder cancer. Infusions of the suspension of these mycobacteria are currently considered as a standard element of therapy in the oncological treatment of high–intermediate risk cancer, where the exact immune mechanism underlying this therapy is not entirely clear, and the pattern and timing of bacterial administration is the subject of ongoing testing [40].
Upon entry into the host organism, the pathogen provokes a response from the cells of the immune system. The presence of mycobacteria induces a response from both innate and acquired immune cells. Innate immunity is based on cells constantly circulating in the bloodstream, such as monocytes, natural killer (NK) cells, or neutrophils, as well as macrophages and dendritic cells. They recognize the antigens of the pathogen through specific receptors, which provoke a cascade of reactions, such as the release of cytokines or phagocytosis. On the other hand, acquired immunity is based on the process of pathogen recognition by lymphocytes, which among others consequently leads to the production of antibodies against this pathogen and the formation of memory cells storing a “picture” of its antigens. Vaccination is based on this process [41]. The BCG vaccine is also known for its non-specific properties [42][43][44][45].
How BCG immunotherapy affects the tumor is still not fully understood. Studies in animal models have shown that BCG interacts with the epithelium lining the bladder. Certain surface structures of the bacterial cell wall interact with fibronectin in the epithelium. Two possible scenarios of this interaction have been described. The first one indicates a physicochemical interaction that damages the glycosaminoglycan layer and provides BCG with easier access to the bladder wall and facilitates binding to fibronectin. The second scenario concerns specific receptor/ligand binding via fibronectin. It seems the presence of antigen 85 and fibronectin attachment protein (FAP) is required for BCG retention and targeting cells [46]. After the introduction of BCG into the bladder, the activation of epithelial cells and antigen-presenting cells that produce cytokines and chemokines is observed. As a result, granulocytes and mononuclear cells begin to flow into the bladder. After the intravesical administration of BCG, the appearance of macrophages, dendritic cells, lymphocytes, and neutrophils is observed in the bladder wall [46]. In vitro studies with the use of human NIMBC cancer cell lines showed that BCG increases the production of IL-6 and IL-8, GM-CSF (granulocyte-macrophage colony-stimulating factor), and TNF. Furthermore, data obtained from studies on human subjects showed that after the introduction of BCG, neutrophils, macrophages, monocytes, T and B lymphocytes, and NK cells are present in the bladder, accompanied by increased levels of IL-1β, IL-8, IL-15, IL-18, CXCL10 (CXC motif chemokine ligand 10), CC motif chemokine ligand (CCL)2, CCL3, and GM-CSF. Neutrophils can directly affect tumor cells through their phagocytic activity and the ability to produce reactive oxygen species, secretion of lytic enzymes, and factors inducing apoptosis, e.g., TRAIL (TNF-related apoptosis-inducing ligand). In the case of successful immunotherapy, intensive secretion of IL-2, IL-12, IFN-γ, TNF-α, and TNF-β was observed, whereas in the case of failure the production of IL-4, IL-5, IL-6, and IL-10 was noticed [46].
5. Conclusions
References
- Brenner, D.R.; McLaughlin, J.R.; Hung, R.J. Previous lung diseases and lung cancer risk: A systematic review and meta-analysis. PLoS ONE 2011, 6, e17479.
- Yu, Y.H.; Liao, C.C.; Hsu, W.H.; Chen, H.J.; Liao, W.C.; Muo, C.H.; Sung, F.C.; Chen, C.Y. Increased lung cancer risk among patients with pulmonary tuberculosis: A population cohort study. J. Thorac. Oncol. 2011, 6, 32–37.
- Seo, G.H.; Kim, M.J.; Seo, S.; Hwang, B.; Lee, E.; Yun, Y.; Choi, M.; Kim, M.; Kim, J.W.; Kim, E.S.; et al. Can-cer-specific incidence rates of tuberculosis: A 5-year nationwide population-based study in a country with an intermediate tu-berculosis burden. Medicine 2016, 95, e4919.
- Vento, S.; Lanzafame, M. Tuberculosis and cancer: A complex and dangerous liaison. Lancet Oncol. 2011, 12, 520–522.
- Simonsen, D.F.; Farkas, D.K.; Horsburgh, C.R.; Thomsen, R.W.; Sørensen, H.T. Increased risk of active tuberculosis after cancer diagnosis. J. Infect. 2017, 74, 590–598.
- Liang, H.-Y.; Li, X.-L.; Yu, X.-S.; Guan, P.; Yin, Z.-H.; He, Q.-C.; Zhou, B.-S. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: A systematic review. Int. J. Cancer 2009, 125, 2936–2944.
- Oh, C.M.; Roh, Y.H.; Lim, D.; Kong, H.J.; Cho, H.; Hwangbo, B.; Won, Y.J.; Jung, K.W.; Oh, K. Pulmonary tuberculosis is associated with elevated risk of lung cancer in Korea: The nationwide cohort study. J. Cancer 2020, 11, 1899–1906.
- Wu, C.-Y.; Hu, H.-Y.; Pu, C.-Y.; Huang, N.; Shen, H.-C.; Li, C.-P.; Chou, Y.-J. Aerodigestive tract, lung and haematological cancers are risk factors for tuberculosis: An 8-year population-based study. Int. J. Tuberc. Lung Dis. 2011, 15, 125–130.
- Skowroński, M.; Iwanik, K.; Halicka, A.; Barinow-Wojewódzki, A. Squamous cell lung cancer in a male with pulmonary tuberculosis. Pneumonol. Alergol. Pol. 2015, 83, 298–302.
- Cukic, V. The association between lung carcinoma and tuberculosis. Med. Arch. 2017, 71, 212–214.
- Engels, E.A.; Shen, M.; Chapman, R.S.; Pfeiffer, R.M.; Yu, Y.Y.; He, X.; Lan, Q. Tuberculosis and subsequent risk of lung cancer in Xuanwei, China. Int. J. Cancer 2009, 124, 1183–1187.
- Cicènas, S.; Vencevičius, V. Lung cancer in patients with tuberculosis. World J. Surg. Oncol. 2007, 5, 22.
- Bhatt, M.; Kant, S.; Bhaskar, R. Pulmonary tuberculosis as differential diagnosis of lung cancer. South Asian J. Cancer 2012, 1, 36–42.
- McCusker, C.; Warrington, R. Primary immunodeficiency. Allergy Asthma Clin. Immunol. 2011, 7 Suppl 1, S11.
- Hauck, F.; Gennery, A.R.; Seidel, M.G. Editorial: The relationship between cancer predisposition and primary immunodeficiency. Front. Immunol. 2019, 30, 1781.
- Stanley, S.A.; Cox, J.S. Host-pathogen interactions during Mycobacterium tuberculosis infections. Curr. Top. Microbiol. Immunol. 2013, 374, 211–241.
- Ehrt, S.; Schnappinger, D. Mycobacterial survival strategies in the phagosome: Defence against host stresses. Cell Microbiol. 2009, 11, 1170–1178.
- Guirado, E.; Schlesinger, L.S. Modeling the Mycobacterium tuberculosis granuloma—the critical battlefield in host immunity and disease. Front. Immunol. 2013, 4, 98.
- Dalgleish, A.G.; O’Byrne, K.J. Chronic immune activation and inflammation in the pathogenesis of AIDS and cancer. Adv. Cancer Res. 2002, 84, 231–276.
- Melnikova, V.O.; Ananthaswamy, H.N. Cellular and molecular events leading to the development of skin cancer. Mutat. Res. 2005, 571, 91–106.
- Azad, N.; Rojanasakul, Y.; Vallyathan, V. Inflammation and lung cancer: Roles of reactive oxygen/nitrogen species. J. Toxicol. Environ. Health B Crit. Rev. 2008, 11, 1–15.
- Ames, B.N.; Gold, L.S.; Willett, W.C. The causes and prevention of cancer. Proc. Natl. Acad. Sci. USA 1995, 92, 5258–5265.
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006, 4, 221–233.
- Bowden, G.T. Prevention of non-melanoma skin cancer by targeting ultraviolet-b-light signalling. Nat. Rev. Cancer 2004, 4, 23–35.
- Broussard, G.W.; Norris, M.B.; Schwindt, A.R.; Fournie, J.W.; Winn, R.N.; Kent, M.L.; Ennis, D.G. Chronic Mycobacterium marinum infection acts as a tumor promoter in Japanese Medaka (Oryziaslatipes). Comp. Biochem. Physiol. C Toxicol. Pharm. 2009, 149, 152–160.
- Teventiyanon, T.; Ratanaharathorn, V.; Leoparait, J. Mucoepidermoid carcinoma of the lung presenting as cavitary lesion. J. Med. Assoc. Thail. 2004, 87, 988–991.
- Yilmaz, A.; Gungor, S.; Damadoglu, E.; Axoy, F.; Aibatly, A. Coexisting bronchial carcinoid tumor and pulmonary tuberculosis in the same lobe: A case report. Tuberk Toraks 2004, 52, 369–372.
- Weitzman, S.A.; Weitberg, A.B.; Clark, E.P.; Stossel, T.P. Phagocytes as carcinogens: Malignant transformation produced by human neutrophils. Science 1985, 227, 1231–1233.
- Shacter, E.; Beecham, E.J.; Covey, J.M.; Kohn, K.W.; Potter, M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis 1988, 9, 2297–2304.
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R.; et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003, 35, 238–245.
- Zhuang, J.C.; Lin, D.; Lin, C.; Jethwaney, D.; Wogan, G.N. Genotoxicity associated with NO production in macrophages and co-cultured target cells. Free Radic. Biol. Med. 2002, 33, 94–102.
- Kim, M.Y.; Wogan, G.N. Mutagenesis of thesupFGene of pSP189 Replicating in AD293 Cells Cocultivated with Activated Macrophages: Roles of Nitric Oxide and Reactive Oxygen Species. Chem. Res. Toxicol. 2006, 19, 1483–1491.
- Molina-Romero, C.; Arrieta, O.; Hernaández-Pando, R. Tuberculosis and lung cancer. Salud Publica Mex. 2019, 61, 286–291.
- Ardies, C.M. Inflammation as cause for scar cancers of the lung. Integr. Cancer Ther. 2003, 2, 238–246.
- Alsaif, H.S.; Hassan, A.; Refai, O.; Awary, K.; Kussaibi, H.; Ismail, M.H.; Alghnimi, I. Concomitant hepatic tuberculosis and hepatocellular carcinoma: A case report and review of the literature. BMC Surg. 2021, 21, 2.
- El-Mahallawy, H.A.; Eissa, S.A.; Refeh, N.G.; Salem, A.E.S.; Eissa, S.A.; Allian, S.A. Tuberculosis in cancer patients: Role of newer techniques in relation to conventional diagnostic methods. J. Adv. Res. 2010, 1, 157–162.
- Anguille, S.; Smits, E.L.; Lion, E.; van Tendeloo, V.F.; Berneman, Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014, 15, 257–267.
- Bickels, J.; Kollender, Y.; Merinsky, O.; Meller, I. Coley’s toxin: Historical perspective. Isr. Med. Assoc. J. 2002, 4, 471–472.
- McCarthy, E.F. The toxin of William, B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158.
- Kawai, K.; Miyazaki, J.; Joraku, A.; Nishiyama, H.; Akaza, H. Bacillus Calmette–Guerin (BCG) immunotherapy for bladder cancer: Current understanding and perspectives on engineered BCG vaccine. Cancer Sci. 2013, 104, 22–27.
- Blok, B.A.; Arts, R.J.; van Crevel, R.; Benn, C.S.; Netea, M.G. Trained innate immunity as underlying mechanism for the long-term, nonspecific effects of vaccines. J. Leukocyt. Biol. 2015, 98, 347–356.
- Garly, M.L.; Martins, C.L.; Balé, C.; Baldé, M.A.; Hedegaard, K.L.; Gustafson, P.; Lisse, I.M.; Whittle, H.C.; Aaby, P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine 2003, 21, 2782–2790.
- Roth, A.; Gustafson, P.; Nhaga, A.; Djana, Q.; Poulsen, A.; Garly, M.L.; Jensen, H.; Sodemann, M.; Rodriques, A.; Aaby, P. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 2005, 34, 540–547.
- Aaby, P.; Roth, A.; Ravn, H.; Napirna, B.M.; Rodrigues, A.; Lisse, I.M.; Stensballe, L.; Diness, B.R.; Lausch, K.R.; Lund, N. Randomized trial of BCG vaccination at birth to low-birth-weight children: Beneficial nonspecific effects in the neonatal period? J. Infect. Dis. 2011, 204, 245–252.
- Biering-Sørensen, S.; Aaby, P.; Napirna, B.M.; Roth, A.; Ravn, H.; Rodrigues, A.; Whittle, H.; Benn, C.S. Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr. Infect. Dis. J. 2012, 31, 306–308.
- Pettenati, C.; Ingresoll, M.A. Mechanisms of BCG immunotherapy and its outlook for bladder cancer. Nat. Rev. Urol. 2018, 15, 615–625.
- Pearl, R. Cancer and tuberculosis. Am. J. Epidemiol. 1929, 9, 97–159.
- Kamat, A.M.; Lamm, D.L. Immunotherapy for bladder cancer. Curr. Urol. Rep. 2001, 2, 62–69.
- Lamm, D.L. Bacillus Calmette-Guerin immunotherapy for bladder cancer. J. Urol. 1985, 134, 40–46.




