The adult NSPCs (e.g., progenitor cells, neuroblasts, ependymal cells, NG2+ glia) are present in the stem cell niches of the brain, spinal cord, and retina. Major cell types present in the general NSPC niche include neurons, oligodendrocytes, astrocytes, pericytes, and endothelial cells. Neural stem cells primarily reside in the neural niches of the CNS, whereas progenitor cells can be found throughout the CNS due to increased migratory capacity [
15,
16,
17,
18].
Additional contributors to the microenvironment of NSPCs in CNS niches include cerebrospinal fluid (CSF), the extracellular matrix (ECM), and vasculature. The CSF consists of neurotrophic/growth factors, transcription factors, and ECM molecules required for NSPC guidance and is important for cell migration, morphogenesis, growth, and development [
19]. The ECM provides mechanical support and regulates extracellular signaling environments. Moreover, proteoglycan and glycoprotein composition varies to influence signaling and bioavailability, motivating NSPC behavior within the stem cell niche [
20].
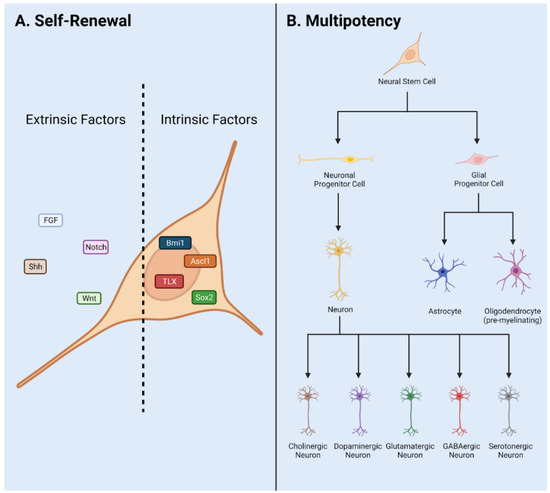
Cellular cross talk between the stem cells and specified cell types contribute to the symphony of cascading signals regulating NSPC behavior. NSPC populations in the stem cell niche are highly regulated to produce neuronal or glial lineage cell types [
21]. The vasculature also regulates neurogenesis in the adult CNS by transport of infiltrating biochemical signals to interact with NSPCs [
22]. In this way, intrinsic and extrinsic signals regulate neurogenesis, generated via cross talk with cells, vasculature, ECM via external forces, and CSF in the neural niche. Intrinsic signals include master transcription factors such as Sox2 and REST [
23]. Extrinsic signals include neurotrophic/trophic and growth factors, neurotransmitters, and signaling pathways such as Wnt and Notch.
When networks of neural cell types responsible for a regulated signaling microenvironment are damaged, NSPCs exhibit extreme behavior [
24]. This is due to distinctly different signals or lack of signals required to regulate pools of active or quiescent NSPCs. Traumatic injury stimulates NSPCs to proliferate rapidly and produce cells which contribute to the glial scar border and upregulate angiogenesis in addition to neurogenic activities [
10]. Preferential survival of transplanted NSCs was observed in geographical areas of high-density vasculature, which is said to play an essential role in the survival and maintenance of NSPCs in the injured spinal cord [
22].
NSPCs often generate new non-functional networks of cells in response to injury which inhibits neural regeneration [
25]. Altered niche activity may contribute to segregation of the injury but does not lead to regeneration of functional tissue. Differences in traumatic injury type and grade in the CNS result in significant changes in neurogenesis in one or more niches [
24]. The heterogeneity of cell populations affected by traumatic injury result in clinical inconsistencies between cases. Further, the neurogenic niches of the brain, spinal cord, or retina exhibit regionally distinct niche composition before and after traumatic injury (
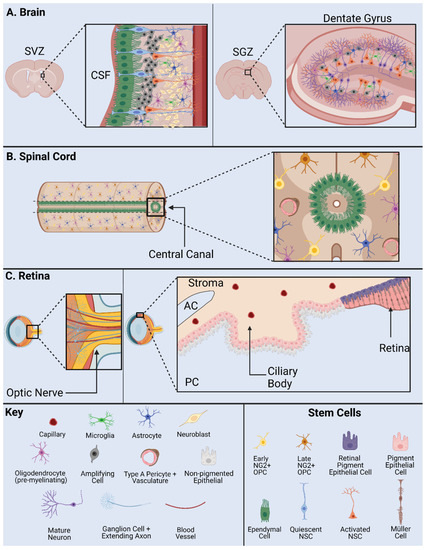
Figure 2).
Figure 2. NSPC Niche in mammals: the SVZ and SGZ in the brain (
A); the ependymal cells and NG2 cells in the spinal cord (
B); and the base of the optic nerve, the Müller glia, and the pigment epithelium in the retina (
C). AC, anterior chamber; CSF, cerebrospinal fluid; PC, posterior chamber; SVZ, subventricular zone; SGZ, subgranular zone. Adapted from Cutler and Kokovay, 2020 [
26] (
A); Sabelström et al., 2014 [
27], Andreotti et al., 2019; Picoli et al., 2019 [
28,
29] (
B); Yoshida et al., 2000 [
30] (
C).
2.1. Adult NSPCs in the Brain
The mammalian brain contains two primary neurogenic niches, i.e., the SGZ of the hippocampus and the SVZ of the lateral ventricles [
31]. The hypothalamus serves as a third neurogenic niche conserved in some species but is nonexistent in humans [
32]. Each distinct niche contains specific populations of NSPCs and differing functions.
The hippocampal neurogenic niche is present at the base of the hippocampus within the dentate gyrus (DG) in the SGZ (
Figure 2A). In this niche, NSPCs are required for maintenance of the hippocampal tissue homeostasis, learning, and memory. Major stem cells in this neurogenic niche are radial glial-like cells (RGLs) which maintain neurogenic activity into adulthood [
33]. Key cell types include OPCs, neuroblasts, immature/mature neurons, and oligodendrocytes. As a note, OPC populations in this niche include NG2+ cells.
The neurogenic niche along the walls of the lateral ventricles is located in the SVZ (
Figure 2A). The lateral ventricle niche can be separated into two different geographical regions in the tissue: 1. dorsal, 2. lateral. Both dorsal and lateral components are in direct contact with pools of CSF, where the ependymal cell layer serves as a border between CSF and niche NSPCs [
34]. This allows regulated contact between the ventricular cavities and undifferentiated progeny. Internal mechanisms direct NSPC behavior via fluid flow of CSF in the lateral ventricles [
19]. NSPCs include astrocytes, neuronal/and glial progenitor subtypes, and neuroblasts [
35,
36,
37]. Progenitors can be subdivided into further populations based on gene mapping analysis in both domains. Transcriptional patterning in temporal and spatial arrangements shows distinct NSPC populations [
38]. Differential gene expression is driven by cell niche based signaling. Major signals include the Wnt/B-catenin and sonic hedgehog (Shh) pathway and are important to maintain regulatory behavior [
39].
Adult neurogenesis in the hippocampus is dictated by intrinsic and extrinsic cues [
40]. Signaling is initiated by surrounding cell types and vasculature in addition to master transcription factors Oct4, Sox2, and CREB. Signals from the Notch, Wnt, Shh, and other pathways direct neurogenesis in the SGZ.
The rostral migratory stream is a migration pathway for neuroblasts from the SVZ to the olfactory bulb and is present in some mammalian species. Conserved signaling pathways direct differentiation and integration of specified neurons and glia into the olfactory bulb. However, this is present to a lesser extent in larger mammalian species such as humans. Migrating neuroblasts from the hippocampal niche have been documented in rodent models to contribute to olfactory bulb mature cell types [
41]. However, in human and primate models these cells are instead generated in the striatum. Damage to the neural niche of the hippocampus has been associated with cognitive deficits in learning and memory.
The hypothalamus neurogenic niche is located near the lateral ventricles below the SVZ, also called the periventricular zone [
42]. Major cell types in this niche include hypothalamic ribbon cells lining the outer wall and monocytes which may present neurogenic potential. Three populations of NSCs have been found in the hypothalamus of animal models including mouse, rat, and monkey including tanycytes, ependymal cells, and small stellate cells [
15]. These populations generate neurons and glia throughout life in the hypothalamic parenchyma. Neurogenesis in this region occurs at a lesser incidence in comparison with the two classic niches, hippocampal SGZ and lateral ventricles SVZ. This may translate into functional significance in murine models via control of energy metabolism.
In the injured brain, specific regulation of quiescence/survival in NSPCs has been attributed to the small glycoprotein lactadherin, growth factors vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF2), and Notch and Wnt pathways [
39,
43,
44]. Proliferation is regulated by lactadherin, amyloid precursor protein, neurotrophic factor Tumor necrosis factor alpha (TNFa), growth factors FGF2, and VEGF, chemokine CX3CL1, and pathways Shh, Notch, and Wnt [
45]. Migration is regulated by growth factor VEGF, chemokines CCR2 and CX3CL1, and the Wnt pathway [
44]. Differentiation is regulated by growth factors FGF2 and VEGF, chemokines CCR2 and CX3CL1, as well as Notch and Shh pathways. Integration is regulated by growth factor VEGF and chemokine CX3CL1 [
43]. Injury-induced or altered signals contribute to the enhanced proliferation, aberrant progenitor migration, ineffective integration, and reduced dendritic branching observed in TBI and SCI.
Using a combination of transgenic mouse model and single-cell RNA-seq analysis, distinct adult NSPC populations were identified in the SVZ [
46]. In this study, GFP+ cells represent Nestin+ stem cell populations in the adult. Four groups of NSCs and three groups of progenitor cells were characterized with in vivo and in vitro RNA-seq studies of the SVZ neurogenic niche [
46]. Immunostaining and imaging analysis revealed distinct subgroups of cells separated by signal intensity: high GFP, low GFP and no GFP, and co-labeled with specific markers such as DCX and GLAST. Further, RNA-seq analysis isolated cells into profiles of quiescent and active stem cells in addition to stem cell markers, e.g., Sox2, Ascl1, and DCX. Groups of cells are also separated anatomically, further supporting the existence of distinct populations. NSPC heterogeneity has also been demonstrated using stem cell markers including Gli1 and Ascl1 in both dividing and nondividing NSPCs [
47]. The utility of these NSPC populations is unknown, but clear differences exist in gene expression profile.
2.2. Adult NPSCs in the Spinal Cord
The mammalian spinal cord contains one neurogenic niche in the ependyma of the central canal in which stem cells are present in an undifferentiated and self-renewable state (
Figure 2B). The central canal serves as a continuation of the lateral ventricles into the spinal cord, while the ependymal cells serve as the bridge and a major regulatory element between the CSF and the stem cell niche [
48]. The central canal neurogenic niche is lined with multiple populations of ependymal cells and CSF contacting neurons [
49]. Ependymal cell populations can be further characterized into cells with short basal processes and cells with long extended processes. Other major components of the niche include NG2+ cells, vasculature, astroglial cells, and oligodendrocytes. Populations of progenitors in the spinal cord are indicated by markers Olig2, PDGFRa, and NG2 [
50]. In addition, the ependymal cell layer is surrounded by supporting mature cell types, while the layer itself contains astroglial cells, NG2+ cells, and Nestin+ undifferentiated stem cells [
49]. In normal physiology, NSPC proliferation is observed in this stem cell niche, indicated by Ki67 antibody staining in numerous studies [
51,
52].
Extrinsic signals guiding adult neurogenesis in the spinal cord include connexin, Notch and Wnt signaling pathways [
18,
53]. Intrinsic signals include neural progenitor transcription factors Nkx6.1, Pax6, and Olig6 [
54,
55,
56]. These signals cohesively create an environment to control NSPC activity and maintain normal pools of immature and mature cell types in quiescent or active states. During injury or disease, NSPCs are subject to altered specific niche-based signals and exhibit skewed behavior. Thus, the neural niche in the central canal of the spinal cord is incredibly unique and maintained by a delicate balance of intrinsic and extrinsic signals.
SCI affects the NSPC stem cell niche in models of contusive, surgical stab, and slice injury at any anatomical level of the spinal cord [
53]. Common clinical SCI disturbs the niche due to equidistant dorsal and ventral positioning of the central canal [
24]. NSPCs proliferate after injury and interact with inflammatory signals to produce the glial scar border, a chemical/physical barrier which segregates the injury and prevents additional damage [
57]. However, this scar also prevents axonal outgrowth into the site of injury and generation of new cell types within the neural lesion. NSPCs proliferate and differentiate into reactive astrocytes in the injured spinal cord and contribute to the glial scar border. In addition to newly generated progeny, resident astrocytes transition to reactive gliosis state and are recruited to the site of injury, lengthen their processes, and fatten to become the scar border [
58]. A multitude of NSPCs in the spinal cord produce progeny of differing lineages to contribute to the glial scar after SCI and TBI.
Two major cell types have been controversially implicated in the NSPC response to injury and pose high therapeutic potential: NG2+ and ependymal cells. Many published studies are in support of the stem-like character or non-stem-like character of these cells. Both NG2+ cells and ependymal cells have been reported to contribute to the formation of the scar border. More recently, NG2+ cells have been shown to contribute to the generation of neurons in the injured spinal cord [
57,
59]. We will discuss the heterogeneity of NSPCs after injury with a focus on the activity of NG2+ and ependymal cells in
Section 4.
2.3. Adult Retinal Stem Cells
Cells from regions of the adult retina such as the retinal pigment epithelium (RPE) [
60,
61], CE [
62,
63,
64,
65,
66], Müller glia cells [
64,
67,
68,
69], iris pigment epithelium [
70,
71] and optic nerve [
61] show stem cell characteristics to varying degrees in humans and rodents (
Figure 2C). Among them, the CE and Müller glia are identified as two main retinal stem cell sources.
A subpopulation of adult human RPE cells is capable of being activated to become RPE retinal stem cells in vitro and differentiated into multipotent stable RPE or mesenchymal lineages [
60]. The optic nerve lamina region (ONLR) in both humans and mice contains a retinal NPC niche [
61]. Adult NPCs in the ONLR exhibit multipotency and generate two types of glia: astrocytes and oligodendrocytes. These populations contribute to enable glial replacement and remyelination in adulthood [
61]. The derived adult rat iris pigment epithelium (IPE) cells have NSPC properties and can differentiate into rod photoreceptor cells under CRX expression [
71]. NeuroD induces human iris cells into rod photoreceptor cells. Moreover, Yuko et al. observed the combination of CRX, RX and NeuroD induces the generation of photoreceptor cells from the derived human IPE cells [
70].
Non-pigmented CE cells show stem cell markers and actively proliferate after photoreceptor cell degeneration or retinal ganglion cell injury in the mouse model [
62,
72]. In the human CE, non-pigmented CE cells are labeled with stem cell markers, e.g., Sox2, Chx10 and Notch1. Non-pigmented CE cells showed proliferative ability under epidermal growth factor (EGF) induction using explants of the human retina [
63]. CE cells including the pigmented cells and non-pigmented cells from human and mouse express NSPC cell markers and characteristics in vitro [
64]. CE cells can be induced into photoreceptor cells, bipolar cells, retinal ganglion cells and Müller glia cells in the mouse model [
65]. In addition, human CE cells can be induced into many types of retinal cells in vitro [
66].
Müller glial cells are also considered as a primary source of retinal stem cells. Bhatia et al. concluded that retinal Müller glia may perform similar functions ascribed to astrocytes, ependymal cells and oligodendrocytes in other regions of the CNS [
64]. Das et al. also stated that Müller glia are the NSCs of the adult retina [
67]. They demonstrated that rat Müller glia have potential to generate retinal neurons in vitro and in vivo. Moreover, they proved the role of Notch and Wnt pathways in regulating this activity. Similarly, in mouse models, Müller glia can be reprogrammed into photoreceptors and retinal ganglion cells under certain culture conditions [
68]. In adult human eyes, no evidence has been found to suggest that Müller glia possess the retinal neuronal regeneration ability in vivo. However, in vitro, these progenitor-type glia can be induced to proliferate and differentiate into retinal neurons and RPE cells [
69]. Human Müller glia-derived stem cells can be differentiated toward the fate of retinal ganglion cell (RGC) precursors using FGF-2 and Notch inhibition [
69]. In summary, Müller glia-derived stem cells can function as NSCs and serve as a potential target of therapy for retinal degenerative disease.
Common retinal diseases/injuries such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD) cause the photoreceptor cell loss and damaged RPE. However, no enhanced differentiation or proliferation was observed after injury [
65]. Damaged cells release growth factors and cytokines which cause the Müller glia cell to differentiate, proliferate and express progenitor cell markers [
73]. The ability of these proliferating Müller cells to regenerate new neurons and repair the injured retina appears to be extremely limited. Regardless, the multipotent stem cells may generate more functional photoreceptor cells and help with the recovery of vision loss in the RP and AMD via transplantation method [
74].
2.4. Heterogeneity between CNS Niches
The perivascular stem cell niche is not technically a NSPC niche, but it interacts with cell types and influences NSPC behavior in all niches, thus contributing to the diversity of NSPC behavior observed in the mammalian CNS. In particular, the retina contains sources of NSPCs such as Müller glia and CE. Major factors unique to the retinal niche include CRX, RX and NeuroD. Interestingly, the retina does not contain ependymal cells, a major controversial stem type cell in the brain and spinal cord. However, NG2+ cells can be found in the retina [
75]. The brain contains NSPC populations such as radial glial-like cells, OPCs, and ependymal cells. However, these populations and their characteristics vary throughout distinct NSPC niches. Major signals unique to the SGZ and SVZ include Shh pathway and transcription factors CREB and Oct4 [
76]. The spinal cord stem cell niche contains both ependymal cells and NG2+ cells. Signals unique to the spinal cord include connexin signaling. The activity and consistency of NG2+ populations vary significantly between the niches of the brain, spinal cord, and retina. Specifically, NG2+ cells in the brain and spinal cord generate oligodendrocyte cell types and consist of glia and pericytes [
77]. However, NG2+ cells in the retina consist of microglia and pericytes [
75]. Ependymal cells also exhibit a variety of diverse behaviors in neurogenic niches of the brain and spinal cord. These controversial stem-like cells will be discussed in the following sections.
Understanding the heterogeneity of these stem cell populations and neurogenic niches is necessary to effectively design therapeutics for SCI, TBI, mechanical/chemical injury, and diseased states such as Glaucoma, Retinitis Pigmentosa, demyelinating diseases, and inflammatory conditions.
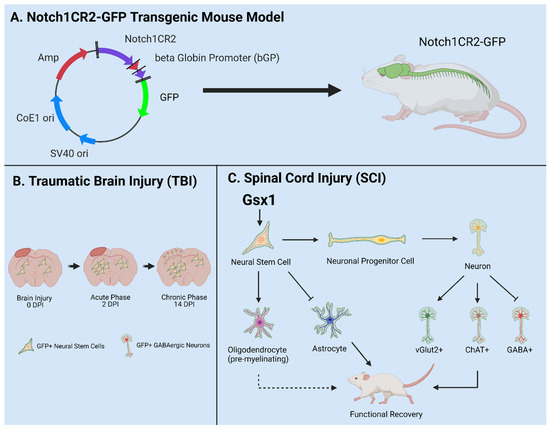
3. Notch1CR2-GFP+ NSPCs in Development and Injury
The canonical Notch signaling pathway is required to regulate the quiescence, proliferation, and differentiation of NSPCs in the CNS [
56,
78,
79,
80]. The Cai lab identified a 399-bp cis-element in the second intron of the Notch1 locus (CR2) [
81]. In the Notch1CR2-GFP transgenic mouse, CR2 directs the reporter GFP expression in the interneuron progenitor cells. The activities of Notch pathway and NSPCs can be traced by the reporter GFP expression (
Figure 3A). The cell fate of GFP tagged interneuron progenitors have been characterized in both normal development and neurological disease/injury conditions, which facilitate the study of the potentials of NSPCs in regenerative medicine [
79,
80,
81,
82]. In these studies, the Cai lab has demonstrated that GFP+ NSPCs preferentially differentiate into interneurons of the brain and spinal cord during embryonic development and in adulthood [
56,
80]. Injury increased the number of GFP+ NSPCs and interneurons at the injury site in a closed head injury model [
80]. These results demonstrate that the endogenous NSPCs in the brain proliferate after injury and differentiate into specific cell fates (
Figure 3B).
Figure 3. Utilities of the Notch1CR2-GFP transgenic mouse line in SCI and TBI models. (
A) Notch1CR2-GFP transgenic mouse model labels NSPCs in the CNS. (
B) Adult NSPCs in the brain proliferate in the acute phase of TBI and differentiate into neurons in the chronic phase of TBI. (
C) In the injured spinal cord, Gsx1 expression promotes adult NSPC proliferation and preferential differentiation into excitatory interneurons and inhibits astrocytes and glial scar formation after injury. Adapted from Tzatzalos, et al., 2012 [
81] (
A), Anderson et al., 2020 [
80] (
B) and Patel et al., 2021 [
79] (
C).
In a more recent study, virus-mediated Gsx1 expression in NSPCs displayed an increased rate of cell proliferation with increased number of GFP+ NSPCs. Gsx1 further promoted neuronal differentiation over glial lineage in the injured spinal cord (
Figure 3C). This resulted in an increased number of neurons, reduced reactive astrocytes and glial scar formation, and improved functional recovery [
79]. Genetic manipulation of NSPCs is a primary therapeutic approach in the field of regenerative medicine [
83]. Many conditions are defined by major cell loss and accompanied by decreased neurogenesis, e.g., SCI, TBI, MS, PD. Engineering NSPCs to increase proliferation and differentiation presents a viable option to promote effective regeneration of lost tissue in the CNS [
84]. Gene/cell therapy can be used to express target genes in host cells, e.g., neurons, astrocytes, NSPCs, and oligodendrocytes [
85,
86]. NSPC specificity can be accomplished via choice of promotor, enhancer, and viral serotype. Common promoters target NSPCs including Nestin, Notch1, NG2, and Sox2. In recent years, forced expression of neurogenic genes (e.g., Ascl1, Gsx1, and Sox11) in stem cell populations promotes cell/tissue regeneration [
79,
87,
88]. Many NSPC subpopulations have been identified, but functional and mechanistic understanding is limited [
89]. Transgenic animal models such as the Notch1CR2-GFP allow in vivo investigation of specific NSPC populations and are vital to develop effective therapeutics in the future [
56,
79,
80,
81,
82].