
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Wenbin Lin | + 2059 word(s) | 2059 | 2021-08-30 10:34:46 | | | |
| 2 | Beatrix Zheng | + 467 word(s) | 2526 | 2021-09-01 14:33:15 | | |
Video Upload Options
Microbially induced carbonate precipitation (MICP) has been proposed as a sustainable approach to solve various environmental, structural, geotechnical and architectural issues. In the last decade, a ubiquitous microbial metabolism, nitrate reduction (also known as denitrification) got attention in MICP research due to its unique added benefits such as simultaneous corrosion inhibition in concrete and desaturation of porous media. The latter even upgraded MICP into a more advanced concept called microbially induced desaturation and precipitation (MIDP) which is being investigated for liquefaction mitigation.
1. Introduction
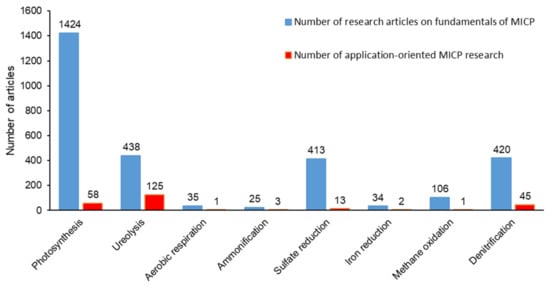
Figure 1 shows that among the metabolic pathways leading to MICP, urea hydrolysis drew significant attention not only in fundamental research describing the MICP mechanism, but it was also the most investigated metabolic pathway to develop new bio-based technologies. The ratio of application-oriented MICP research to fundamental research was considerably low for other metabolic pathways ( Figure 1 ). Therefore, possible advantages of the other metabolic pathways in MICP applications were overlooked.
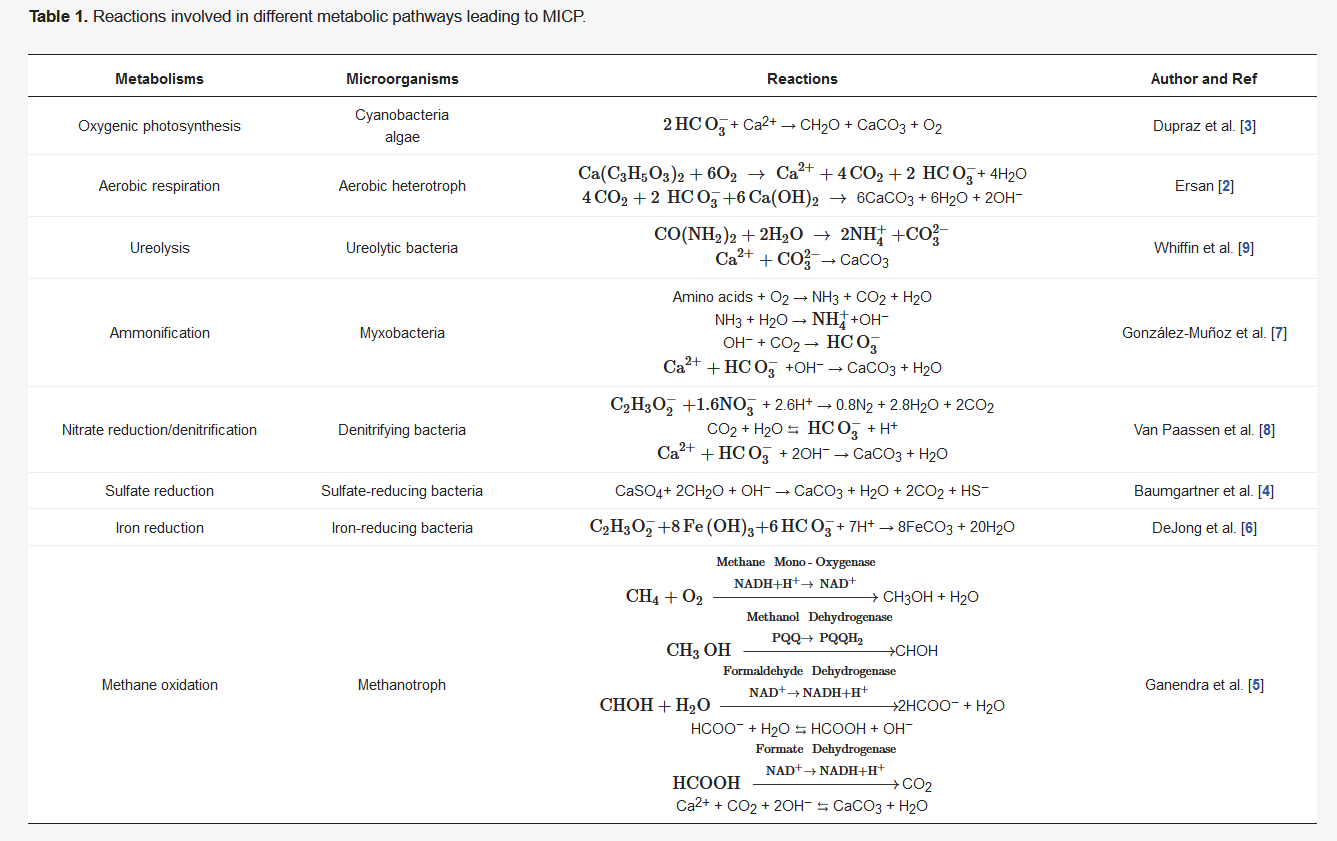
In MICP through denitrification, the denitrifying bacteria are introduced into the soil together with a carbon source as an electron donor, nitrate ( NO 3- ) as a terminal electron acceptor, calcium as a precursor, and the general nutrients for bacterial growth and reproduction. In complete nitrate reduction process, so called denitrification, nitrate is reduced to nitrogen gas and the carbon source is oxidized to carbon dioxide. Since nitrate reduction process inherently removes H + from the environment, the denitrification process leads to the production of alkalinity which further converts part of the produced CO 2 gas into CO 32− ( Table 1 ). Consequently, nitrogen (N 2) and CO 2 gases are generated, while CaCO 3 is precipitated out of the solution in the presence of free Ca 2+ ions ( Table 1 ). If the aforementioned process occurs in a saturated porous environment, biogas production (N 2 and CO 2) and mineral (CaCO 3) precipitation result in partial desaturation of the porous media and thus changes in its hydromechanical behavior. The process of simultaneous desaturation and CaCO 3 precipitation is specific to denitrification pathway and thus MICP through denitrification becomes prominent among the other commonly investigated MICP pathways. In fact, this new process of simultaneous desaturation and CaCO 3 precipitation occurring in porous environments upgraded MICP to a new level named as microbially induced desaturation and precipitation (MIDP) [1][2].
Currently, the potential of the denitrification pathway for MICP applications is overlooked ( Figure 1 ). Both technical studies and review studies focus on MICP via ureolysis and the use of ureolytic pure cultures. Critical reviews on alternative MICP pathways are necessary to create a ground for detailed evaluation of advantages and disadvantages of various MICP pathways in different applications which will pave the way for tailored solutions. Therefore, this review study reveals the potential of denitrification pathway as an alternative to commonly proposed, less sustainable MICP pathways by covering the added benefits (i.e., corrosion inhibition, MIDP) offered by the stepwise occurrence of denitrification based MICP.
This review study consists of four major parts (i) denitrification mechanism and, the activities of denitrifying bacteria related to desaturation and CaCO 3 precipitation, (ii) applications of MICP and MIDP through denitrification, (iii) the challenges involved in the practical applications and (iv) suggestions of future research to overcome those challenges and enable process upscaling and optimization of the novel applications.
2. The Denitrification Mechanism
Organisms that are capable of denitrification, that is, denitrifying bacteria, are widely distributed with a high density in nature. These types of microorganisms are common in a variety of environments, and in agricultural soil they reach a population density of the order of 10 6 microorganisms/g of soil [3]. Typically, denitrifying bacteria constitute about 20% of the total microbial population that can grow under anaerobic conditions and their population corresponds to about 1% to 5% of the overall culturable soil microbiota [4]. More than 50 genera have been identified, including Bacillus , Alcaligenes, Diaphorobacter , Pseudomonas , Spirillum , Paracoccus , Thiobacillus , and Achromobacter [5]. Thus, so far, many studies have used different denitrifying bacteria to link their nitrate reduction activity with either CaCO 3 precipitation or biogas generation or the combination of both (e.g., [6][7][2][3][8]).
Denitrification, or nitrate reduction, is an essential process in the global nitrogen cycle, in which the fixed nitrogen is cycled back into the atmosphere as N 2 gas. Thus, it closes the global nitrogen cycle and keeps the ecosystem in balance. Most denitrifying bacteria undertake denitrification in the presence of organic carbon and nitrate when oxygen is scarce or unavailable [9].
For growth-limited conditions the overall metabolic stoichiometry is equal to the catabolic reaction given in Equation (5).
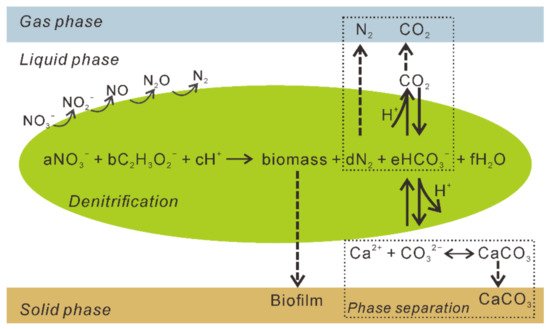
Denitrifying bacteria can use a diverse range of electron donors in natural environments, including pure compounds (methanol, acetone, acetate, glucose, methane, and amino acids), sugars, wastewater, food industry waste, and sludge [5], which favor the generation of dissolved inorganic carbon (DIC). Inorganic carbon dissociates into CO 2, bicarbonate HC O 3- , and CO 32- in aqueous solutions. If a solution with a high pH and total inorganic carbon content contains excess dissolved Ca 2+ , CaCO 3 will precipitate [6], and the system will transfer to the solid phase ( Figure 2 ). The denitrification process can be expressed by the equations using acetate (CH 3COO − ) as the electron donor ( Table 1 ). Furthermore, the production of CaCO 3, the generation of biogases, and the growth of bacteria result in biofilm and biomass accumulation, which favors the formation of a bio-barrier and decreases the permeability of the medium [10]. The full reaction system of the denitrification-based MICP is given in Figure 2 .
3. Potential Applications of Denitrification-Based MICP Biotechnology
| Applications | Process | Microorganisms | Author and Ref | Country/Region |
|---|---|---|---|---|
| Soil reinforcement | MICP | Pseudomonas denitrificans | Karatas [8]; Hamdan [12]; O’Donnell [2]; Hamdan et al. [13] | Netherlands UK USA |
| Castellaniella denitrificans | Van Paassen et al. [6] | |||
| Halomonas halodenitrificans | Martin et al. [14] | |||
| Denitrifiers from natural soil | Pham [1]; Pham et al. [11][15] | |||
| Self-healing concrete | MICP | Nitrate reducing biogranules | Ersan et al. [16][17][18]; Ersan [19] |
Belgium Turkey |
| Diaphorobacter nitroreducens | ||||
| Pseudomonas aeruginosa | ||||
| Sewer corrosion resistant concrete | MICP | Nitrate and sulfate reducing biogranules | Song et al. [20] | Australia |
| Corrosion inhibition of steel in reinforced concrete | MICP | Nitrate reducing biogranules | Ersan et al. [17][21] | Belgium |
| Diaphorobacter nitroreducens | ||||
| Pseudomonas aeruginosa | ||||
| Treatment of industrial wastewater (calcium, nitrate, zinc, nickel, fluoride removal) | MICP | Diaphorobacter nitroreducens | Ersan et al. [16] | Belgium China Japan Spain |
| Pseudomonas aeruginosa | ||||
| Sludge from the biological treatment of leachate | Fernandez-Nava et al. [22] | |||
| Sludge from a Sewage Treatment Plant | ||||
| Acinetobacter sp. | Aoki et al. [23], Fan et al. [24], Liu et al. [25], Su et al. [26] | |||
| Remediation of artwork and historical monuments | MICP | Bacillus cereus | Castanier et al. [27] | France Greece Italy Spain |
| Ranalli et al. [28][29][30]; Bosch-Roig et al. [31] | ||||
| Pseudomonas stutzeri | ||||
| Pseudomonas aeruginosa | Ranalli et al. [28][30] | |||
| Pseudomonas pseudoalcaligenes | Alfano et al. [32] | |||
| Pseudomonas chlororaphis | Daskalakis et al. [33] | |||
| Liquefaction mitigation of soils | MIDP | Paracoccus denitrificans | Rebata-Landa et al. [34] | China USA |
| Acidovorax sp. | He et al. [35][36]; He and Chu [37] | |||
| Mixed culture of bacteria from natural sand | O’Donnell [2]; O’Donnell et al. [38][39] |
4. Challenges in Denitrification-Based MICP/MIDP Biotechnology
Although denitrification-based MICP and MIDP biotechnology have been successfully demonstrated in many laboratory experiments and in several trials in the field, there are several challenges hindering the natural and commercial-scale applications of this technique. Table 3 summarizes the up-to-date challenges in upscaling of the approach as: (i) including the generation of harmful intermediates, (ii) environmental impacts, (iii) monitoring the remediation process, (iv) control of gas generation, (v) the low rate of CaCO 3 precipitation, and (vi) the homogeneous distribution of the treatment impact.
| Challenges in In-Situ Applications | Strategies to Mitigate Those Challenges |
|---|---|
| Generation of harmful intermediates | Avoid by ensuring the completeness of reactions (i.e., proper substrate concentration) Use for other applications (nitrite can be utilized as a commercial anodic rebar corrosion inhibitor) Treat the harmful intermediates on site or collection after the application is done |
| Environmental factors | Stimulation of inactive cells in the field by providing appropriate nutritional conditions Incorporation of a functional isolate or a non-axenic microbial community into the application field to enumerate the number of functional microorganisms combined ureolysis and denitrification process |
| CaCO3 precipitation rate | Proper substrate concentration Applying an optimized substrate regime and residence time Isolate and select more appropriate strains adding iron nanoparticles |
| Controlling of gas generation | Control the generation, distribution, and persistence of the gas applying an optimized substrate regime and residence time proper substrate concentration |
| Obtaining homogeneous treatment | Uniform distribution of microorganisms and solution chemistry Applying an optimized substrate regime and spatial distribution |
| Monitoring | Mathematical model |
The first challenge of this biotechnology is to avoid the accumulation of harmful intermediates by ensuring a complete denitrification reaction. Although the end product of denitrification is harmless nitrogen gas, three toxic intermediates, that is, nitrite, nitric oxide, and nitrous oxide, can accumulate when incomplete microbial nitrate reduction occurs [6]. The only exception to this is that the intermediate nitrite is functional as a commercial anodic rebar corrosion inhibitor in microbial self-healing concrete applications [17][21].
Environmental factors, including pH, temperature, pressure, the concentrations of nutrients (electron donors/acceptors), and the abundance of operative microorganisms in the microbial community vary significantly in the natural soils. In contrast to laboratory experiments, in which most parameters can be controlled, these environmental factors are extremely complex and interfere with each other in natural soils. They affect the activities of the denitrifying bacteria and the generation and transportation of the denitrification reaction products. Thus, another challenge in the application of MICP and MIDP technologies is to design monitoring systems for field applications to quantify the influences of the complexities of these factors in natural soils and subsequent design of suitable microbial cultures for bioaugmentation of the relevant environment.
In terms of CaCO 3 precipitation, denitrification-based MICP has a slower reaction rate than MICP through ureolysis, so it takes more time for the mechanical properties of soil to reach the desired values [6]. Ureolysis-based MICP has been reported to produce 6% CaCO 3 ( w/w ) in a few days [40][41], whereas denitrification-based MICP only generates an average of 1–3% CaCO 3 ( w/w ) within a few weeks to several months [6][2][11]. Although slow precipitation rates seem like a drawback of MICP through denitrification, they enable maintaining microbial activity without occlusion of microbial cells with the precipitated CaCO 3 crystals. Therefore, applying an optimized substrate regime and residence time can make denitrification based MICP more advantageous over ureolysis in long-term. However, there is no valid optimized procedure for field applications of MICP through denitrification, which remains as an obstacle before the transition of the concept into real life examples.
5. Suggestions for Future Work
The findings evaluated in this paper demonstrate that microbial induced desaturation and/or precipitation through denitrification possesses a great potential to solve a wide range of environmental, geotechnical, architectural and structural problems under anoxic conditions in a sustainable, environmentally friendly, and cost-effective manner. Promising MICP-driven applications include microbial self-healing concrete with a corrosion inhibition property, bioremediation of artwork and monuments, treatment of high strength industrial wastewater and soil reinforcement. Most importantly, liquefaction mitigation is a novel and unique MIDP-driven application specific to denitrification pathway.
Along with other microbiological processes, such as urea hydrolysis, aerobic respiration and sulfate reduction, denitrification-based MICP has initiated a revolution in various civil engineering applications. However, there are still many challenges that are needed to be addressed before this biotechnology can be commercialized.
Further exploratory studies should be conducted to enhance the efficacy of the in-situ biogas and biomineral production at the microbial level and at the field scale ( Table 3 ). Ureolytic bacteria ( Sporosarcina pasteurii ) is recognized as the most suitable microbe for MICP via ureolysis, but no specific denitrifying bacteria is widely accepted to be the most useful for denitrification-based MICP. Therefore, initial efforts should be made to isolate and select a model organism or develop a microbiome with superior carbonate precipitation yield (i.e., denitrification abilities). Furthermore, more tightly controlled experiments focusing on the key factors would be useful for understanding, optimizing, and successfully developing denitrification technologies. One key factor is the substrate concentration, namely, of the electron acceptor (nitrate) and the organic carbon donor (e.g., formate, acetate, methanol, and ethanol), which affect the conversion rate of the denitrification reactions and the production of the intermediates. Other key factors include, but are not limited to, temperature, pH, pressure, grain size distribution, and salinity. Considering the complexity of natural soils and groundwater, a novel method, which may be helpful in future research, is a combination of metabolic pathways in a way that one process dominates the conditions in which nitrate and carbon source are present under anoxic conditions, and the latter process dominates when the environment is oxic. In addition, special efforts should be made to evaluate the long-term efficacy of denitrification-based MICP and MIDP in different applications. Currently, many studies are working on adding some environmentally friendly additives like nanoparticles and mainly iron nanoparticles for the removal of wastewater contamination [42][43][44]. The results are proving that these nanoparticles have a positive effect on the anaerobic digestion process and the bacterial growth [42], which in turn could have a positive effect on the denitrification process, thereby, efforts could be made to test the efficiency of MICP as well as MIDP by adding iron nanoparticles to the reaction systems. Finally, although a biogeochemical model (no-flow condition), has been developed to simulate the process of MIDP via denitrification by O’Donnell et al. [45], which is an upgraded version of the model created by O’Donnell et al. [2], mathematical models should be further studied to account for continuous flow.
The successful development and implementation of the denitrification-based MICP and MIDP processes described in this paper could also be used for other applications. Owing to their abundance in subsurface soils and groundwater, denitrifying bacteria and denitrification based MICP can be exploited for co-precipitation of minerals and metals enabling in-situ remediation of metal contaminants and radionuclides in anoxic conditions.
References
- Pham, V.P. Bio-Based Ground Improvement through Microbial Induced Desaturation and Precipitation (midp). Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2017.
- O’Donnell, S.T.; Hamdan, N.; Rittmann, B.E.; Kavazanjian, E., Jr. A stoichiometric model for biogeotechnical soil improvement. In Geo-Chicago 2016; American Society of Civil Engineers: Reston, VA, USA, 2016.
- Gamble, T.N.; Betlach, M.R.; Tiedje, J.M. Numerically dominant denitrifying bacteria from world soils. Appl. Environ. Microbiol. 1977, 33, 926–939.
- Tiedje, J.M.; Sexstone, A.J.; Myrold, D.D.; Robinson, J.A. Denitrification: Ecological niches, competition and survival. Anton. Leeuw. Int. J. G. 1982, 48, 569–583.
- Zumft, W.G. The denitrifying prokaryotes. In The Prokaryotes; Balows, A., Ed.; Springer: New York, NY, USA, 1992.
- Van Paassen, L.A.; Daza, C.M.; Staal, M.; Sorokin, D.Y.; van der Zon, W.; van Loosdrecht, M.C.M. Potential soil reinforcement by biological denitrification. Ecol. Eng. 2010, 36, 168–175.
- Zhu, T.; Dittrich, M. Carbonate precipitation through microbial activities in natural environment, and their potential in biotechnology: A review. Front. Bioeng. Biotechnol. 2016, 4, 4.
- Karatas, I. Microbiological Improvement of the Physical Properties of Soils. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2008.
- Ersan, Y.C. Overlooked strategies in exploitation of microorganisms in the field of building materials. In Ecological Wisdom Inspired Restoration Engineering; Springer: Berlin/Heidelberg, Germany, 2019; pp. 19–45.
- Gerlach, R.; Cunningham, A. Influence of microbial biofilms on reactive transport in porous media. AIP Conf. Proc. 2012, 1453, 276–283.
- Pham, V.P.; Nakano, A.; van der Star, W.R.L.; Heimovaara, T.J.; van Paassen, L.A. Applying MICP by denitrification in soils: A process analysis. Environ. Geotech. 2018, 5, 79–93.
- Hamdan, N. Carbonate Mineral Precipitation for Soil Improvement through Microbial Denitrification. Master’s Thesis, Arizona State University, Tempe, AZ, USA, 2013.
- Hamdan, N.; Kavazanjian, E.; Rittmann, B.E.; Karatas, I. Carbonate mineral precipitation for soil improvement through microbial denitrification. Geomicrobio. J. 2017, 34, 139–146.
- Martin, D.; Dodds, K.; Butler, I.B.; Ngwenya, B.T. Carbonate precipitation under pressure for bioengineering in the anaerobic subsurface via denitrification. Environ. Sci. Technol. 2013, 47, 8692–8699.
- Pham, V.P.; van Paassen, L.A.; van der Star, W.R.L.; Heimovaara, T.J. Evaluating strategies to improve process efficiency of denitrification-based micp. J. Geotech. Geoenviron. Eng. 2018, 144.
- Ersan, Y.Ç.; Belie, N.d.; Boon, N. Microbially induced CaCO3 precipitation through denitrification: An optimization study in minimal nutrient environment. Biochem. Eng. J. 2015, 101, 108–118.
- Ersan, Y.C.; Van Tittelboom, K.; Boon, N.; De Belie, N. Nitrite producing bacteria inhibit reinforcement bar corrosion in cementitious materials. Sci. Rep. 2018, 8, 14092.
- Ersan, Y.C.; Palin, D.; Yengec, T.S.B.; Tasdemir, K.; Jonkers, H.M.; Boon, N.; De Belie, N. Volume fraction, thickness, and permeability of the sealing layer in microbial self-healing concrete containing biogranules. Front. Built Environ. 2018, 4.
- Ersan, Y.Ç. Self-healing performance of biogranule containing microbial self-healing concrete under intermittent wet/dry cycles. J. Polytech. 2021, 24, 323–332.
- Song, Y.; Chetty, K.; Garbe, U.; Wei, J.; Bu, H.; O’moore, L.; Li, X.; Yuan, Z.; McCarthy, T.; Jiang, G. A novel granular sludge-based and highly corrosion-resistant bio-concrete in sewers. Sci. Total Environ. 2021, 791, 148270.
- Ersan, Y.Ç.; Verbruggen, H.; De Graeve, I.; Verstraete, W.; De Belie, N.; Boon, N. Nitrate reducing CaCO3 precipitating bacteria survive in mortar and inhibit steel corrosion. Cem. Concr. Res. 2016, 83, 19–30.
- Fernandez-Nava, Y.; Maranon, E.; Soons, J.; Castrillon, L. Denitrification of wastewater containing high nitrate and calcium concentrations. Bioresour. Technol. 2008, 99, 7976–7981.
- Aoki, M.; Noma, T.; Araki, N.; Yamaguchi, T.; Masataka, K.; Hayashi, K. Isolation of Acinetobacter sp. Strain WKDN with capacity of aerobic denitrification and CaCO3 biomineralization and its potential application in dissolved Zn removal. Desalin. Water Treat. 2020, 194, 172–179.
- Fan, Y.; Su, J.; Wang, Z.; Deng, L.; Zhang, H. Impact of C/N ratio on the fate of simultaneous Ca2+ precipitation, F− removal, and denitrification in quartz sand biofilm reactor. Chemosphere 2021, 273, 129667.
- Liu, J.; Amhaj, A.; Su, J.; Wu, Z.; Zhang, R.; Xiong, R. Simultaneous removal of calcium, fluoride, nickel and nitrate using microbial induced calcium precipitation in a biological immobilization reactor. J. Hazard. Mater. 2021, 416, 125776.
- Su, J.; Wu, Z.; Huang, T.; Zhang, H.; Li, J. A new technology for simultaneous calcium–nitrate and fluoride removal in the biofilm reactor. J. Hazard. Mater. 2020, 399, 122846.
- Castanier, S.; Le Métayer-Levrel, G.; Orial, G.; Loubière, J.-F.; Perthuisot, J.-P. Bacterial carbonatogenesis and applications to preservation and restoration of historic property. In Of Microbes and Art; Ciferri, O., Mastromei, G., Tiano, P., Eds.; Springer: Boston, MA, USA, 2000; pp. 203–218.
- Ranalli, G.; Chiavarini, M.; Guidetti, V.; Marsala, F.; Matteini, M.; Zanardini, E.; Sorlini, C. In The use of microorganisms for the removal of nitrates and organic substances on artistic stoneworks. In Proceedings of the 8th International Congress on Deterioration and Conservation of Stone, Berlin, Germany, 30 September–4 October 1996; pp. 1415–1420.
- Ranalli, G.; Chiavarini, M.; Tosini, I.; Zanardini, E.; Sorlini, C. Bioremediation on cultural heritage: Removal of sulphates, nitrates and organic substances. In Of Microbes and Art: The Role of Microbial Communities in the Degradation and Protection of Cul-tural Heritage; Ciferri, O., Tiano, P., Mastromei, G., Eds.; Kluwer Academic-Plenum: New York, NY, USA, 2000; pp. 231–245.
- Ranalli, G.; Alfano, G.; Belli, C.; Lustrato, G.; Colombini, M.P.; Bonaduce, I.; Zanardini, E.; Abbruscato, P.; Cappitelli, F.; Sorlini, C. Biotechnology applied to cultural heritage: Biorestoration of frescoes using viable bacterial cells and enzymes. J. Appl. Microbiol. 2005, 98, 73–83.
- Roig, P.B.; Regidor Ros, J.L.; Estellés, R.M. Biocleaning of nitrate alterations on wall paintings by pseudomonas stutzeri. Int. Biodeterior. Biodegrad. 2013, 84, 266–274.
- Alfano, G.; Lustrato, G.; Belli, C.; Zanardini, E.; Cappitelli, F.; Mello, E.; Sorlini, C.; Ranalli, G. The bioremoval of nitrate and sulfate alterations on artistic stonework: The case-study of matera cathedral after six years from the treatment. Int. Biodeterior. Biodegrad. 2011, 65, 1004–1011.
- Daskalakis, M.I.; Magoulas, A.; Kotoulas, G.; Catsikis, I.; Bakolas, A.; Karageorgis, A.P.; Mavridou, A.; Doulia, D.; Rigas, F. Pseudomonas, pantoea and cupriavidus isolates induce calcium carbonate precipitation for biorestoration of ornamental stone. J. Appl. Microbiol. 2013, 115, 409–423.
- Rebata-Landa, V.; Santamarina, J.C. Mechanical effects of biogenic nitrogen gas bubbles in soils. J. Geotech. Geoenviron. Eng. 2012, 138, 128–137.
- He, J.; Chu, J.; Ivanov, V. Mitigation of liquefaction of saturated sand using biogas. Géotechnique 2013, 63, 267–275.
- He, J.; Chu, J.; Wu, S.; Peng, J. Mitigation of soil liquefaction using microbially induced desaturation. J. Zhejiang Univ. Sci. A 2016, 17, 577–588.
- He, J.; Chu, J. Undrained responses of microbially desaturated sand under monotonic loading. J. Geotech. Geoenviron. Eng. 2014, 140, 04014003.
- O’Donnell, S.T.; Rittmann, B.E.; Kavazanjian, E. MIDP: Liquefaction mitigation via microbial denitrification as a two-stage process. I: Desaturation. J. Geotech. Geoenviron. Eng. 2017, 143, 04017094.
- O’Donnell, S.T.; Kavazanjian, E.; Rittmann, B.E. MIDP: Liquefaction mitigation via microbial denitrification as a two-stage process. II: MICP. J. Geotech. Geoenviron. Eng. 2017, 143, 04017095.
- Chu, J.; Stabnikov, V.; Ivanov, V. Microbially induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol. J. 2012, 29, 544–549.
- Yang, Z.; Cheng, X. A performance study of high-strength microbial mortar produced by low pressure grouting for the reinforcement of deteriorated masonry structures. Constr. Build. Mater. 2013, 41, 505–515.
- Amen, T.W.M.; Eljamal, O.; Khalil, A.M.E.; Matsunaga, N. Biochemical methane potential enhancement of domestic sludge digestion by adding pristine iron nanoparticles and iron nanoparticles coated zeolite compositions. J. Environ. Chem. Eng. 2017, 5, 5002–5013.
- Eljamal, O.; Shubair, T.; Tahara, A.; Sugihara, Y.; Matsunaga, N. Iron based nanoparticles-zeolite composites for the removal of cesium from aqueous solutions. J. Mol. Liq. 2019, 277, 613–623.
- Eljamal, O.; Thompson, I.P.; Maamoun, I.; Shubair, T.; Eljamal, K.; Lueangwattanapong, K.; Sugihara, Y. Investigating the design parameters for a permeable reactive barrier consisting of nanoscale zero-valent iron and bimetallic iron/copper for phosphate removal. J. Mol. Liq. 2020, 299.
- O’Donnell, S.T.; Hall, C.A.; Kavazanjian, E.; Rittmann, B.E. Biogeochemical model for soil improvement by denitrification. J. Geotech. Geoenviron. Eng. 2019, 145, 04019091.




