1.1. Ovarian Cancer Is a Serious Problem in Gynaecological Oncology
Ovarian cancer (OC) is a major life-threatening problem in the field of gynecological oncology. Globally, it stands as the foremost cause of death in women accounting for approximately 239,000 newly diagnosed cases and over 150,000 deaths per year
[1]. Recent reports in the United States estimated 22,240 new cases with ovarian cancer and 14,070 deaths owing to the disease
[2]. Notably, the highest incidence and mortality rates have been linked to Eastern and Central Europe
[1]. Therefore, great efforts are required to improve the therapeutic outcomes for diseased women. Additionally, thorough understanding of the molecular mechanisms and key elements contributing the disease is substantial in combating ovarian cancer
[3].
Indeed, ovarian tumors can arise from three ovarian cell types namely, surface epithelium, sex cord stromal cells and germ cells
[4]. Epithelial tumors account for 90% of ovarian malignancies while non-epithelial tumors including sex cord stromal and germ cell tumors represent 10% of the diagnosed cases. Epithelial ovarian cancer (EOC) are histologically categorized into serous, endometrioid, clear cell and mucinous carcinomas; the serous type itself is subclassified into high grade serous carcinoma (HGSC), low grade serous carcinoma (LGSC) and serous tubal intraepithelial carcinoma (STIC)
[3] (a brief classification of OC histology is illustrated in
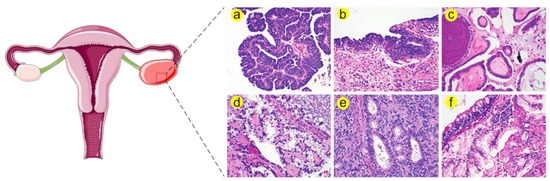
Figure 1).
Figure 1. Histological stratification of ovarian cancer
a. (
a) High grade serous carcinoma (HGSC) is distinguished by increased nuclear atypia, high nuclear-to-cytoplasmic ratio and abundant mitosis. (
b) Serous tubal intraepithelial carcinoma (STIC) resembles HGSC in many morphological aspects such as severe atypia, defective cellular polarity and mitoses. Therefore, STIC is believed to be a precursor of HGSC. (
c) Low grade serous carcinoma (LGSC) is characterized by increased papillae, mild nuclear atypia and low nuclear-to-cytoplasmic ratio. (
d) Clear cell carcinoma exhibits large tumor cell sizes and frequent clearing of the cytoplasm together with stromal hyalinization. (
e) Endometrioid adenocarcinoma can be differentiated by gland formation that recapitulates endometrial glands. This type is further categorized according to cellular architecture and nuclear atypia. (
f) Mucinous adenocarcinoma is characterized by increased cellular mucin and formation of goblet cells.
a Histological images are adapted from Nature Reviews Disease Primers
[3].
OC is often diagnosed at relatively old age of life, with a median age of 63 years in the US women population (
https://seer.cancer.gov/statfacts/html/ovary.html). In addition, current data show that 59% of the cases have metastatic forms of the disease, while only 15% are diagnosed at the local stage. Of particular importance, early detection of ovarian malignancies is associated with higher cure rates, with a five-year survival exceeding 92% for localized ovarian cancer, whereas late stage diagnosis of the metastatic disease lowers cure rates to 20%
[5][6].
The standard treatment protocol for human ovarian cancer includes maximal cytoreductive surgical debulking followed by the platinum-based chemotherapy. Concurrent with surgical cytoreduction, staging of the disease remains important
[7][8]. Current therapeutic regimens to the first-line treatment which involve bevacizumab and paclitaxel have shown improved survival among patients with OC
[7][9]. Unfortunately, despite initial remarkable response to chemotherapy, the majority of advanced OC cases recur after primary drug treatment with fatal outcome
[10]. According to Ovarian Cancer Research Alliance (OCRA), current reports show that patients diagnosed at stages I and II have a recurrence chance of 10% and 30%, respectively, whereas the chance of recurrence in those of stage III and IV ranges between 70% and 95% (
https://ocrahope.org/patients/about-ovarian-cancer/recurrence/).
Multiple treatment approaches have been adapted for management of relapsed ovarian cancer. For instance, agents targeting angiogenesis include Bevacizumab, a monoclonal antibody that binds human vascular endothelial growth factor (VEGF) and inhibits its activity. Cediranib is an oral VEGF receptor and c-KIT inhibitor that displays antitumor activity in relapsed EOC in phase I/II studies. Trebananib is a peptide that suppresses angiogenesis by inhibiting angiopoietin-1 and -2. Moreover, other treatment strategies involve PARP inhibitors (PARPi) which render PARP enzymes no more capable of performing DNA repair processes and ultimately leading to synthetic lethality
[11]. These PARP inhibitors include olaparib (AZD2281), niraparib (MK4827), rucaparib (CO338, AGO14699, and PF01367338), veliparib (ABT-888) and talazoparib (BMN 673)
[11]. However, it should be noted that that PARP inhibitors have mostly been successful and are approved for patients with platinum sensitive ovarian carcinoma rather than resistant disease
[12]. Furthermore, recent reports show that sorafenib, a pleiotropic tyrosine kinase inhibitor that inhibits pathways mediated by angiogenic and growth stimulating factors, could significantly increase the progression-free survival in platinum-resistant OC patients compared to placebo
[13].
A common significant problem in the treatment of women with advanced OC is therapeutic resistance. To enhance therapeutic outcomes in recurrent OC, it would be beneficial to generate drugs or therapeutic combinations that would overwhelm the resistance and increase the response to the main therapy. Therefore, targeting the molecular mechanisms underlying such drug resistance in OC is highly recommended and may allow for optimum treatment
[14].
Recent research highlights the implication of heat shock proteins (HSPs) in malignant processes and their association with drug resistance in cancer. HSPs are known as stress inducible molecules that are highly conserved across prokaryotic and eukaryotic species ranging from bacteria to human
[15][16]. These molecules are well known for their molecular chaperone activities including protein folding, anti-aggregation of proteins and cellular protein trafficking
[17][18][19][20]. Expression of HSPs is either constitutive or induced by various physiological, environmental and pathological factors including thermal stress, hypoxia, inflammation, toxic agents, heavy metals and cancer
[21]. In response to variant stresses, members of HSPs are mostly regulated by a physiological process collectively known as “heat shock response (HSR)”, which involves heat shock factor 1 (HSF1) as a key player
[22][23][24].
1.2. Heat Shock Proteins (HSPs) Are Multifamily Chaperones Implicated in Several Malignancies
HSPs have been traditionally grouped into six main families according to their molecular weight
[25][26]. These include small HSPs (sHSPs), HSP40 (DNAJ), chaperonin or HSP60, HSP70, HSP90 and large HSPs (HSP110 and glucose-regulated protein 170, GRP170)
[27]. Due to growing numbers of HSP members and their diverse and/or overlapping structures and functions, Kampinga et al. have set a new classification of HSP families which includes alphabet letters A, B, C, D, E, H rather than the traditional molecular weight system
[27], see also
Table 1.
Table 1. General overview of human heat shock proteins (HSP) families and common members
[27].
| HSP Family |
Recent Name |
Number of Members |
Common Members and Their Alternative Names |
| HSP110 |
HSPH |
4 |
HSPH1 (HSP105) |
| HSPH2 (HSP110, HSPA4 and APG-2) |
| HSPH4 (HYOU1/Grp170, ORP150 and HSP12A) |
| HSP90 |
HSPC |
5 |
HSPC2 (HSP90α, HSP90AA2, HSPCA and HSPCAL3) |
| HSPC3 (HSP90β, HSP90AB1, HSPC2, HSPCB, D6S182, HSP90B, FLJ26984) |
| HSPC4 (GRP94, HSP90B1, GP96, ECGP, TRA1, endoplasmin) |
| HSPC5 (TRAP1, HSP75, HSP90L) |
| HSP70 |
HSPA |
13 |
HSPA1A (HSP70-1, HSP72 and HSPA1) |
| HSPA1B (HSP70-2) |
| HSPA5 (BIP, GRP78 and MIF2) |
| HSPA6 (Heat shock 70kD protein 6 and HSP70B′) |
| HSPA8 (HSC70, HSC71, HSP71 and HSP73) |
| HSPA9 (GRP75, HSPA9B, MOT, MOT2, PBP74 and mot-2) |
| Chaperonins |
HSPD and HSPE |
14 |
HSPD1 (HSP60 and GroEL) |
| HSPE1 (HSP10, chaperonin 10 and GroES) |
| HSP40 |
DNAJ |
50 |
DNAJA1 (DJ-2, DjA1, HDJ2, HSDJ, HSJ2, HSPF4 and hDJ-2) |
| DNAJB1 (HSPF1 and HSP40) |
| DNAJC1 (MTJ1, ERdj1, ERj1p and Dnajl1) |
| sHSPs |
HSPB |
11 |
HSPB1 (HSP27, HSP28, HSP25, HS.76067, DKFZp586P1322, CMT2F and HMN2B) |
| HSPB4 (CRYAA, crystallin alpha A and CRYA1) |
| HSPB5 (CRYAB, crystallin alpha B and CRYA2) |
High expression levels of HSPs have been reported in many cancers, including breast, head and neck, gallbladder, colorectal, skin, liver, colon, renal, prostate as well as ovarian cancer
[21][28]. Of particular interest, HSPs play dual complex role in apoptosis via promoting or counteracting cell death. For instance, HSPs have been shown to activate apoptotic mediators such as pro-caspase 3
[29][30] and conversely, they bind and inhibit several molecules at different levels in the apoptotic pathway
[31]. Among the anti-apoptotic events is the blockade of cytochrome C and SMAC Diablo release from the mitochondria by HSP27 besides antagonizing caspase 3 and 9
[32][33][34]. HSP27 can also suppress other apoptotic death receptor pathways, including TNFα, Fas and TRAIL
[35]. Similarly, HSP70 inhibits apoptosis by interfering with the c-jun kinase pathway and preventing cytochrome C release from mitochondria
[34][36] (See
Figure 2). Moreover, HSPs have been found to chaperone several oncogenes including mutant P53 and prevent its degradation, thus evading the classical apoptotic pathway and resulting in cancer cell survival
[37][38][39]. Furthermore, increased levels of certain HSPs conferred drug resistance in many cancers including prostate
[40][41], liver
[42], lung
[43], colon
[44], head and neck
[45] and ovarian cancer
[6].
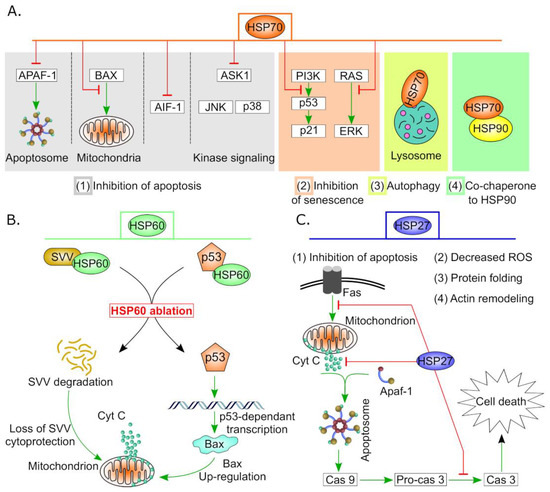
Figure 2. Anti-apoptotic and cell survival activities of some HSPs in cancer. (
A) Variant roles of HSP70 in carcinogenesis. High expression of HSP70 in tumor cells suppresses apoptosis by (1) hindering APAF1 recruitment to apoptosome, interfering with BAX translocation to mitochondria, downregulation of AIF1 and other stress-related kinases. Additionally, (2) HSP70 regulates both p53-dependent and -independent senescence pathways, (3) supports autophagy by stabilization of lysosomal membrane and finally (4) it forms complex with HSP90 which is essential for efficient functionality. (
B) HSP60 controls apoptosis by stabilizing mitochondrial survivin (SVV) and hindering P53 pro-apoptotic actions. HSP60 ablation results in degradation of SVV and activation of the mitochondrial apoptotic pathway. In addition, silencing of HSP60 increases P53 stability and subsequently, triggers p53-dependent transcription of apoptotic proteins such as BAX which promotes cell death
[46]. (
C) HSP27 performs multiple functions in cancer including protein folding, actin remodeling, minimizing oxidative stress and inhibition of apoptosis. Sample anti-apoptotic events of HSP27 are indicated by red blunt arrows.
Having anti-apoptotic properties and drug resistance characteristics, overexpression of HSPs in variant malignancies has been correlated to cell survival, tumor progression and metastasis as well as poor prognosis
[47][48]. Therefore, most studies consider HSPs not only as diagnostic/prognostic markers but also as ideal therapeutic targets for cancer therapy
[21][49][50]By virtue of the increasing interest in HSPs as a potential drug target for cancer treatment among gynaecologists, we focused on the function of HSPs in ovarian cancer and highlighted their roles in carcinogenesis and therapeutic resistance. We will start by briefly discussing the general function of HSPs in ovaries in both physiological and pathological conditions.
2. Biological Functions of HSPs in Healthy and Diseased Ovaries
Previous in vitro and in vivo studies have demonstrated the importance of HSPs in the development of normal ovaries, growth of ovarian follicles and their resistance to stress conditions. In swine, thermal stress and serum deprivation induced high transcription levels of HSP70.2, HSP72 and HSP105/110 in both granulosa cells and ovarian follicles. Moreover, the expression levels of the respective HSPs was reduced following hormonal treatment highlighting the regulation of stress related changes by hormones in ovarian tissues
[51]. In rat, treatment of immature granulosa cells with follicle stimulating hormone (FSH) resulted in cell rounding concurrent with activation of p38 mitogen activated protein kinase (MAPK) pathway and HSP27 phosphorylation
[52].
Notably, chaperones including HSP90 and HSP70 play a key role in regulation of the function of steroid hormones by modulating their receptor activity such as estrogen receptor (ER), progesterone receptor (PR) and androgen receptor (AR)
[40][53][54][55]. Expression of HSP70 has been also described in normal ovaries where a chaperone complex including HSP70 and HSP90 is suggested to bind steroid receptors and regulate their function
[56]. For that, two hypotheses have been suggested to modulate steroid receptor function; the first hypothesizes the association of a chaperone heterocomplex including HSP90, HSP70, HSP40, p23, etc. to the unbound receptors and keep them in an inactive state
[57]. Binding of the ligand to steroid receptor results in dissociation of the complex and release of HSP70 and HSP90 chaperones. Since proliferation of the growing follicles in proestrus occurs because of sex steroids, HSP70 has been thought as inhibitor of steroidal effects
[56]. Additionally, elevated HSP70 levels have been shown to repress steroid biosynthesis and secretion
[58][59]. Heat shocked rat luteal cells lost their ability to synthesize or secrete LH-sensitive progesterone and 20α-dihydroprogesterone after treatment with 8-bromo-cAMP and forskolin
[58]. Conversely, the other hypothesis postulates that HSP90/HSP70 machinery is essential for maintaining the appropriate conformation required for hormone-binding activity of the receptor
[60]. Therefore, it is collectively apparent that HSPs modulate ovarian physiology via controlling sex steroid receptors functionality as well as regulating apoptotic mechanisms
[6][61][62].
On the other hand, HSPs have been associated with cystic ovarian disease (COD). Expression profiles of HSP27, HSP70, HSP60 and HSP90 revealed abundant levels of HSP27 in primary, secondary, tertiary and cystic follicles and diminished in atretic follicles
[63]. Furthermore, overexpression of HSP70, HSP60 and HSP90 has been noticed in tertiary and atretic follicles. As a conclusion, the aberrant expression of HSPs in ovarian cysts is suggested to counteract apoptosis and delay regression of cystic follicles
[63][64]. Interestingly, the herbicide atrazine, which dysregulates estrous cycle in rats and impairs folliculogenesis, has been shown to reduce expression of HSP90 and increase follicular atresia
[65]. Additionally, in rats, ACTH or cold stress-induced polycystic ovary syndrome (PCOS) reveal a significant elevation of the expression of HSP90 and abnormal ovarian morphology compared to the control group
[66]. Furthermore, proteomic studies in women with PCOS have demonstrated two-fold increase in HSP90B1 levels suggesting a role in promoting cell survival and suppression of apoptosis
[67].