
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesca Mariani | + 3032 word(s) | 3032 | 2021-09-21 05:03:39 | | | |
| 2 | Lindsay Dong | + 84 word(s) | 3116 | 2021-09-22 03:57:32 | | |
Video Upload Options
Mediterranean wild edible plants (MWEPs) and their antimicrobial properties have been known from ancient times, and nowadays, a growing number of people have rediscovered them as natural remedies for common infections. One of the problems concerning their use is the heterogeneity of the protocols used to extract and analyze the properties of their active principles; such heterogeneity still marks the overall set of scientific studies on MWEPs, not to mention the enormous heterogeneity that characterizes the properties of plants at the outset. We reviewed the current literature on medicinal value of Mediterranean native edible plants trying to emphasize both the weaknesses and the opportunities of these plants. The majority of the reviewed MWEPs can inhibit both Gram-negative and Gram-positive bacteria, and fungi.
1. Introduction
2. Antimicrobial Properties of Mediterranean Wild Edible Plants
2.1. Results of Syntheses
2.1.1. The Antimicrobial Effects Reported for the Most Studied Species
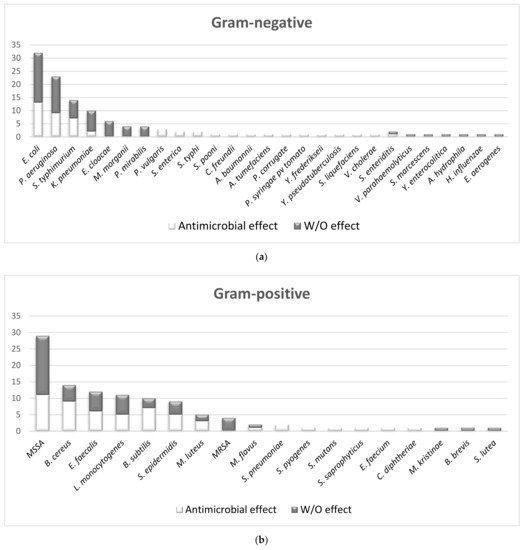
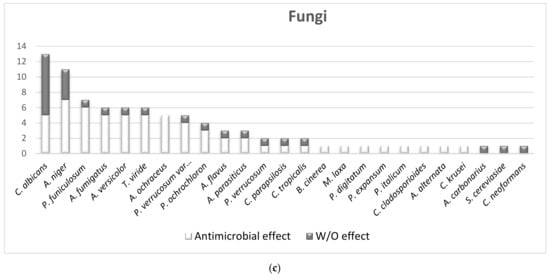
2.1.2. The Overall Picture of MWEPs Antimicrobial Effects on Gram-Positive Bacteria, Gram-Negative Bacteria, Fungi
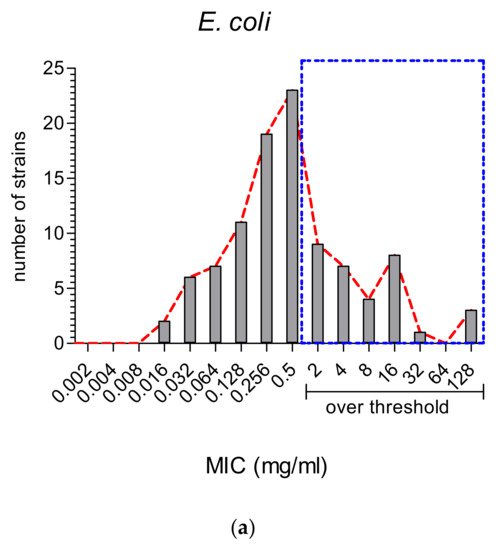
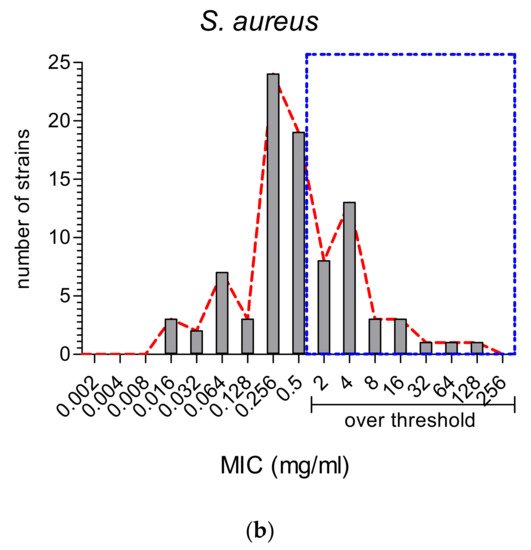
2.2. The Comparison of MIC Values
2.3. Antioxidant vs. Antimicrobial Properties: Direct or Inverse Association?
2.4. MWEPs Useful Properties
2.4.1. Antimicrobial Efficacy
2.4.2. MWEPs vs. Antibiotic-Resistant Species
2.4.3. Antioxidant vs. Antimicrobial Properties
2.4.4. Implications of the Results for Practice, Policy, and Future Research
highly effective in inhibiting bacteria and fungi.
Finally, for what concerns the pathogens analysed, we should include at least the more characterized Gram-positive (S. aureus, MRSA) and Gram-negative (E. coli, P. aeruginosa) bacteria, which are responsible for the major number of infections in humans, in particular for community-associated infections.
3. Conclusions
We found that, out of seventy-four MWEPs species, fifty-one (69%) belong to eight out of the twenty-five botanical families analysed in this review (see table S1). In particular, Asteraceae, Apiaceae, Brassicaceae, Caryophyllaceae and Lamiaceae contain more than eight of the species most studied, and most of them display antimicrobial properties.
It is true that we still know very little about MWEPs, and it is necessary to standardize the protocols to further study these plants.
It is advisable to avoid the extinction of MWEPs cultivation; however, the agronomic practices must be as closer as possible to the in situ growth, to preserve the active principles.
The study of their effect on viruses must be increased.
On the other hand, MWEPs can inhibit both Gram-negative and Gram-positive bacteria, and fungi. Importantly, the effective MIC on Gram-negative is significantly below the stringent threshold we employed, and some extracts do inhibit clinical isolates.
Their common antioxidant and metal chelating properties, despite some controversy, exert a positive effect on human and domestic animals’ immune system, further helping them to face infections.
Despite the problems listed, MWEPs constitute a very important source of antimicrobial compounds to be explored.
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance, 2016. Wellcome Trust and HM Government. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 14 April 2021).
- Shankar, D.; Patwardhan, B. AYUSH for New India: Vision and strategy. J. Ayurveda Integr. Med. 2017, 8, 137–139.
- Li, J.; Li, J.; Zhang, F. The immunoregulatory effects of Chinese herbal medicine on the maturation and function of dendritic cells. J. Ethnopharmacol. 2015, 171, 184–195.
- Panagouleas, C.; Skaltsa, H.; Lazari, D.; Skaltsounis, A.-L.; Sokovic, M. Antifungal Activity of Secondary Metabolites of Centaurea raphanina ssp. mixta, Growing Wild in Greece. Pharm. Biol. 2003, 41, 266–270.
- Rayan, M.; Abu-Farich, B.; Basha, W.; Rayan, A.; Abu-Lafi, S. Correlation between Antibacterial Activity and Free-Radical Scavenging: In-Vitro Evaluation of Polar/Non-Polar Extracts from 25 Plants. Processes 2020, 8, 117.
- Najjaa, H.; Neffati, M.; Ammar, E.; Fattouch, S. Antimicrobial properties of Allium roseum L.: A wild edible species in Southern Tunisia. Acta Hortic. 2010, 853, 323–328.
- Najjaa, H.; Ammar, E.; Neffati, M. Antimicrobial activities of protenic extracts of Allium roseum L., a wild edible species in North Africa. J. Food Agric. Environ. 2009, 7, 150–154.
- Hussain, A.; Qarshi, I.A.; Liaqat, R.; Akhtar, S.; Aziz, I.; Ullah, I.; Shinwari, Z.K. Antimicrobial potential of leaf and fruit extracts and oil of wild and cultivated edible olive. Pak. J. Bot. 2014, 46, 1463–1468.
- Gatto, M.A.; Ippolito, A.; Linsalata, V.; Cascarano, N.A.; Nigro, F.; Vanadia, S.; Di Venere, D. Activity of extracts from wild edible herbs against postharvest fungal diseases of fruit and vegetables. Postharvest Biol. Technol. 2011, 61, 72–82.
- Najjaa, H.; Zouari, S.; Ammar, E.; Neffati, M. Phytochemical screening and antibacterial properties of Allium roseum L., A WILD EDIBLE SPECIES IN NORTH AFRICA. J. Food Biochem. 2011, 35, 699–714.
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.; Di Gioia, F.; Tzortzakis, N.; Ivanov, M.; Sokovic, M.; Barros, L.; et al. Wild and Cultivated Centaurea raphanina subsp. mixta: A Valuable Source of Bioactive Compounds. Antioxidants 2020, 9, 314.
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Pereira, C.; Calhelha, R.C.; Ivanov, M.; Sokovic, M.D.; Ferreira, I.C.; Barros, L. The Effect of Nitrogen Fertigation and Harvesting Time on Plant Growth and Chemical Composition of Centaurea raphanina subsp. mixta (DC.) Runemark. Molecules 2020, 25, 3175.
- Petropoulos, S.; Fernandes, Â.; Dias, M.; Pereira, C.; Calhelha, R.; Ivanov, M.; Sokovic, M.; Ferreira, I.; Barros, L. Effects of Growing Substrate and Nitrogen Fertilization on the Chemical Composition and Bioactive Properties of Centaurea raphanina ssp. mixta (DC.) Runemark. Agronomy 2021, 11, 576.
- Xia, D.-Z.; Yu, X.-F.; Zhu, Z.-Y.; Zou, Z.-D. Antioxidant and antibacterial activity of six edible wild plants (Sonchus spp.) in China. Nat. Prod. Res. 2011, 25, 1893–1901.
- Petropoulos, S.A.; Fernandes, Â.; Tzortzakis, N.; Sokovic, M.; Ciric, A.; Barros, L.; Ferreira, I.C. Bioactive compounds content and antimicrobial activities of wild edible Asteraceae species of the Mediterranean flora under commercial cultivation conditions. Food Res. Int. 2019, 119, 859–868.
- Iyda, J.H.; Fernandes, Â.; Ferreira, F.D.; Alves, M.J.; Pires, T.C.S.P.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of the wild edible plant Raphanus raphanistrum L. Food Res. Int. 2019, 121, 714–722.
- Aboukhalaf, A.; El Amraoui, B.; Tabatou, M.; Da Rocha, J.M.F.; Belahsen, R. Screening of the antimicrobial activity of some extracts of edible wild plants in Morocco. Funct. Foods Health Dis. 2020, 10, 265.
- Khammassi, M.; Loupassaki, S.; Tazarki, H.; Mezni, F.; Slama, A.; Tlili, N.; Zaouali, Y.; Mighri, H.; Jamoussi, B.; Khaldi, A. Variation in essential oil composition and biological activities of Foeniculum vulgare Mill. populations growing widely in Tunisia. J. Food Biochem. 2018, 42, e12532.
- Mulani, M.S.; Kamble, E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539.
- Zengin, G.; Mahomoodally, M.F.; Aktumsek, A.; Ceylan, R.; Uysal, S.; Mocan, A.; Yilmaz, M.A.; Picot-Allain, C.M.N.; Ćirić, A.; Glamočlija, J.; et al. Functional constituents of six wild edible Silene species: A focus on their phytochemical profiles and bioactive properties. Food Biosci. 2018, 23, 75–82.
- Akgunlu, S.; Sekeroglu, N.; Koca-Caliskan, U.; Ozkutlu, F.; Ozcelik, B.; Kulak, M.; Gezici, S. Research on selected wild edible vegetables: Mineral content and antimicrobial potentials. Ann. Phytomed. Int. J. 2016, 5, 50–57.
- Ivashchenko, I.V. Chemical composition of essential oil and antimicrobial properties of Chrysantemum coronarium (Asteraceae). Biosyst. Divers. 2017, 25, 119–123.
- Ušjak, L.; Petrović, S.; Drobac, M.; Sokovic, M.; Stanojković, T.; Ciric, A.; Niketić, M. Edible wild plant Heracleum pyrenaicum subsp. orsinii as a potential new source of bioactive essential oils. J. Food Sci. Technol. 2017, 54, 2193–2202.
- Anzabi, Y.; Aghdam, V.B.; Makoui, M.H.; Anvarian, M.; Mousavinia, M.N. Evaluation of antibacterial properties of edible oils and extracts of a native plant, Ziziphora clinopodioides (mountains’ Kakoty), on bacteria isolated from urinary tract infections. Life Sci. J. 2013, 10 (Suppl. 4), 121–127.
- Shehadeh, M.; Jaradat, N.; Al-Masri, M.; Zaid, A.N.; Hussein, F.; Khasati, A.; Suaifan, G.; Darwish, R. Rapid, cost-effective and organic solvent-free production of biologically active essential oil from Mediterranean wild Origanum syriacum. Saudi Pharm. J. 2019, 27, 612–618.
- Kayiran, S.D.; Ozkan, E.E.; Kara, E.M.; Yilmaz, M.A.; Zengin, G.; Boga, M. Comprehensive analysis of an uninvestigated wild edible medicinal garlic species from Turkey: Allium macrochaetum Boiss. & Hausskn. J. Food Biochem. 2019, 43, e12928.
- Mhamdi, B.; Abbassi, F.; Abdelly, C. Chemical composition, antioxidant and antimicrobial activities of the edible medicinal Ononis natrix growing wild in Tunisia. Nat. Prod. Res. 2015, 29, 1157–1160.
- Najjaa, H.; Zerria, K.; Fattouch, S.; Ammar, E.; Neffati, M. Antioxidant and Antimicrobial Activities of Allium roseum L. “Lazoul,” A Wild Edible Endemic Species in North Africa. Int. J. Food Prop. 2011, 14, 371–380.
- Khan, H.; Jan, S.A.; Javed, M.; Shaheen, R.; Khan, Z.; Ahmad, A.; Safi, S.Z.; Imran, M. Nutritional Composition, Antioxidant and Antimicrobial Activities of Selected Wild Edible Plants. J. Food Biochem. 2015, 40, 61–70.
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C. Phenolic Profile and Bioactive Properties of Carissa macrocarpa (Eckl.) A.DC: An In Vitro Comparative Study between Leaves, Stems, and Flowers. Molecules 2019, 24, 1696.
- Nayaka, N.; Sasadara, M.; Sanjaya, D.; Yuda, P.; Dewi, N.; Cahyaningsih, E.; Hartati, R. Piper betle (L.): Recent Review of Antibacterial and Antifungal Properties, Safety Profiles, and Commercial Applications. Molecules 2021, 26, 2321.
- Anand, U.; Nandy, S.; Mundhra, A.; Das, N.; Pandey, D.K.; Dey, A. A review on antimicrobial botanicals, phytochemicals and natural resistance modifying agents from Apocynaceae family: Possible therapeutic approaches against multidrug resistance in pathogenic microorganisms. Drug Resist. Updates 2020, 51, 100695.
- Heinrich, M.; Prieto, J. Diet and healthy ageing 2100: Will we globalise local knowledge systems? Ageing Res. Rev. 2008, 7, 249–274.
- Adedapo, A.; Jimoh, F.; Afolayan, A. Comparison of the nutritive value and biological activities of the acetone, methanol and water extracts of the leaves of Bidens pilosa and Chenopodium album. Acta Pol. Pharm. 2011, 68, 83–92.
- Coruh, I.; Gormez, A.A.; Ercisli, S.; Bilen, S. Total phenolics, mineral elements, antioxidant and antibacterial activities of some edible wild plants in Turkey. Asian J. Chem. 2007, 19, 5755–5762.
- Karaman, K.; Polat, B.; Ozturk, I.; Sagdic, O.; Ozdemir, C. Volatile Compounds and Bioactivity of Eremurus spectabilis (Ciris), a Turkish Wild Edible Vegetable. J. Med. Food 2011, 14, 1238–1243.
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhães, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Front. Pharmacol. 2020, 11, 581840.
- Peterfalvi, A.; Miko, E.; Nagy, T.; Reger, B.; Simon, D.; Miseta, A.; Czéh, B.; Szereday, L. Much More Than a Pleasant Scent: A Review on Essential Oils Supporting the Immune System. Molecules 2019, 24, 4530.




