Mediterranean wild edible plants (MWEPs) and their antimicrobial properties have been known from ancient times, and nowadays, a growing number of people have rediscovered them as natural remedies for common infections. One of the problems concerning their use is the heterogeneity of the protocols used to extract and analyze the properties of their active principles; such heterogeneity still marks the overall set of scientific studies on MWEPs, not to mention the enormous heterogeneity that characterizes the properties of plants at the outset. We reviewed the current literature on medicinal value of Mediterranean native edible plants trying to emphasize both the weaknesses and the opportunities of these plants. The majority of the reviewed MWEPs can inhibit both Gram-negative and Gram-positive bacteria, and fungi.
- wild edible plants
- antimicrobial effect
- Mediterranean plant
- Gram+ bacteria
- Gram− bacteria
- extraction protocols
- bioactive compounds
- essential oils
1. Introduction
2. Antimicrobial Properties of Mediterranean Wild Edible Plants
2.1. Results of Syntheses
2.1.1. The Antimicrobial Effects Reported for the Most Studied Species
2.1.2. The Overall Picture of MWEPs Antimicrobial Effects on Gram-Positive Bacteria, Gram-Negative Bacteria, Fungi
- How many Gram-negative bacteria are sensitive to MWEPs extracts?
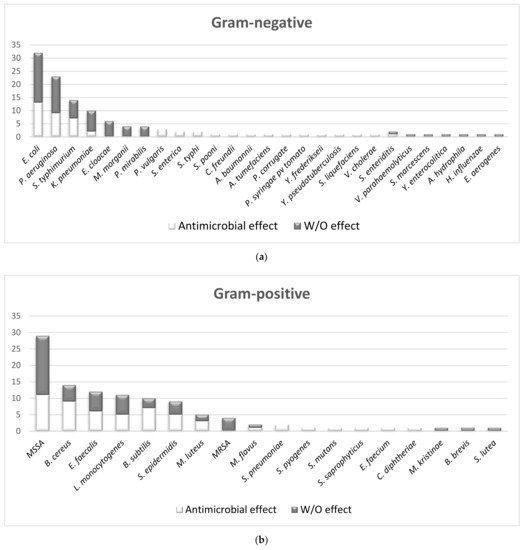
- 2.
-
How many Gram-positive bacteria are sensitive to MWEPs extracts?Overall, we found that 15 species, out of 18 of Gram-positive bacteria, were sensitive to the MWEPs extracts, representing 83% of the species analyzed in this review.In Figure 1Figure 4b, all of the Gram-positive species are shown and whether or not the MWEPs were efficacious. For the main bacteria of this group, which is composed of S. aureus Methicillin Sensible (MSSA), B. cereus, E. faecalis, L. monocytogenes, B. subtilis, and S. epidermidis, the studies with MIC ≤ 0.5 mg/mL were slightly more in number than those over this threshold. This is in accordance with the higher sensitivity of Gram-positive bacteria to antimicrobial drugs, but the use of natural products might also help to lower the antibiotic doses in human treatments.The three species not inhibited by MWEPs extracts were analyzed only in one study each: they are environmental bacteria, such as B. brevis and S. lutea, or opportunistic bacterial infection in immune compromised hosts, such as M. kristinae. Again, the studies concerning these bacteria are very few.3.
- 3.
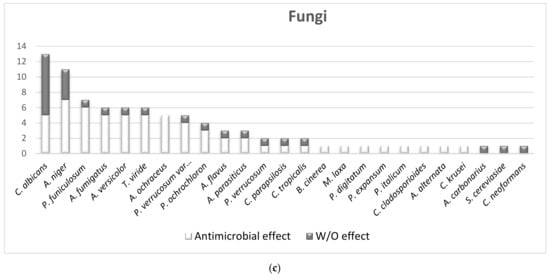
How many fungi are susceptible to MWEPs extracts?For fungal species, 88% were sensitive according to 38 studies.In Figure 1Figure 4c, all the fungi species are shown and whether or not the MWEPs were efficacious. It is quite clear that MWEPs were fairly efficient in inhibiting fungal growth, given that the number of studies demonstrating antimicrobial activity was higher than those proving the absence of such effect.2.2. The Comparison of MIC Values
Most studies analyzed both E. coli (Gram-negative) and S. aureus (Gram-positive); therefore, we could compare all the MIC values reported for these two main species (Figure 2Figure 5).It is well known that Gram-negative bacteria are more resistant to antibiotic treatments and that they embody 67% of the ESKAPE group of ABR bacterial species [20][45]. It is then important to note that the majority of MWEPs extracts displayed MIC values (Figure 2Figure 5a) below the very stringent threshold adopted in our selection (see Materials and Methods).Instead, S. aureus showed MIC values distributed at the turn of the threshold value (as shown in Figure 2Figure 5b).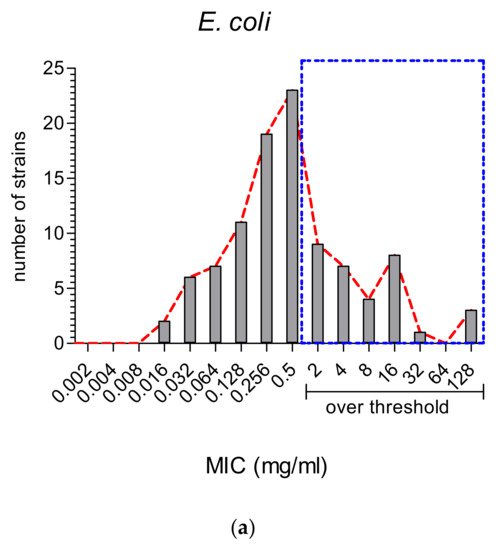
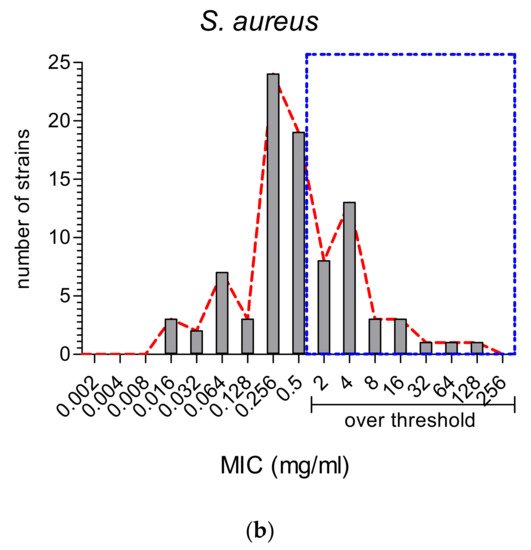 Figure 2. MIC values (in mg/mL) reported in the thirty-eight studies for MWEPs extracts vs. the two main and most studied pathogenic bacteria: (a) the Gram-negative E. coli and (b) the Gram-positive S. aureus.Only three studies analyzed the antimicrobial properties of the plants against clinically isolated strains [8][21][22][33,39,44]. It is worth noting that, in all the comparisons between collection species and the clinically isolated strains, the MIC values were not always higher for the latter (16 vs. 256 μg/mL for the most effective plants, such as Chenopodium album), but in some cases were significantly lower, even lower than conventional antibiotics (as for Silene conoidea, 0.01 vs. 0.1 mg/mL ampicillin).Finally, there were only six studies using essential oils (EO), among which the EO distilled from C. coronarium [23][35] was the only one where MIC values were given in EO dilutions (v/v). Overall, the EOs were shown to be highly efficient in inhibiting bacterial and fungal growth [24][25][26][27][12,25,28,36], displaying in some cases, as compared with routinely employed antibiotics, a similar (vs. streptomycin) or even higher efficiency (vs. ampicillin) vs. Gram-negative bacteria [28][9]. The only exception was EO of F. vulgare, which did not show any antibacterial activity [19][31].
Figure 2. MIC values (in mg/mL) reported in the thirty-eight studies for MWEPs extracts vs. the two main and most studied pathogenic bacteria: (a) the Gram-negative E. coli and (b) the Gram-positive S. aureus.Only three studies analyzed the antimicrobial properties of the plants against clinically isolated strains [8][21][22][33,39,44]. It is worth noting that, in all the comparisons between collection species and the clinically isolated strains, the MIC values were not always higher for the latter (16 vs. 256 μg/mL for the most effective plants, such as Chenopodium album), but in some cases were significantly lower, even lower than conventional antibiotics (as for Silene conoidea, 0.01 vs. 0.1 mg/mL ampicillin).Finally, there were only six studies using essential oils (EO), among which the EO distilled from C. coronarium [23][35] was the only one where MIC values were given in EO dilutions (v/v). Overall, the EOs were shown to be highly efficient in inhibiting bacterial and fungal growth [24][25][26][27][12,25,28,36], displaying in some cases, as compared with routinely employed antibiotics, a similar (vs. streptomycin) or even higher efficiency (vs. ampicillin) vs. Gram-negative bacteria [28][9]. The only exception was EO of F. vulgare, which did not show any antibacterial activity [19][31].2.3. Antioxidant vs. Antimicrobial Properties: Direct or Inverse Association?
Even if most studies underpin the role of the antioxidant and reducing power of a MWEP by conferring it with effective antimicrobial capacity, there are still contradictory results to be evaluated. Overall, we cannot draw a conclusion yet.For example, Sonchus species, with the highest reducing power, are the very same species displaying the highest antimicrobial properties [15][7]. C. raphanina cultivated plants have less polyphenol content than the wild plants, which correlates with their lower antimicrobial capacity [12][17].In Allium roseum the authors found a direct association between antimicrobial properties and total phenolic compounds (TPCs) in the extracts made with different parts of the plant (either leaves, flowers, or bulbs) [29][21]. For Silene spp., the order of descending antimicrobial properties was compared with the respective order for metal chelating potential [21][39]. While it is true that the most antimicrobial species (S. conoidea) are also the most powerful in chelating metals, we must acknowledge that the descending order for both features in the six Silene species does not allow a direct association between them to be established. In another example [23][35], P. hydropiper proved to have a high level of antioxidant properties but weak antimicrobial properties: according to our threshold, none of the extracts were able to inhibit bacterial growth. In another study of MWEPs, the total antioxidant and free radical-scavenging activities of plant species showed a linear correlation with the total phenolics. For example, M. polymorpha was found to be the most active, and high antioxidant activity was observed for G. laevigata. Nonetheless, the antibacterial activity of methanolic extracts of all the plant species was lower than that of the positive control (streptomycin) against the tested bacterial species [30][41].On the other hand, in a study on C. macrocarpa, it is interesting to note strong correlation values (0.7–0.9 and >0.9) between reducing power, total flavan-3-ols (TF3O), total phenolic compounds (TPC), and inhibiting properties vs. some Gram-positive bacteria tested (E. faecalis, L. monocytogenes, MRSA, and MSSA), even if the MIC values were over the threshold we set. Conversely, for DPPH scavenging activity, the strong correlation values were associated with total phenolic acids (TPA), total flavonols (TF), and inhibiting properties vs. E. faecalis and MRSA only [31][42].Finally, the authors of another study wondered whether the results they had obtained could lead to the conclusion that there is no correlation between antibacterial activity against S. mutans and free radical scavenging [5][24]. However, an in-depth analysis revealed that the extracts of plants that exhibited an EC50 (amount of antioxidant necessary to decrease the initial DPPH absorbance by 50%) ≤ 100 ppm showed some degree of enrichment (4 orders of magnitude) in antibacterial activity.From the analysis of the studies’ results, it is also evident that geographical location of plant collections (such as altitude in the case of F. vulgare) and extraction procedures also have substantial effects on the activity of the extracts [9][34].2.4. MWEPs Useful Properties
2.4.1. Antimicrobial Efficacy
1. Gram-negative bacteria are sensitive to MWEPs extracts.-
Gram-negative bacteria are sensitive to MWEPs extracts.
A total of 70% of the assayed Gram-negative species were inhibited by MWEPs extracts. For E. coli, most inhibiting MIC values were significantly below our stringent threshold. This effective antimicrobial property might suggest their possible use in co-administration with antibiotics as a means to fight Gram-negative bacterial infections.Some of the Gram-negative bacteria not inhibited by MWEPs are opportunistic and responsible for urinary tract infections in immunocompromised hosts or in the event of catheter insertions, and the search for inhibiting natural products derived from MWEPs is definitely worthwhile.2.- 2.
Gram-positive bacteria are sensitive to MWEPs extracts.A total of 83% of the assayed Gram-positive species were inhibited by MWEPs extracts. For S. aureus, the inhibiting MIC values were distributed at the turn of the threshold value, most of them being below the threshold.3.- 3.
Fungi are sensitive to MWEPs extracts.Regarding fungi, 88% of the species analyzed in the thirty-eight studies were sensitive to MWEPs extracts. Approximately 300 fungal species on Earth are known to cause illnesses, such as Candida spp. and dermatophytes. In the food industry, bacteria and fungi cause problems during product processing and storage [32][50]. MWEPs could be a new safe and effective antimicrobial agent that could be applied in many fields.2.4.2. MWEPs vs. Antibiotic-Resistant Species
We report some examples of the advantages of the widening use of MWEPs extracts, also co-administered with conventional antibiotics to decrease the typically required drug amount and to contribute to slowing down the increase in antibiotic resistance [33][51].For example, Bacillus cereus is an opportunistic bacterium that became a serious cause of nosocomial infections [34][4]. Nine studies (see Table S3) reported in this review provide experimental data of MWEPs effective against this bacterium that could become pathogenic in immunocompromised hosts [16][35][24][13][12][36][7][8][21][8,11,12,16,17,30,32,33,39].Additionally, regarding Enterococcus faecalis, which is responsible for urinary tract infections and under surveillance for resistance to aminoglycosides, six studies (see Table S3) reported MWEPs extracts were effective in inhibiting its growth [28][29][8][27][21][22][9,21,33,36,39,44].Another pathogen belonging to the ESKAPE group of ABR bacteria is Klebsiella pneumoniae, in which two studies (see Table S3) reported effective inhibitory activity of MWEPs extracts [37][25][18,25].Altogether, the studies here reviewed provide natural tools for inhibiting the growth of five out six ESKAPE pathogens, and the only one missing is an Enterobacter, for which we could include only one study.2.4.3. Antioxidant vs. Antimicrobial Properties
In the thirty-eight studies reported, there is still some controversy concerning the type of association between the antioxidant power of MWPs and their corresponding antimicrobial power, which is an issue deserving further investigation. Instead, what is clearer so far is the adjuvant role of natural antioxidant compounds in maintaining the good performance of the immune system [38][39][52,53]. This might suggest that even if the MWEPs antioxidant properties are not always the ones solely responsible for the killing of microbes, they certainly improve the capacity of the immune system to orchestrate the microbicide response of the infected host.2.4.4. Implications of the Results for Practice, Policy, and Future Research
MWEPs are being more and more rediscovered by consumers, both in Mediterranean and Northern European countries [34][4]. Their use in the daily diet might provide important micronutrients and healthy active components. We should start, then, to face the overwhelming heterogeneity that characterizes the way in which MWEPs are studied, in order to more systematically and consistently document their precious properties.If it does not sound conceited, we would like to propose a list of steps to be shared and hopefully discussed inside the scientific community in order to reduce the great heterogeneity of the studies on MWEPs (as is also very often indicated in the EU Pharmacopoeia).We should all put our best efforts forward to perform the very same tests to describe the antimicrobial properties of plant extracts. This means that at least the disk diffusion test and the MIC/MBC test should be included in our future studies, in order to allow comparison with similar studies.Concerning the protocols for extraction, in order to allow comparison with similar studies, we should include at least alcoholic, hydro-alcoholic, and aqueous extracts from the very same part of the plant analyzed.For aromatic plants, we should include the analysis of essential oils, which are highly effective in inhibiting bacteria and fungi.Finally, concerning the pathogens analyzed, we should include at least the more characterized Gram-positive (S. aureus, MRSA) and Gram-negative (E. coli, P. aeruginosa) bacteria, which arehighly effective in inhibiting bacteria and fungi.
Finally, for what concerns the pathogens analysed, we should include at least the more characterized Gram-positive (S. aureus, MRSA) and Gram-negative (E. coli, P. aeruginosa) bacteria, which are responsible for the major number of infections in humans, in particular for community-associated infections.
3. Conclusions
Conclusions.
We found that, out of seventy-four MWEPs species, fifty-one (69%) belong to eight out of the twenty-five botanical families analysed in this review (see table S1). In particular, Asteraceae, Apiaceae, Brassicaceae, Caryophyllaceae and Lamiaceae contain more than eight of the species most studied, and most of them display antimicrobial properties.
It is true that we still know very little about MWEPs, and it is necessary to standardize the protocols to further study these plants.
It is advisable to avoid the extinction of MWEPs cultivation; however, the agronomic practices must be as closer as possible to the in situ growth, to preserve the active principles.
The study of their effect on viruses must be increased.
On the other hand, MWEPs can inhibit both Gram-negative and Gram-positive bacteria, and fungi. Importantly, the effective MIC on Gram-negative is significantly below the stringent threshold we employed, and some extracts do inhibit clinical isolates.
Their common antioxidant and metal chelating properties, despite some controversy, exert a positive effect on human and domestic animals’ immune system, further helping them to face infections.
Despite the problems listed, MWEPs constitute a very important source of antimicrobial compounds to be explored.
