
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Li Cai | + 4171 word(s) | 4171 | 2021-08-20 14:17:21 | | | |
| 2 | Conner Chen | -21 word(s) | 4150 | 2021-09-03 09:58:31 | | |
Video Upload Options
Adult neural stem and progenitor cells (NSPCs) contribute to learning, memory, maintenance of homeostasis, energy metabolism and many other essential processes. They are highly heterogeneous populations that require input from a regionally distinct microenvironment including a mix of neurons, oligodendrocytes, astrocytes, ependymal cells, NG2+ glia, vasculature, cerebrospinal fluid (CSF), and others. The diversity of NSPCs is present in all three major parts of the CNS, i.e., the brain, spinal cord, and retina. Intrinsic and extrinsic signals, e.g., neurotrophic and growth factors, master transcription factors, and mechanical properties of the extracellular matrix (ECM), collectively regulate activities and characteristics of NSPCs: quiescence/survival, proliferation, migration, differentiation, and integration.
1. Introduction
During development, neural stem cells (NSCs) are responsible for the formation of the central nervous system (CNS). Initially, NSCs, also called neuroepithelial cells, differentiate into radial glial cells and proliferate into pools of neural progenitor cells (NPCs) [1]. NSC refers to an uncommitted cell with differentiation potential into the neurons and glia of the CNS. NSC is defined by two essential characteristics: self-renewal and multipotency [2]. These neural stem and progenitor cells (NSPCs) represent both populations and are established as the only self-renewing cell type in the adult CNS. NSPCs migrate and differentiate into highly specified networks of neurons via neurogenesis, and oligodendrocytes and astrocytes are generated via gliogenesis [3][4] (Figure 1). Thus, NSPCs are a major research thrust in the field of regenerative medicine. Extrinsic and intrinsic factors such as neurotrophic/growth factors, transcription factors, and canonical pathways guide neurogenesis and gliogenesis during development and adulthood.
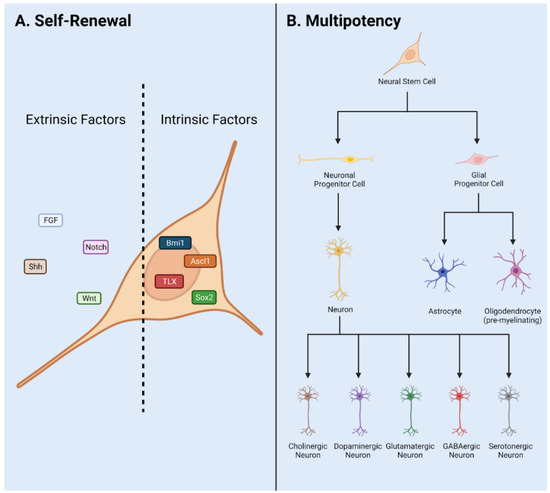
Figure 1. NSPC characteristics in adult mammals. (A) Self renewal requires input via extrinsic and intrinsic factors. These include signaling pathways Notch, Wnt, and Shh, and transcription factors Sox2, Ascl1, Bmi1, Tlx, and neurotransmitters and neurotrophic/trophic growth factors. (B) Multipotency allows NSPCs to differentiate into a variety of cell fates such as Neurons, Astrocytes, and Oligodendrocytes. Adapted from Navarro Quiroz et al., 2018 [5].
For the past 50 years, the topic of endogenous adult neurogenesis has been highly debated. This began with the initial discovery of adult mammalian neurogenesis in 1962 by Joseph Altman and has continued with noteworthy publications supporting the existence or non-existence of adult neurogenesis in mammals [6]. In the adult CNS, neurogenesis plays a primary role in essential processes such as learning, memory, maintenance of tissue homeostasis, and many others.
Heterogeneous populations of NSPCs exist in the neurogenic niches of the brain, spinal cord, and retina. Primary NSPCs are found in the subventricular zone (SVZ) and subgranular zone (SGZ) of the brain and include radial glial-like cells, NG2+/oligodendrocyte progenitor cells (OPCs), and Foxj1+ ependymal cells. Both OPCs and ependymal cell populations can be found in the spinal cord. In the adult retina, potential sources of NSPCs include Müller glia cells and the ciliary epithelium (CE).
NSPC response to CNS injury is extraordinarily complex and dependent upon the extent and location of injury. Injuries are most often contusion or blunt force-based and primarily result from sporting or vehicular accidents. Traumatic brain injuries (TBI) habitually damage two central niches: SGZ of the hippocampus and SVZ of the lateral ventricles. Damage to these regions can result in consequences including aberrant migration of NSPC progeny cells, incorrect dendritic branching, enhanced progenitor cell proliferation, ineffective integration of cells into networks of tissue, and many others. Spinal cord injury (SCI) may affect the neurogenic niche of the central canal resulting in differing contributions of NSPC populations to the glial scar. In addition, large differences in injury pathophysiology occur as a direct result of injury-mediated proliferation and altered differentiation.
In the eye, retinal injury results from chemical or mechanical damage and is highly dependent on NSPC activity. Traumatic mechanical injury of the eye results in severe morphological and functional changes in the eye structure including retinal detachment in humans [7]. Common retinal degenerative diseases include retinitis pigmentosa (RP), age-related macular degeneration (AMD) and glaucoma. Retinal degeneration affects photoreceptors, retinal ganglion cells and retinal pigment epithelium (RPE) to cause vision loss at varying degrees and eventual blindness.
Adult neurogenesis in heterogeneous NSPC populations has been implicated in demyelinating, inflammatory, and neurodegenerative conditions such as Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), and schizophrenia [8][9][10]. Early onset AD has been largely attributed to two genetic mutations, APP and presenilins (PS) [11]. Gene knock-out and knock-in mouse models show decreased neurogenesis, learning, and memory associated with upregulation of PS genes [12]. PD is characterized by progressive degeneration of dopaminergic neurons and PD-associated transgenic animal models have shown increased neurogenesis in dopaminergic neurons [13]. MS is defined by oligodendrocyte loss and axonal degeneration/demyelination [14]. A reduction in progenitor proliferation in the SVZ was observed in the lesion model of MS. Neuronal loss or axonal damage is characteristic of these conditions, thus modulation of adult neurogenesis, the generation of new neurons, has been proposed as a prospective treatment.
Neurogenic activity of the brain, spinal cord, and retina may facilitate the generation of functional networks of integrated tissue in damaged or diseased areas. Overall, NSPCs play an essential role in injuries or degenerative disorders that are largely affected by neurogenesis and disruptions in cell behavior such as traumatic brain injury (TBI), spinal cord injury (SCI), retinal injury, multiple sclerosis (MS), and schizophrenia [8][9][10]. Thus, an in-depth understanding of neurogenesis throughout the CNS will facilitate effective stem cell oriented therapeutic development.
2. Neural Stem/Progenitor Cells
The adult NSPCs (e.g., progenitor cells, neuroblasts, ependymal cells, NG2+ glia) are present in the stem cell niches of the brain, spinal cord, and retina. Major cell types present in the general NSPC niche include neurons, oligodendrocytes, astrocytes, pericytes, and endothelial cells. Neural stem cells primarily reside in the neural niches of the CNS, whereas progenitor cells can be found throughout the CNS due to increased migratory capacity [15][16][17][18].
Additional contributors to the microenvironment of NSPCs in CNS niches include cerebrospinal fluid (CSF), the extracellular matrix (ECM), and vasculature. The CSF consists of neurotrophic/growth factors, transcription factors, and ECM molecules required for NSPC guidance and is important for cell migration, morphogenesis, growth, and development [19]. The ECM provides mechanical support and regulates extracellular signaling environments. Moreover, proteoglycan and glycoprotein composition varies to influence signaling and bioavailability, motivating NSPC behavior within the stem cell niche [20].
Cellular cross talk between the stem cells and specified cell types contribute to the symphony of cascading signals regulating NSPC behavior. NSPC populations in the stem cell niche are highly regulated to produce neuronal or glial lineage cell types [21]. The vasculature also regulates neurogenesis in the adult CNS by transport of infiltrating biochemical signals to interact with NSPCs [22]. In this way, intrinsic and extrinsic signals regulate neurogenesis, generated via cross talk with cells, vasculature, ECM via external forces, and CSF in the neural niche. Intrinsic signals include master transcription factors such as Sox2 and REST [23]. Extrinsic signals include neurotrophic/trophic and growth factors, neurotransmitters, and signaling pathways such as Wnt and Notch.
When networks of neural cell types responsible for a regulated signaling microenvironment are damaged, NSPCs exhibit extreme behavior [24]. This is due to distinctly different signals or lack of signals required to regulate pools of active or quiescent NSPCs. Traumatic injury stimulates NSPCs to proliferate rapidly and produce cells which contribute to the glial scar border and upregulate angiogenesis in addition to neurogenic activities [10]. Preferential survival of transplanted NSCs was observed in geographical areas of high-density vasculature, which is said to play an essential role in the survival and maintenance of NSPCs in the injured spinal cord [22].
NSPCs often generate new non-functional networks of cells in response to injury which inhibits neural regeneration [25]. Altered niche activity may contribute to segregation of the injury but does not lead to regeneration of functional tissue. Differences in traumatic injury type and grade in the CNS result in significant changes in neurogenesis in one or more niches [24]. The heterogeneity of cell populations affected by traumatic injury result in clinical inconsistencies between cases. Further, the neurogenic niches of the brain, spinal cord, or retina exhibit regionally distinct niche composition before and after traumatic injury (Figure 2).
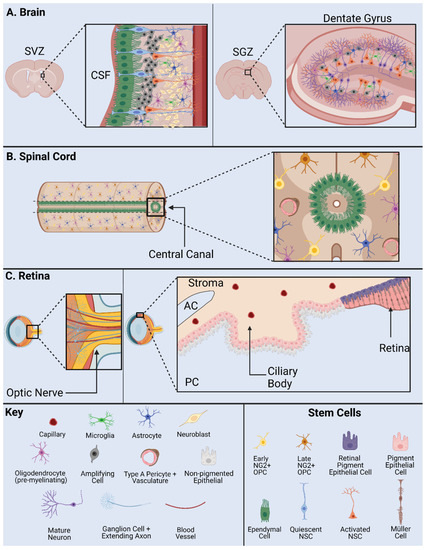
Figure 2. NSPC Niche in mammals: the SVZ and SGZ in the brain (A); the ependymal cells and NG2 cells in the spinal cord (B); and the base of the optic nerve, the Müller glia, and the pigment epithelium in the retina (C). AC, anterior chamber; CSF, cerebrospinal fluid; PC, posterior chamber; SVZ, subventricular zone; SGZ, subgranular zone. Adapted from Cutler and Kokovay, 2020 [26] (A); Sabelström et al., 2014 [27], Andreotti et al., 2019; Picoli et al., 2019 [28][29] (B); Yoshida et al., 2000 [30] (C).
2.1. Adult NSPCs in the Brain
The mammalian brain contains two primary neurogenic niches, i.e., the SGZ of the hippocampus and the SVZ of the lateral ventricles [31]. The hypothalamus serves as a third neurogenic niche conserved in some species but is nonexistent in humans [32]. Each distinct niche contains specific populations of NSPCs and differing functions.
The hippocampal neurogenic niche is present at the base of the hippocampus within the dentate gyrus (DG) in the SGZ (Figure 2A). In this niche, NSPCs are required for maintenance of the hippocampal tissue homeostasis, learning, and memory. Major stem cells in this neurogenic niche are radial glial-like cells (RGLs) which maintain neurogenic activity into adulthood [33]. Key cell types include OPCs, neuroblasts, immature/mature neurons, and oligodendrocytes. As a note, OPC populations in this niche include NG2+ cells.
The neurogenic niche along the walls of the lateral ventricles is located in the SVZ (Figure 2A). The lateral ventricle niche can be separated into two different geographical regions in the tissue: 1. dorsal, 2. lateral. Both dorsal and lateral components are in direct contact with pools of CSF, where the ependymal cell layer serves as a border between CSF and niche NSPCs [34]. This allows regulated contact between the ventricular cavities and undifferentiated progeny. Internal mechanisms direct NSPC behavior via fluid flow of CSF in the lateral ventricles [19]. NSPCs include astrocytes, neuronal/and glial progenitor subtypes, and neuroblasts [35][36][37]. Progenitors can be subdivided into further populations based on gene mapping analysis in both domains. Transcriptional patterning in temporal and spatial arrangements shows distinct NSPC populations [38]. Differential gene expression is driven by cell niche based signaling. Major signals include the Wnt/B-catenin and sonic hedgehog (Shh) pathway and are important to maintain regulatory behavior [39].
Adult neurogenesis in the hippocampus is dictated by intrinsic and extrinsic cues [40]. Signaling is initiated by surrounding cell types and vasculature in addition to master transcription factors Oct4, Sox2, and CREB. Signals from the Notch, Wnt, Shh, and other pathways direct neurogenesis in the SGZ.
The rostral migratory stream is a migration pathway for neuroblasts from the SVZ to the olfactory bulb and is present in some mammalian species. Conserved signaling pathways direct differentiation and integration of specified neurons and glia into the olfactory bulb. However, this is present to a lesser extent in larger mammalian species such as humans. Migrating neuroblasts from the hippocampal niche have been documented in rodent models to contribute to olfactory bulb mature cell types [41]. However, in human and primate models these cells are instead generated in the striatum. Damage to the neural niche of the hippocampus has been associated with cognitive deficits in learning and memory.
The hypothalamus neurogenic niche is located near the lateral ventricles below the SVZ, also called the periventricular zone [42]. Major cell types in this niche include hypothalamic ribbon cells lining the outer wall and monocytes which may present neurogenic potential. Three populations of NSCs have been found in the hypothalamus of animal models including mouse, rat, and monkey including tanycytes, ependymal cells, and small stellate cells [15]. These populations generate neurons and glia throughout life in the hypothalamic parenchyma. Neurogenesis in this region occurs at a lesser incidence in comparison with the two classic niches, hippocampal SGZ and lateral ventricles SVZ. This may translate into functional significance in murine models via control of energy metabolism.
In the injured brain, specific regulation of quiescence/survival in NSPCs has been attributed to the small glycoprotein lactadherin, growth factors vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF2), and Notch and Wnt pathways [39][43][44]. Proliferation is regulated by lactadherin, amyloid precursor protein, neurotrophic factor Tumor necrosis factor alpha (TNFa), growth factors FGF2, and VEGF, chemokine CX3CL1, and pathways Shh, Notch, and Wnt [45]. Migration is regulated by growth factor VEGF, chemokines CCR2 and CX3CL1, and the Wnt pathway [44]. Differentiation is regulated by growth factors FGF2 and VEGF, chemokines CCR2 and CX3CL1, as well as Notch and Shh pathways. Integration is regulated by growth factor VEGF and chemokine CX3CL1 [43]. Injury-induced or altered signals contribute to the enhanced proliferation, aberrant progenitor migration, ineffective integration, and reduced dendritic branching observed in TBI and SCI.
Using a combination of transgenic mouse model and single-cell RNA-seq analysis, distinct adult NSPC populations were identified in the SVZ [46]. In this study, GFP+ cells represent Nestin+ stem cell populations in the adult. Four groups of NSCs and three groups of progenitor cells were characterized with in vivo and in vitro RNA-seq studies of the SVZ neurogenic niche [46]. Immunostaining and imaging analysis revealed distinct subgroups of cells separated by signal intensity: high GFP, low GFP and no GFP, and co-labeled with specific markers such as DCX and GLAST. Further, RNA-seq analysis isolated cells into profiles of quiescent and active stem cells in addition to stem cell markers, e.g., Sox2, Ascl1, and DCX. Groups of cells are also separated anatomically, further supporting the existence of distinct populations. NSPC heterogeneity has also been demonstrated using stem cell markers including Gli1 and Ascl1 in both dividing and nondividing NSPCs [47]. The utility of these NSPC populations is unknown, but clear differences exist in gene expression profile.
2.2. Adult NPSCs in the Spinal Cord
The mammalian spinal cord contains one neurogenic niche in the ependyma of the central canal in which stem cells are present in an undifferentiated and self-renewable state (Figure 2B). The central canal serves as a continuation of the lateral ventricles into the spinal cord, while the ependymal cells serve as the bridge and a major regulatory element between the CSF and the stem cell niche [48]. The central canal neurogenic niche is lined with multiple populations of ependymal cells and CSF contacting neurons [49]. Ependymal cell populations can be further characterized into cells with short basal processes and cells with long extended processes. Other major components of the niche include NG2+ cells, vasculature, astroglial cells, and oligodendrocytes. Populations of progenitors in the spinal cord are indicated by markers Olig2, PDGFRa, and NG2 [50]. In addition, the ependymal cell layer is surrounded by supporting mature cell types, while the layer itself contains astroglial cells, NG2+ cells, and Nestin+ undifferentiated stem cells [49]. In normal physiology, NSPC proliferation is observed in this stem cell niche, indicated by Ki67 antibody staining in numerous studies [51][52].
Extrinsic signals guiding adult neurogenesis in the spinal cord include connexin, Notch and Wnt signaling pathways [18][53]. Intrinsic signals include neural progenitor transcription factors Nkx6.1, Pax6, and Olig6 [54][55][56]. These signals cohesively create an environment to control NSPC activity and maintain normal pools of immature and mature cell types in quiescent or active states. During injury or disease, NSPCs are subject to altered specific niche-based signals and exhibit skewed behavior. Thus, the neural niche in the central canal of the spinal cord is incredibly unique and maintained by a delicate balance of intrinsic and extrinsic signals.
SCI affects the NSPC stem cell niche in models of contusive, surgical stab, and slice injury at any anatomical level of the spinal cord [53]. Common clinical SCI disturbs the niche due to equidistant dorsal and ventral positioning of the central canal [24]. NSPCs proliferate after injury and interact with inflammatory signals to produce the glial scar border, a chemical/physical barrier which segregates the injury and prevents additional damage [57]. However, this scar also prevents axonal outgrowth into the site of injury and generation of new cell types within the neural lesion. NSPCs proliferate and differentiate into reactive astrocytes in the injured spinal cord and contribute to the glial scar border. In addition to newly generated progeny, resident astrocytes transition to reactive gliosis state and are recruited to the site of injury, lengthen their processes, and fatten to become the scar border [58]. A multitude of NSPCs in the spinal cord produce progeny of differing lineages to contribute to the glial scar after SCI and TBI.
Two major cell types have been controversially implicated in the NSPC response to injury and pose high therapeutic potential: NG2+ and ependymal cells. Many published studies are in support of the stem-like character or non-stem-like character of these cells. Both NG2+ cells and ependymal cells have been reported to contribute to the formation of the scar border. More recently, NG2+ cells have been shown to contribute to the generation of neurons in the injured spinal cord [57][59].
2.3. Adult Retinal Stem Cells
Cells from regions of the adult retina such as the retinal pigment epithelium (RPE) [60][61], CE [62][63][64][65][66], Müller glia cells [64][67][68][69], iris pigment epithelium [70][71] and optic nerve [61] show stem cell characteristics to varying degrees in humans and rodents (Figure 2C). Among them, the CE and Müller glia are identified as two main retinal stem cell sources.
A subpopulation of adult human RPE cells is capable of being activated to become RPE retinal stem cells in vitro and differentiated into multipotent stable RPE or mesenchymal lineages [60]. The optic nerve lamina region (ONLR) in both humans and mice contains a retinal NPC niche [61]. Adult NPCs in the ONLR exhibit multipotency and generate two types of glia: astrocytes and oligodendrocytes. These populations contribute to enable glial replacement and remyelination in adulthood [61]. The derived adult rat iris pigment epithelium (IPE) cells have NSPC properties and can differentiate into rod photoreceptor cells under CRX expression [71]. NeuroD induces human iris cells into rod photoreceptor cells. Moreover, Yuko et al. observed the combination of CRX, RX and NeuroD induces the generation of photoreceptor cells from the derived human IPE cells [70].
Non-pigmented CE cells show stem cell markers and actively proliferate after photoreceptor cell degeneration or retinal ganglion cell injury in the mouse model [62][72]. In the human CE, non-pigmented CE cells are labeled with stem cell markers, e.g., Sox2, Chx10 and Notch1. Non-pigmented CE cells showed proliferative ability under epidermal growth factor (EGF) induction using explants of the human retina [63]. CE cells including the pigmented cells and non-pigmented cells from human and mouse express NSPC cell markers and characteristics in vitro [64]. CE cells can be induced into photoreceptor cells, bipolar cells, retinal ganglion cells and Müller glia cells in the mouse model [65]. In addition, human CE cells can be induced into many types of retinal cells in vitro [66].
Müller glial cells are also considered as a primary source of retinal stem cells. Bhatia et al. concluded that retinal Müller glia may perform similar functions ascribed to astrocytes, ependymal cells and oligodendrocytes in other regions of the CNS [64]. Das et al. also stated that Müller glia are the NSCs of the adult retina [67]. They demonstrated that rat Müller glia have potential to generate retinal neurons in vitro and in vivo. Moreover, they proved the role of Notch and Wnt pathways in regulating this activity. Similarly, in mouse models, Müller glia can be reprogrammed into photoreceptors and retinal ganglion cells under certain culture conditions [68]. In adult human eyes, no evidence has been found to suggest that Müller glia possess the retinal neuronal regeneration ability in vivo. However, in vitro, these progenitor-type glia can be induced to proliferate and differentiate into retinal neurons and RPE cells [69]. Human Müller glia-derived stem cells can be differentiated toward the fate of retinal ganglion cell (RGC) precursors using FGF-2 and Notch inhibition [69]. In summary, Müller glia-derived stem cells can function as NSCs and serve as a potential target of therapy for retinal degenerative disease.
Common retinal diseases/injuries such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD) cause the photoreceptor cell loss and damaged RPE. However, no enhanced differentiation or proliferation was observed after injury [65]. Damaged cells release growth factors and cytokines which cause the Müller glia cell to differentiate, proliferate and express progenitor cell markers [73]. The ability of these proliferating Müller cells to regenerate new neurons and repair the injured retina appears to be extremely limited. Regardless, the multipotent stem cells may generate more functional photoreceptor cells and help with the recovery of vision loss in the RP and AMD via transplantation method [74].
2.4. Heterogeneity between CNS Niches
The perivascular stem cell niche is not technically a NSPC niche, but it interacts with cell types and influences NSPC behavior in all niches, thus contributing to the diversity of NSPC behavior observed in the mammalian CNS. In particular, the retina contains sources of NSPCs such as Müller glia and CE. Major factors unique to the retinal niche include CRX, RX and NeuroD. Interestingly, the retina does not contain ependymal cells, a major controversial stem type cell in the brain and spinal cord. However, NG2+ cells can be found in the retina [75]. The brain contains NSPC populations such as radial glial-like cells, OPCs, and ependymal cells. However, these populations and their characteristics vary throughout distinct NSPC niches. Major signals unique to the SGZ and SVZ include Shh pathway and transcription factors CREB and Oct4 [76]. The spinal cord stem cell niche contains both ependymal cells and NG2+ cells. Signals unique to the spinal cord include connexin signaling. The activity and consistency of NG2+ populations vary significantly between the niches of the brain, spinal cord, and retina. Specifically, NG2+ cells in the brain and spinal cord generate oligodendrocyte cell types and consist of glia and pericytes [77]. However, NG2+ cells in the retina consist of microglia and pericytes [75]. Ependymal cells also exhibit a variety of diverse behaviors in neurogenic niches of the brain and spinal cord. These controversial stem-like cells will be discussed in the following sections.
Understanding the heterogeneity of these stem cell populations and neurogenic niches is necessary to effectively design therapeutics for SCI, TBI, mechanical/chemical injury, and diseased states such as Glaucoma, Retinitis Pigmentosa, demyelinating diseases, and inflammatory conditions.
3. Notch1CR2-GFP+ NSPCs in Development and Injury
The canonical Notch signaling pathway is required to regulate the quiescence, proliferation, and differentiation of NSPCs in the CNS [56][78][79][80]. The Cai lab identified a 399-bp cis-element in the second intron of the Notch1 locus (CR2) [81]. In the Notch1CR2-GFP transgenic mouse, CR2 directs the reporter GFP expression in the interneuron progenitor cells. The activities of Notch pathway and NSPCs can be traced by the reporter GFP expression (Figure 3A). The cell fate of GFP tagged interneuron progenitors have been characterized in both normal development and neurological disease/injury conditions, which facilitate the study of the potentials of NSPCs in regenerative medicine [79][80][81][82]. In these studies, the Cai lab has demonstrated that GFP+ NSPCs preferentially differentiate into interneurons of the brain and spinal cord during embryonic development and in adulthood [56][80]. Injury increased the number of GFP+ NSPCs and interneurons at the injury site in a closed head injury model [80]. These results demonstrate that the endogenous NSPCs in the brain proliferate after injury and differentiate into specific cell fates (Figure 3B).
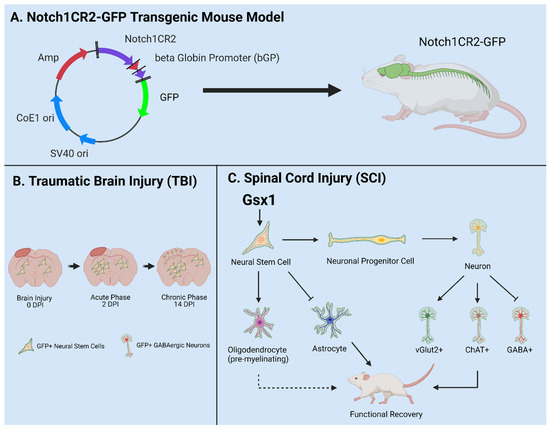
Figure 3. Utilities of the Notch1CR2-GFP transgenic mouse line in SCI and TBI models. (A) Notch1CR2-GFP transgenic mouse model labels NSPCs in the CNS. (B) Adult NSPCs in the brain proliferate in the acute phase of TBI and differentiate into neurons in the chronic phase of TBI. (C) In the injured spinal cord, Gsx1 expression promotes adult NSPC proliferation and preferential differentiation into excitatory interneurons and inhibits astrocytes and glial scar formation after injury. Adapted from Tzatzalos, et al., 2012 [81] (A), Anderson et al., 2020 [80] (B) and Patel et al., 2021 [79] (C).
In a more recent study, virus-mediated Gsx1 expression in NSPCs displayed an increased rate of cell proliferation with increased number of GFP+ NSPCs. Gsx1 further promoted neuronal differentiation over glial lineage in the injured spinal cord (Figure 3C). This resulted in an increased number of neurons, reduced reactive astrocytes and glial scar formation, and improved functional recovery [79]. Genetic manipulation of NSPCs is a primary therapeutic approach in the field of regenerative medicine [83]. Many conditions are defined by major cell loss and accompanied by decreased neurogenesis, e.g., SCI, TBI, MS, PD. Engineering NSPCs to increase proliferation and differentiation presents a viable option to promote effective regeneration of lost tissue in the CNS [84]. Gene/cell therapy can be used to express target genes in host cells, e.g., neurons, astrocytes, NSPCs, and oligodendrocytes [85][86]. NSPC specificity can be accomplished via choice of promotor, enhancer, and viral serotype. Common promoters target NSPCs including Nestin, Notch1, NG2, and Sox2. In recent years, forced expression of neurogenic genes (e.g., Ascl1, Gsx1, and Sox11) in stem cell populations promotes cell/tissue regeneration [79][87][88]. Many NSPC subpopulations have been identified, but functional and mechanistic understanding is limited [89]. Transgenic animal models such as the Notch1CR2-GFP allow in vivo investigation of specific NSPC populations and are vital to develop effective therapeutics in the future [56][79][80][81][82].
References
- Kawaguchi, A. Neuronal Delamination and Outer Radial Glia Generation in Neocortical Development. Front. Cell Dev. Biol. 2020, 8, 623573.
- Molofsky, A.V.; Pardal, R.; Iwashita, T.; Park, I.K.; Clarke, M.F.; Morrison, S.J. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 2003, 425, 962–967.
- Bond, A.M.; Berg, D.A.; Lee, S.; Garcia-Epelboim, A.S.; Adusumilli, V.S.; Ming, G.L.; Song, H. Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions. Brain Sci. 2020, 10, 909.
- Miller, F.D.; Gauthier, A.S. Timing is everything: Making neurons versus glia in the developing cortex. Neuron 2007, 54, 357–369.
- Navarro Quiroz, E.; Navarro Quiroz, R.; Ahmad, M.; Gomez Escorcia, L.; Villarreal, J.L.; Fernandez Ponce, C.; Aroca Martinez, G. Cell Signaling in Neuronal Stem Cells. Cells 2018, 7, 75.
- Altman, J. Are new neurons formed in the brains of adult mammals? Science 1962, 135, 1127–1128.
- Potockova, A.; Strmen, P.; Krasnik, V.; Olah, Z. Mechanical injuries of the eye. Bratisl Lek Listy 2010, 111, 329–335.
- Park, I.H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-specific induced pluripotent stem cells. Cell 2008, 134, 877–886.
- Akkermann, R.; Beyer, F.; Kury, P. Heterogeneous populations of neural stem cells contribute to myelin repair. Neural Regen. Res. 2017, 12, 509–517.
- Li, Y.; Chang, S.; Li, W.; Tang, G.; Ma, Y.; Liu, Y.; Yuan, F.; Zhang, Z.; Yang, G.Y.; Wang, Y. cxcl12-engineered endothelial progenitor cells enhance neurogenesis and angiogenesis after ischemic brain injury in mice. Stem Cell Res. Ther. 2018, 9, 139.
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer's disease. Mol. Neurodegener. 2011, 6, 85.
- Gadadhar, A.; Marr, R.; Lazarov, O. Presenilin-1 regulates neural progenitor cell differentiation in the adult brain. J. Neurosci. 2011, 31, 2615–2623.
- Hoglinger, G.U.; Rizk, P.; Muriel, M.P.; Duyckaerts, C.; Oertel, W.H.; Caille, I.; Hirsch, E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004, 7, 726–735.
- Chang, A.; Smith, M.C.; Yin, X.; Fox, R.J.; Staugaitis, S.M.; Trapp, B.D. Neurogenesis in the chronic lesions of multiple sclerosis. Brain 2008, 131, 2366–2375.
- Fernandez-Castaneda, A.; Gaultier, A. Adult oligodendrocyte progenitor cells—Multifaceted regulators of the CNS in health and disease. Brain Behav. Immun. 2016, 57, 1–7.
- Mallett, C.L.; Shuboni-Mulligan, D.D.; Shapiro, E.M. Tracking Neural Progenitor Cell Migration in the Rodent Brain Using Magnetic Resonance Imaging. Front. Neurosci. 2018, 12, 995.
- Nelson, B.R.; Hodge, R.D.; Daza, R.A.; Tripathi, P.P.; Arnold, S.J.; Millen, K.J.; Hevner, R.F. Intermediate progenitors support migration of neural stem cells into dentate gyrus outer neurogenic niches. Elife 2020, 9, e53777.
- Tang, C.; Wang, M.; Wang, P.; Wang, L.; Wu, Q.; Guo, W. Neural Stem Cells Behave as a Functional Niche for the Maturation of Newborn Neurons through the Secretion of PTN. Neuron 2019, 101, 32–44.e36.
- Alonso, M.I.; Gato, A. Cerebrospinal fluid and neural stem cell niche control. Neural Regen Res. 2018, 13, 1546–1547.
- Ahmed, M.; Ffrench-Constant, C. Extracellular Matrix Regulation of Stem Cell Behavior. Curr Stem Cell Rep. 2016, 2, 197–206.
- Spitzer, S.O.; Sitnikov, S.; Kamen, Y.; Evans, K.A.; Kronenberg-Versteeg, D.; Dietmann, S.; de Faria, O., Jr.; Agathou, S.; Karadottir, R.T. Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 2019, 101, 459–471.e455.
- Karakatsani, A.; Shah, B.; Ruiz de Almodovar, C. Blood Vessels as Regulators of Neural Stem Cell Properties. Front. Mol. Neurosci. 2019, 12, 85.
- Surzenko, N.; Crowl, T.; Bachleda, A.; Langer, L.; Pevny, L. SOX2 maintains the quiescent progenitor cell state of postnatal retinal Muller glia. Development 2013, 140, 1445–1456.
- Falnikar, A.; Stratton, J.; Lin, R.; Andrews, C.E.; Tyburski, A.; Trovillion, V.A.; Gottschalk, C.; Ghosh, B.; Iacovitti, L.; Elliott, M.B.; et al. Differential Response in Novel Stem Cell Niches of the Brain after Cervical Spinal Cord Injury and Traumatic Brain Injury. J. Neurotrauma 2018, 35, 2195–2207.
- Griffin, J.M.; Bradke, F. Therapeutic repair for spinal cord injury: Combinatory approaches to address a multifaceted problem. EMBO Mol. Med. 2020, 12, e11505.
- Cutler, R.R.; Kokovay, E. Rejuvenating subventricular zone neurogenesis in the aging brain. Curr. Opin. Pharmacol. 2020, 50, 1–8.
- Sabelstrom, H.; Stenudd, M.; Frisen, J. Neural stem cells in the adult spinal cord. Exp. Neurol. 2014, 260, 44–49.
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. Neural stem cell niche heterogeneity. Semin Cell Dev. Biol. 2019, 95, 42–53.
- Picoli, C.C.; Coimbra-Campos, L.M.C.; Guerra, D.A.P.; Silva, W.N.; Prazeres, P.; Costa, A.C.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; Birbrair, A. Pericytes Act as Key Players in Spinal Cord Injury. Am. J. Pathol. 2019, 189, 1327–1337.
- Yoshida, M.; Takeuchi, M.; Streilein, J.W. Participation of pigment epithelium of iris and ciliary body in ocular immune privilege. 1. Inhibition of T-cell activation in vitro by direct cell-to-cell contact. Invest. Ophthalmol. Vis. Sci. 2000, 41, 811–821.
- Martin-Suarez, S.; Valero, J.; Muro-Garcia, T.; Encinas, J.M. Phenotypical and functional heterogeneity of neural stem cells in the aged hippocampus. Aging Cell 2019, 18, e12958.
- Pellegrino, G.; Trubert, C.; Terrien, J.; Pifferi, F.; Leroy, D.; Loyens, A.; Migaud, M.; Baroncini, M.; Maurage, C.A.; Fontaine, C.; et al. A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and gray mouse lemur (Microcebus murinus). J. Comp. Neurol. 2018, 526, 1419–1443.
- Le Belle, J.E.; Kornblum, H.I. Interactive Regulation of Neuronal Development by Hippocampal Stem Cell Niche Populations. Neuron 2019, 101, 1–2.
- Nascimento, M.A.; Sorokin, L.; Coelho-Sampaio, T. Fractone Bulbs Derive from Ependymal Cells and Their Laminin Composition Influence the Stem Cell Niche in the Subventricular Zone. J. Neurosci. 2018, 38, 3880–3889.
- Doetsch, F.; Caille, I.; Lim, D.A.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716.
- Kirschenbaum, B.; Doetsch, F.; Lois, C.; Alvarez-Buylla, A. Adult subventricular zone neuronal precursors continue to proliferate and migrate in the absence of the olfactory bulb. J. Neurosci. 1999, 19, 2171–2180.
- Figueres-Onate, M.; Sanchez-Villalon, M.; Sanchez-Gonzalez, R.; Lopez-Mascaraque, L. Lineage Tracing and Cell Potential of Postnatal Single Progenitor Cells In Vivo. Stem Cell Rep. 2019, 13, 700–712.
- Dulken, B.W.; Leeman, D.S.; Boutet, S.C.; Hebestreit, K.; Brunet, A. Single-Cell Transcriptomic Analysis Defines Heterogeneity and Transcriptional Dynamics in the Adult Neural Stem Cell Lineage. Cell Rep. 2017, 18, 777–790.
- Chavali, M.; Klingener, M.; Kokkosis, A.G.; Garkun, Y.; Felong, S.; Maffei, A.; Aguirre, A. Non-canonical Wnt signaling regulates neural stem cell quiescence during homeostasis and after demyelination. Nat. Commun. 2018, 9, 36.
- Ma, D.K.; Kim, W.R.; Ming, G.L.; Song, H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann. NY Acad. Sci. 2009, 1170, 664–673.
- Kjell, J.; Fischer-Sternjak, J.; Thompson, A.J.; Friess, C.; Sticco, M.J.; Salinas, F.; Cox, J.; Martinelli, D.C.; Ninkovic, J.; Franze, K.; et al. Defining the Adult Neural Stem Cell Niche Proteome Identifies Key Regulators of Adult Neurogenesis. Cell Stem Cell 2020, 26, 277–293.e278.
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820.
- Thau-Zuchman, O.; Shohami, E.; Alexandrovich, A.G.; Leker, R.R. Vascular endothelial growth factor increases neurogenesis after traumatic brain injury. J. Cereb. Blood Flow Metab. 2010, 30, 1008–1016.
- Zhang, J.Y.; Lee, J.H.; Gu, X.; Wei, Z.Z.; Harris, M.J.; Yu, S.P.; Wei, L. Intranasally Delivered Wnt3a Improves Functional Recovery after Traumatic Brain Injury by Modulating Autophagic, Apoptotic, and Regenerative Pathways in the Mouse Brain. J. Neurotrauma 2018, 35, 802–813.
- Xing, C.; Lo, E.H. Help-me signaling: Non-cell autonomous mechanisms of neuroprotection and neurorecovery. Prog. Neurobiol 2017, 152, 181–199.
- Xie, X.P.; Laks, D.R.; Sun, D.; Poran, A.; Laughney, A.M.; Wang, Z.; Sam, J.; Belenguer, G.; Farinas, I.; Elemento, O.; et al. High-resolution mouse subventricular zone stem-cell niche transcriptome reveals features of lineage, anatomy, and aging. Proc. Natl. Acad. Sci. USA 2020, 117, 31448–31458.
- Bottes, S.; Jaeger, B.N.; Pilz, G.A.; Jorg, D.J.; Cole, J.D.; Kruse, M.; Harris, L.; Korobeynyk, V.I.; Mallona, I.; Helmchen, F.; et al. Long-term self-renewing stem cells in the adult mouse hippocampus identified by intravital imaging. Nat. Neurosci. 2021, 24, 225–233.
- Mahuzier, A.; Shihavuddin, A.; Fournier, C.; Lansade, P.; Faucourt, M.; Menezes, N.; Meunier, A.; Garfa-Traore, M.; Carlier, M.F.; Voituriez, R.; et al. Ependymal cilia beating induces an actin network to protect centrioles against shear stress. Nat. Commun. 2018, 9, 2279.
- Marichal, N.; Reali, C.; Trujillo-Cenoz, O.; Russo, R.E. Spinal Cord Stem Cells In Their Microenvironment: The Ependyma as a Stem Cell Niche. Adv. Exp. Med. Biol. 2017, 1041, 55–79.
- Nishiyama, A.; Komitova, M.; Suzuki, R.; Zhu, X. Polydendrocytes (NG2 cells): Multifunctional cells with lineage plasticity. Nat. Rev. Neurosci. 2009, 10, 9–22.
- Barnabe-Heider, F.; Goritz, C.; Sabelstrom, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisen, J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 2010, 7, 470–482.
- Ortiz-Alvarez, G.; Daclin, M.; Shihavuddin, A.; Lansade, P.; Fortoul, A.; Faucourt, M.; Clavreul, S.; Lalioti, M.E.; Taraviras, S.; Hippenmeyer, S.; et al. Adult Neural Stem Cells and Multiciliated Ependymal Cells Share a Common Lineage Regulated by the Geminin Family Members. Neuron 2019, 102, 159–172.e157.
- Fabbiani, G.; Reali, C.; Valentin-Kahan, A.; Rehermann, M.I.; Fagetti, J.; Falco, M.V.; Russo, R.E. Connexin Signaling Is Involved in the Reactivation of a Latent Stem Cell Niche after Spinal Cord Injury. J. Neurosci. 2020, 40, 2246–2258.
- Ghazale, H.; Ripoll, C.; Leventoux, N.; Jacob, L.; Azar, S.; Mamaeva, D.; Glasson, Y.; Calvo, C.F.; Thomas, J.L.; Meneceur, S.; et al. RNA Profiling of the Human and Mouse Spinal Cord Stem Cell Niches Reveals an Embryonic-like Regionalization with MSX1(+) Roof-Plate-Derived Cells. Stem Cell Rep. 2019, 12, 1159–1177.
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallieres, N.; Lessard, M.; Janelle, M.E.; Vernoux, N.; Tremblay, M.E.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019, 10, 518.
- Li, Y.; Tzatzalos, E.; Kwan, K.Y.; Grumet, M.; Cai, L. Transcriptional Regulation of Notch1 Expression by Nkx6.1 in Neural Stem/Progenitor Cells during Ventral Spinal Cord Development. Sci. Rep. 2016, 6, 38665.
- Hesp, Z.C.; Yoseph, R.Y.; Suzuki, R.; Jukkola, P.; Wilson, C.; Nishiyama, A.; McTigue, D.M. Proliferating NG2-Cell-Dependent Angiogenesis and Scar Formation Alter Axon Growth and Functional Recovery After Spinal Cord Injury in Mice. J. Neurosci. 2018, 38, 1366–1382.
- Hackett, A.R.; Yahn, S.L.; Lyapichev, K.; Dajnoki, A.; Lee, D.H.; Rodriguez, M.; Cammer, N.; Pak, J.; Mehta, S.T.; Bodamer, O.; et al. Injury type-dependent differentiation of NG2 glia into heterogeneous astrocytes. Exp. Neurol. 2018, 308, 72–79.
- Tai, W.; Wu, W.; Wang, L.L.; Ni, H.; Chen, C.; Yang, J.; Zang, T.; Zou, Y.; Xu, X.M.; Zhang, C.L. In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell 2021, 28, 923–937.e4.
- Salero, E.; Blenkinsop, T.A.; Corneo, B.; Harris, A.; Rabin, D.; Stern, J.H.; Temple, S. Adult human RPE can be activated into a multipotent stem cell that produces mesenchymal derivatives. Cell Stem Cell 2012, 10, 88–95.
- Bernstein, S.L.; Guo, Y.; Kerr, C.; Fawcett, R.J.; Stern, J.H.; Temple, S.; Mehrabian, Z. The optic nerve lamina region is a neural progenitor cell niche. Proc. Natl. Acad. Sci. USA 2020, 117, 19287–19298.
- Nickerson, P.E.; Emsley, J.G.; Myers, T.; Clarke, D.B. Proliferation and expression of progenitor and mature retinal phenotypes in the adult mammalian ciliary body after retinal ganglion cell injury. Invest. Ophthalmol. Vis. Sci 2007, 48, 5266–5275.
- Bhatia, B.; Singhal, S.; Lawrence, J.M.; Khaw, P.T.; Limb, G.A. Distribution of Muller stem cells within the neural retina: Evidence for the existence of a ciliary margin-like zone in the adult human eye. Exp. Eye Res. 2009, 89, 373–382.
- Bhatia, B.; Singhal, S.; Jayaram, H.; Khaw, P.T.; Limb, G.A. Adult retinal stem cells revisited. Open Ophthalmol. J. 2010, 4, 30–38.
- Aladdad, A.M.; Kador, K.E. Adult Stem Cells, Tools for Repairing the Retina. Curr. Ophthalmol. Rep. 2019, 7, 21–29.
- Coles, B.L.; Angenieux, B.; Inoue, T.; Del Rio-Tsonis, K.; Spence, J.R.; McInnes, R.R.; Arsenijevic, Y.; van der Kooy, D. Facile isolation and the characterization of human retinal stem cells. Proc. Natl. Acad. Sci. USA 2004, 101, 15772–15777.
- Das, A.V.; Mallya, K.B.; Zhao, X.; Ahmad, F.; Bhattacharya, S.; Thoreson, W.B.; Hegde, G.V.; Ahmad, I. Neural stem cell properties of Muller glia in the mammalian retina: Regulation by Notch and Wnt signaling. Dev. Biol. 2006, 299, 283–302.
- Zhao, X.F.; Wan, J.; Powell, C.; Ramachandran, R.; Myers, M.G., Jr.; Goldman, D. Leptin and IL-6 family cytokines synergize to stimulate Muller glia reprogramming and retina regeneration. Cell Rep. 2014, 9, 272–284.
- Singhal, S.; Bhatia, B.; Jayaram, H.; Becker, S.; Jones, M.F.; Cottrill, P.B.; Khaw, P.T.; Salt, T.E.; Limb, G.A. Human Muller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med. 2012, 1, 188–199.
- Seko, Y.; Azuma, N.; Kaneda, M.; Nakatani, K.; Miyagawa, Y.; Noshiro, Y.; Kurokawa, R.; Okano, H.; Umezawa, A. Derivation of human differential photoreceptor-like cells from the iris by defined combinations of CRX, RX and NEUROD. PLoS ONE 2012, 7, e35611.
- Haruta, M.; Kosaka, M.; Kanegae, Y.; Saito, I.; Inoue, T.; Kageyama, R.; Nishida, A.; Honda, Y.; Takahashi, M. Induction of photoreceptor-specific phenotypes in adult mammalian iris tissue. Nat. Neurosci. 2001, 4, 1163–1164.
- Nishiguchi, K.M.; Kaneko, H.; Nakamura, M.; Kachi, S.; Terasaki, H. Generation of immature retinal neurons from proliferating cells in the pars plana after retinal histogenesis in mice with retinal degeneration. Mol. Vis. 2009, 15, 187–199.
- Tropepe, V.; Coles, B.L.; Chiasson, B.J.; Horsford, D.J.; Elia, A.J.; McInnes, R.R.; van der Kooy, D. Retinal stem cells in the adult mammalian eye. Science 2000, 287, 2032–2036.
- Li, T.; Lewallen, M.; Chen, S.; Yu, W.; Zhang, N.; Xie, T. Multipotent stem cells isolated from the adult mouse retina are capable of producing functional photoreceptor cells. Cell Res. 2013, 23, 788–802.
- Wohl, S.G.; Schmeer, C.W.; Friese, T.; Witte, O.W.; Isenmann, S. In situ dividing and phagocytosing retinal microglia express nestin, vimentin, and NG2 in vivo. PLoS ONE 2011, 6, e22408.
- Marcuzzo, S.; Isaia, D.; Bonanno, S.; Malacarne, C.; Cavalcante, P.; Zacheo, A.; Laquintana, V.; Denora, N.; Sanavio, B.; Salvati, E.; et al. FM19G11-Loaded Gold Nanoparticles Enhance the Proliferation and Self-Renewal of Ependymal Stem Progenitor Cells Derived from ALS Mice. Cells 2019, 8, 279.
- Kang, S.H.; Fukaya, M.; Yang, J.K.; Rothstein, J.D.; Bergles, D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010, 68, 668–681.
- Alexson, T.O.; Hitoshi, S.; Coles, B.L.; Bernstein, A.; van der Kooy, D. Notch signaling is required to maintain all neural stem cell populations--irrespective of spatial or temporal niche. Dev. Neurosci. 2006, 28, 34–48.
- Patel, M.; Li, Y.; Anderson, J.; Castro-Pedrido, S.; Skinner, R.; Lei, S.; Finkel, Z.; Rodriguez, B.; Esteban, F.; Lee, K.B.; et al. Gsx1 promotes locomotor functional recovery after spinal cord injury. Mol. Ther. 2021, 29, 2469–2482.
- Anderson, J.; Patel, M.; Forenzo, D.; Ai, X.; Cai, C.; Wade, Q.; Risman, R.; Cai, L. A novel mouse model for the study of endogenous neural stem and progenitor cells after traumatic brain injury. Exp. Neurol. 2020, 325.
- Tzatzalos, E.; Smith, S.M.; Doh, S.T.; Hao, H.; Li, Y.; Wu, A.; Grumet, M.; Cai, L. A cis-element in the Notch1 locus is involved in the regulation of gene expression in interneuron progenitors. Dev. Biol. 2012, 372, 217–228.
- Patel, M.; Anderson, J.; Lei, S.; Finkel, Z.; Rodriguez, B.; Esteban, F.; Risman, R.; Li, Y.; Lee, K.B.; Lyu, Y.L.; et al. Nkx6.1 enhances neural stem cell activation and attenuates glial scar formation and neuroinflammation in the adult injured spinal cord. Exp. Neurol. 2021, 113826.
- Nandoe Tewarie, R.S.; Hurtado, A.; Bartels, R.H.; Grotenhuis, A.; Oudega, M. Stem cell-based therapies for spinal cord injury. J. Spinal Cord Med. 2009, 32, 105–114.
- Winner, B.; Winkler, J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2015, 7, a021287.
- Castle, M.J.; Turunen, H.T.; Vandenberghe, L.H.; Wolfe, J.H. Controlling AAV Tropism in the Nervous System with Natural and Engineered Capsids. Methods Mol. Biol. 2016, 1382, 133–149.
- Juttner, J.; Szabo, A.; Gross-Scherf, B.; Morikawa, R.K.; Rompani, S.B.; Hantz, P.; Szikra, T.; Esposti, F.; Cowan, C.S.; Bharioke, A.; et al. Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat. Neurosci. 2019, 22, 1345–1356.
- Battiste, J.; Helms, A.W.; Kim, E.J.; Savage, T.K.; Lagace, D.C.; Mandyam, C.D.; Eisch, A.J.; Miyoshi, G.; Johnson, J.E. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development 2007, 134, 285–293.
- Wang, Z.; Reynolds, A.; Kirry, A.; Nienhaus, C.; Blackmore, M.G. Overexpression of Sox11 promotes corticospinal tract regeneration after spinal injury while interfering with functional recovery. J. Neurosci. 2015, 35, 3139–3145.
- Mizrak, D.; Levitin, H.M.; Delgado, A.C.; Crotet, V.; Yuan, J.; Chaker, Z.; Silva-Vargas, V.; Sims, P.A.; Doetsch, F. Single-Cell Analysis of Regional Differences in Adult V-SVZ Neural Stem Cell Lineages. Cell Rep. 2019, 26, 394–406.e395.




