Ovarian cancer is a serious cause of death in gynecological oncology. Delayed diagnosis and poor survival rates associated with late stages of the disease are major obstacles against treatment efforts. Heat shock proteins (HSPs) are stress responsive molecules known to be crucial in many cancer types including ovarian cancer. Clusterin (CLU), a unique chaperone protein with analogous oncogenic criteria to HSPs, has also been proven to confer resistance to anti-cancer drugs. Indeed, these chaperone molecules have been implicated in diagnosis, prognosis, metastasis and aggressiveness of various cancers. However, relative to other cancers, there is limited body of knowledge about the molecular roles of these chaperones in ovarian cancer. Here, we shed light on the diverse roles of HSPs as well as related chaperone proteins like CLU in the pathogenesis of ovarian cancer and elucidate their potential as effective drug targets.
- ovarian cancer
- heat shock proteins (HSPs)
- clusterin
- therapeutic resistance
- HSP inhibitors
- ovarian cancer treatment
1. Introduction
1.1. Ovarian Cancer Is a Serious Problem in Gynaecological Oncology
1.2. Heat Shock Proteins (HSPs) Are Multifamily Chaperones Implicated in Several Malignancies
| HSP Family | Recent Name | Number of Members | Common Members and Their Alternative Names |
|---|---|---|---|
| HSP110 | HSPH | 4 | HSPH1 (HSP105) |
| HSPH2 (HSP110, HSPA4 and APG-2) | |||
| HSPH4 (HYOU1/Grp170, ORP150 and HSP12A) | |||
| HSP90 | HSPC | 5 | HSPC2 (HSP90α, HSP90AA2, HSPCA and HSPCAL3) |
| HSPC3 (HSP90β, HSP90AB1, HSPC2, HSPCB, D6S182, HSP90B, FLJ26984) | |||
| HSPC4 (GRP94, HSP90B1, GP96, ECGP, TRA1, endoplasmin) | |||
| HSPC5 (TRAP1, HSP75, HSP90L) | |||
| HSP70 | HSPA | 13 | HSPA1A (HSP70-1, HSP72 and HSPA1) |
| HSPA1B (HSP70-2) | |||
| HSPA5 (BIP, GRP78 and MIF2) | |||
| HSPA6 (Heat shock 70kD protein 6 and HSP70B′) | |||
| HSPA8 (HSC70, HSC71, HSP71 and HSP73) | |||
| HSPA9 (GRP75, HSPA9B, MOT, MOT2, PBP74 and mot-2) | |||
| Chaperonins | HSPD and HSPE | 14 | HSPD1 (HSP60 and GroEL) |
| HSPE1 (HSP10, chaperonin 10 and GroES) | |||
| HSP40 | DNAJ | 50 | DNAJA1 (DJ-2, DjA1, HDJ2, HSDJ, HSJ2, HSPF4 and hDJ-2) |
| DNAJB1 (HSPF1 and HSP40) | |||
| DNAJC1 (MTJ1, ERdj1, ERj1p and Dnajl1) | |||
| sHSPs | HSPB | 11 | HSPB1 (HSP27, HSP28, HSP25, HS.76067, DKFZp586P1322, CMT2F and HMN2B) |
| HSPB4 (CRYAA, crystallin alpha A and CRYA1) | |||
| HSPB5 (CRYAB, crystallin alpha B and CRYA2) |
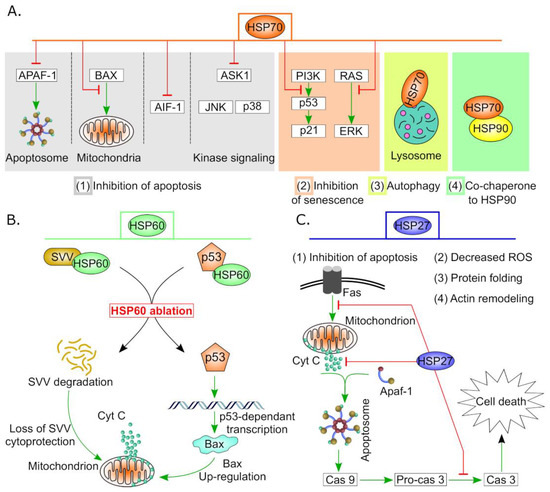
2. Biological Functions of HSPs in Healthy and Diseased Ovaries
This entry is adapted from the peer-reviewed paper 10.3390/cancers11091389
References
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Boil. Med. 2017, 14, 9–32.
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296.
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Prim. 2016, 2, 16061.
- Romero, I.; Bast, R.C. Minireview: Human Ovarian Cancer: Biology, Current Management, and Paths to Personalizing Therapy. Endocrinology 2012, 153, 1593–1602.
- Bast, R.C.; Urban, N.; Shridhar, V.; Smith, D.; Zhang, Z.; Skates, S.; Lu, K.; Liu, J.; Fishman, D.; Mills, G. Early Detection of Ovarian Cancer: Promise and Reality. Cancer Treat. Res. 2002, 107, 61–97.
- Stope, M.; Koensgen, D.; Burchardt, M.; Concin, N.; Zygmunt, M.; Mustea, A.; Information, P.E.K.F.C. Jump in the fire—Heat shock proteins and their impact on ovarian cancer therapy. Crit. Rev. Oncol. 2016, 97, 152–156.
- Cortez, A.J.; Tudrej, P.; Kujawa, K.A.; Lisowska, K.M. Advances in ovarian cancer therapy. Cancer Chemother. Pharmacol. 2018, 81, 17–38.
- Monk, B.J.; Anastasia, P.J. Ovarian Cancer: Current Treatment and Patient Management. J. Adv. Pract. Oncol. 2016, 7, 271–273.
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. ICON7 Investigators A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011, 365, 2484–2496.
- Ozols, R.F.; Bundy, B.N.; Greer, B.E.; Fowler, J.M.; Clarke-Pearson, D.; Burger, R.A.; Mannel, R.S.; DeGeest, K.; Hartenbach, E.M.; Baergen, R. Phase III Trial of Carboplatin and Paclitaxel Compared With Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2003, 21, 3194–3200.
- Mullen, M.M.; Kuroki, L.M.; Thaker, P.H. Novel treatment options in platinum-sensitive recurrent ovarian cancer: A review. Gynecol. Oncol. 2019, 152, 416–425.
- Jiang, X.; Li, X.; Li, W.; Bai, H.; Zhang, Z. PARP inhibitors in ovarian cancer: Sensitivity prediction and resistance mechanisms. J. Cell. Mol. Med. 2019, 23, 2303–2313.
- Chekerov, R.; Hilpert, F.; Mahner, S.; El-Balat, A.; Harter, P.; De Gregorio, N.; Fridrich, C.; Markmann, S.; Potenberg, J.; Lorenz, R.; et al. Sorafenib plus topotecan versus placebo plus topotecan for platinum-resistant ovarian cancer (TRIAS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018, 19, 1247–1258.
- Cohen, M.; Dromard, M.; Petignat, P. Heat shock proteins in ovarian cancer: A potential target for therapy. Gynecol. Oncol. 2010, 119, 164–166.
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266.
- Lindquist, S. The heat-shock response. Annu. Rev. Biochem. 1986, 55, 1151–1191.
- Hartl, F.U. Molecular chaperones in cellular protein folding. Nature 1996, 381, 571–580.
- Calderwood, S.K.; Khaleque, M.A.; Sawyer, D.B.; Ciocca, D.R. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem. Sci. 2006, 31, 164–172.
- Mogk, A.; Bukau, B.; Kampinga, H.H. Cellular Handling of Protein Aggregates by Disaggregation Machines. Mol. Cell 2018, 69, 214–226.
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Boil. 2019, 20, 421–435.
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978.
- Dai, C.; Whitesell, L.; Rogers, A.B.; Lindquist, S. Heat Shock Factor 1 Is a Powerful Multifaceted Modifier of Carcinogenesis. Cell 2007, 130, 1005–1018.
- Ciocca, D.R.; Arrigo, A.P.; Calderwood, S.K. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: An update. Arch. Toxicol. 2013, 87, 19–48.
- Vihervaara, A.; Sistonen, L. HSF1 at a glance. J. Cell Sci. 2014, 127, 261–266.
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256.
- Jee, H. Size dependent classification of heat shock proteins: A mini-review. J. Exerc. Rehabil. 2016, 12, 255–259.
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111.
- Jego, G.; Hazoume, A.; Seigneuric, R.; Garrido, C. Targeting heat shock proteins in cancer. Cancer Lett. 2013, 332, 275–285.
- Samali, A.; Cai, J.; Zhivotovsky, B.; Jones, D.P.; Orrenius, S. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999, 18, 2040–2048.
- Xanthoudakis, S.; Roy, S.; Rasper, D.; Hennessey, T.; Aubin, Y.; Cassady, R.; Tawa, P.; Ruel, R.; Rosen, A.; Nicholson, D.W. Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J. 1999, 18, 2049–2056.
- Kennedy, D.; Jäger, R.; Mosser, D.D.; Samali, A. Regulation of apoptosis by heat shock proteins. IUBMB Life 2014, 66, 327–338.
- Calderwood, S.K.; Gong, J. Heat Shock Proteins Promote Cancer: It’s a Protection Racket. Trends Biochem. Sci. 2016, 41, 311–323.
- Chauhan, D.; Li, G.; Hideshima, T.; Podar, K.; Mitsiades, C.; Mitsiades, N.; Catley, L.; Tai, Y.T.; Hayashi, T.; Shringarpure, R.; et al. Hsp27 inhibits release of mitochondrial protein Smac in multiple myeloma cells and confers dexamethasone resistance. Blood 2003, 102, 3379–3386.
- Garrido, C.; Brunet, M.; Didelot, C.; Zermati, Y.; Schmitt, E.; Kroemer, G. Heat Shock Proteins 27 and 70: Anti-Apoptotic Proteins with Tumorigenic Properties. Cell Cycle 2006, 5, 2592–2601.
- Arrigo, A.-P.; Gibert, B. HspB1 dynamic phospho-oligomeric structure dependent interactome as cancer therapeutic target. Curr. Mol. Med. 2012, 12, 1151–1163.
- Beere, H.M.; Wolf, B.B.; Cain, K.; Mosser, D.D.; Mahboubi, A.; Kuwana, T.; Tailor, P.; Morimoto, R.I.; Cohen, G.M.; Green, D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000, 2, 469–475.
- Alexandrova, E.M.; Marchenko, N.D. Mutant p53—Heat Shock Response Oncogenic Cooperation: A New Mechanism of Cancer Cell Survival. Front. Endocrinol. 2015, 6, 53.
- Yamamoto, S.; Iwakuma, T. Regulators of Oncogenic Mutant TP53 Gain of Function. Cancers 2018, 11, 4.
- Wawrzynow, B.; Zylicz, A.; Zylicz, M. Chaperoning the guardian of the genome. The two-faced role of molecular chaperones in p53 tumor suppressor action. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2018, 1869, 161–174.
- Azad, A.A.; Zoubeidi, A.; Gleave, M.E.; Chi, K.N. Targeting heat shock proteins in metastatic castration-resistant prostate cancer. Nat. Rev. Urol. 2015, 12, 26–36.
- Hoter, A.; Rizk, S.; Naim, H.Y. The Multiple Roles and Therapeutic Potential of Molecular Chaperones in Prostate Cancer. Cancers 2019, 11, 1194.
- Wang, C.; Zhang, Y.; Guo, K.; Wang, N.; Jin, H.; Liu, Y.; Qin, W. Heat shock proteins in hepatocellular carcinoma: Molecular mechanism and therapeutic potential. Int. J. Cancer 2016, 138, 1824–1834.
- Hendriks, L.E.; Dingemans, A.-M.C. Heat shock protein antagonists in early stage clinical trials for NSCLC. Expert Opin. Investig. Drugs 2017, 26, 541–550.
- Kimura, A.; Ogata, K.; Altan, B.; Yokobori, T.; Mochiki, E.; Yanai, M.; Kogure, N.; Yanoma, T.; Suzuki, M.; Bai, T.; et al. Nuclear heat shock protein 110 expression is associated with poor prognosis and hyperthermo-chemotherapy resistance in gastric cancer patients with peritoneal metastasis. World J. Gastroenterol. 2017, 23, 7541–7550.
- Yin, X.; Zhang, H.; Burrows, F.; Shores, C.G. Potent Activity of a Novel Dimeric Heat Shock Protein 90 Inhibitor against Head and Neck Squamous Cell Carcinoma In vitro and In vivo. Clin. Cancer Res. 2005, 11, 3889–3896.
- Ghosh, J.C.; Dohi, T.; Kang, B.H.; Altieri, D.C. Hsp60 regulation of tumor cell apoptosis. J. Biol. Chem. 2008, 283, 5188–5194.
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperon 2005, 10, 86–103.
- Wang, J.; Cui, S.; Zhang, X.; Wu, Y.; Tang, H. High Expression of Heat Shock Protein 90 Is Associated with Tumor Aggressiveness and Poor Prognosis in Patients with Advanced Gastric Cancer. PLoS ONE 2013, 8, e62876.
- Narayanankutty, V.; Narayanankutty, A.; Nair, A. Heat Shock Proteins (HSPs): A Novel Target for Cancer Metastasis Prevention. Curr. Drug Targets 2019, 20, 727–737.
- Milani, A.; Basirnejad, M.; Bolhassani, A. Heat-shock proteins in diagnosis and treatment: An overview of different biochemical and immunological functions. Immunotherapy 2019, 11, 215–239.
- Sirotkin, A.V.; Bauer, M. Heat shock proteins in porcine ovary: Synthesis, accumulation and regulation by stress and hormones. Cell Stress Chaperones 2011, 16, 379–387.
- Maizels, E.T.; Cottom, J.; Jones, J.C.R.; Hunzicker-Dunn, M. Follicle Stimulating Hormone (FSH) Activates the p38 Mitogen-Activated Protein Kinase Pathway, Inducing Small Heat Shock Protein Phosphorylation and Cell Rounding in Immature Rat Ovarian Granulosa Cells. Endocrinology 1998, 139, 3353–3356.
- Salvetti, N.R.; Mazzini, R.A.; Taboada, A.F.; Ortega, H.H.; Acosta, J.C.; Gimeno, E.J.; Müller, L.A. Estrogen Receptors α and β and Progesterone Receptors in Normal Bovine Ovarian Follicles and Cystic Ovarian Disease. Veter.-Pathol. 2007, 44, 373–378.
- D’Haeseleer, M.; Van Poucke, M.; Broeck, W.V.D. Cell-specific Localization of Oestrogen Receptor beta (ESR2) mRNA within Various Bovine Ovarian Cell Types Using In situ Hybridization. Anat. Histol. Embryol. 2005, 34, 265–272.
- Stope, M.B.; Sauermann, A.; Rönnau, C.; Zimmermann, U.; Walther, R.; Burchardt, M. Androgen receptor and heat shock proteins in progression of prostate cancer cells. Int. J. Clin. Pharmacol. Ther. 2012, 50, 65–67.
- Salvetti, N.; Baravalle, C.; Mira, G.; Gimeno, E.; Dallard, B.; Rey, F.; Ortega, H. Heat Shock Protein 70 and Sex Steroid Receptors in the Follicular Structures of Induced Ovarian Cysts. Reprod. Domest. Anim. 2009, 44, 805–814.
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Boil. Med. 2003, 228, 111–133.
- Khanna, A.; Aten, R.F.; Behrman, H.R. Heat shock protein induction blocks hormone-sensitive steroidogenesis in rat luteal cells. Steroids 1994, 59, 4–9.
- Khanna, A.; Aten, R.F.; Behrman, H.R. Physiological and pharmacological inhibitors of luteinizing hormone-dependent steroidogenesis induce heat shock protein-70 in rat luteal cells. Endocrinology 1995, 136, 1775–1781.
- Koshiyama, M.; Konishi, I.; Nanbu, K.; Nanbu, Y.; Mandai, M.; Komatsu, T.; Yamamoto, S.; Mori, T.; Fujii, S. Immunohistochemical localization of heat shock proteins HSP70 and HSP90 in the human endometrium: Correlation with sex steroid receptors and Ki-67 antigen expression. J. Clin. Endocrinol. Metab. 1995, 80, 1106–1112.
- Isobe, N.; Yoshimura, Y. Deficient proliferation and apoptosis in the granulosa and theca interna cells of the bovine cystic follicle. J. Reprod. Dev. 2007, 53, 1119–1124.
- Koshiyama, M.; Konishi, I.; Mandai, M.; Komatsu, T.; Yamamoto, S.; Nanbu, K.; Mori, T. Immunohistochemical analysis of p53 protein and 72 kDa heat shock protein (HSP72) expression in ovarian carcinomas. Virchows Arch. 1995, 425, 603–609.
- Velazquez, M.M.; Alfaro, N.S.; Dupuy, C.R.; Salvetti, N.R.; Rey, F.; Ortega, H.H. Heat shock protein patterns in the bovine ovary and relation with cystic ovarian disease. Anim. Reprod. Sci. 2010, 118, 201–209.
- Velázquez, M.M.; Alfaro, N.S.; Salvetti, N.R.; Stangaferro, M.L.; Rey, F.; Panzani, C.G.; Ortega, H.H. Levels of heat shock protein transcripts in normal follicles and ovarian follicular cysts. Reprod. Boil. 2011, 11, 276–283.
- Juliani, C.; Silva-Zacarin, E.; Santos, D.; Boer, P. Effects of atrazine on female Wistar rats: Morphological alterations in ovarian follicles and immunocytochemical labeling of 90kDa heat shock protein. Micron 2008, 39, 607–616.
- Park, E.; Cockrem, J.F.; Han, K.-H.; Kim, D.-H.; Jung, M.-H.; Chu, J.-P. Stress-induced activation of ovarian heat shock protein 90 in a rat model of polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2012, 38, 396–407.
- Li, L.; Mo, H.; Zhang, J.; Zhou, Y.; Peng, X.; Luo, X. The Role of Heat Shock Protein 90B1 in Patients with Polycystic Ovary Syndrome. PLoS ONE 2016, 11, e0152837.
