
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rajesh Singh | + 2328 word(s) | 2328 | 2021-08-24 11:51:29 | | | |
| 2 | Santosh Kumar Singh | Meta information modification | 2328 | 2021-08-27 17:26:50 | | | | |
| 3 | Vivi Li | Meta information modification | 2328 | 2021-08-30 04:59:42 | | | | |
| 4 | Vivi Li | Meta information modification | 2328 | 2021-08-30 05:00:05 | | |
Video Upload Options
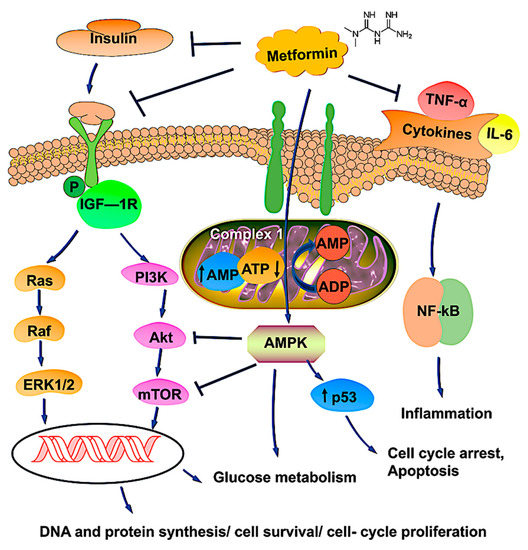
Since multiple reports established an association between diabetes mellitus and various cancers, emerging studies have surfaced to understand the effects of metformin as an anti-cancer agent. Although there was previous, but conflicting evidence, of a relationship between diabetes and ovarian cancer (OvCa), recent studies have supported this association. The mechanism of cancer development in patients with diabetes is likely to involve hyperglycemia, hyperinsulinemia, chronic inflammation, reactive oxygen species, regulation of cellular homeostasis, and activation of various pathways that lead to tumor cell proliferation. Preclinical evidence indicating that metformin, a medication commonly used to treat type 2 diabetes mellitus, may protect against OvCa. Metformin exerts anti-cancer properties by activating the MAPK pathway, inhibiting the PI3K/AKT/mTOR pathway, increasing tumor suppressor genes, inducing G2/M cycle arrest, and various other processes. Several studies have shown the efficacy of metformin as an adjunct with standard chemotherapeutic agents due to its synergistic effects on OvCa cells.
1. Introduction
2. Theories Regarding the Biological Link between DM and OvCa
3. Antineoplastic Mechanisms of Metformin on OvCa Cells
4. The Future of Metformin as an Adjunct for OvCa Therapy
| Disease Condition | Treatment | Summary | Trial Phase Status | Clinical Trial Number |
|---|---|---|---|---|
| Advanced stage OvCa | Metformin with carboplatin/paclitaxel | mTOR pathway inhibition, p53-induced apoptosis. | Phase1 | NCT02312661 |
| Advanced stage OvCa | Paclitaxel, carboplatin, and oral metformin | Increased synergy without compromising patient tolerability. | Phase 2 | NCT02437812 |
| Advanced epithelial OvCa in Stages IIIa–-IV | Metformin, acetylsalicylic acid, olaparib, and letrozole |
Women with advanced (stage IIIa-IV) OvCa of the histologic subtype high-grade serous carcinoma (HGSOC) are going through a diagnostic laparoscopy. They will receive treatment with a study agent for 10–14 days before surgery. The study is randomized and unblinded. | Early Phase 1 | NCT03378297 |
| Complex endometrial hyperplasia with atypia grade 1 endometrial endometrioid adenocarcinoma |
Levonorgestrel and metformin | Metformin is an effective treatment for early-stage endometrial cancer and endometrial hyperplasia with atypia. | Phase 2 | NCT01686126 |
| Ovarian, fallopian tube, and primary peritoneal cancer | Metformin | To determine if metformin administered in combination with chemotherapy to women with advanced ovarian, primary peritoneal, or fallopian tube cancer will improve recurrence-free survival at 18 months compared to controls. | Phase 2 | NCT01579812 |
| Cancer | Metformin, atorvastatin, doxycycline, and mebendazole |
To determine the effectiveness of a regimen of selected metabolic treatments for patients with cancer in a real-world setting and to conduct exploratory analysis on the relationship between the degree of response and changes in biochemical markers (such as glucose and lipid levels). | Phase 3 | NCT02201381 |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33.
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944.
- Lisio, M.A.; Fu, L.; Goyeneche, A.; Gao, Z.H.; Telleria, C. High-Grade Serous Ovarian Cancer: Basic Sciences, Clinical and Therapeutic Standpoints. Int. J. Mol. Sci. 2019, 20, 952.
- Gajjar, K.; Ogden, G.; Mujahid, M.I.; Razvi, K. Symptoms and risk factors of ovarian cancer: A survey in primary care. ISRN Obstet. Gynecol. 2012, 2012, 754197.
- Shlomai, G.; Neel, B.; LeRoith, D.; Gallagher, E.J. Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J. Clin. Oncol. 2016, 34, 4261–4269.
- Li, A.; Qiu, M.; Zhou, H.; Wang, T.; Guo, W. PTEN, Insulin Resistance and Cancer. Curr. Pharm. Des. 2017, 23, 3667–3676.
- Zhang, D.; Li, N.; Xi, Y.; Zhao, Y.; Wang, T. Diabetes mellitus and risk of ovarian cancer. A systematic review and meta-analysis of 15 cohort studies. Diabetes Res. Clin. Pract. 2017, 130, 43–52.
- Romero, I.L.; McCormick, A.; McEwen, K.A.; Park, S.; Karrison, T.; Yamada, S.D.; Pannain, S.; Lengyel, E. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet. Gynecol. 2012, 119, 61–67.
- Rattan, R.; Giri, S.; Hartmann, L.C.; Shridhar, V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J. Cell Mol. Med. 2011, 15, 166–178.
- Zhu, J.; Zheng, Y.; Zhang, H.; Sun, H. Targeting cancer cell metabolism: The combination of metformin and 2-Deoxyglucose regulates apoptosis in ovarian cancer cells via p38 MAPK/JNK signaling pathway. Am. J. Transl. Res. 2016, 8, 4812–4821.
- Wang, S.B.; Lei, K.J.; Liu, J.P.; Jia, Y.M. Continuous use of metformin can improve survival in type 2 diabetic patients with ovarian cancer: A retrospective study. Medicine 2017, 96, e7605.
- Marrone, K.A.; Zhou, X.; Forde, P.M.; Purtell, M.; Brahmer, J.R.; Hann, C.L.; Kelly, R.J.; Coleman, B.; Gabrielson, E.; Rosner, G.L.; et al. A Randomized Phase II Study of Metformin plus Paclitaxel/Carboplatin/Bevacizumab in Patients with Chemotherapy-Naïve Advanced or Metastatic Nonsquamous Non-Small Cell Lung Cancer. Oncologist 2018, 23, 859–865.
- Adler, A.I.; Weiss, N.S.; Kamb, M.L.; Lyon, J.L. Is diabetes mellitus a risk factor for ovarian cancer? A case-control study in Utah and Washington (United States). Cancer Causes Control 1996, 7, 475–478.
- Gapstur, S.M.; Patel, A.V.; Diver, W.R.; Hildebrand, J.S.; Gaudet, M.M.; Jacobs, E.J.; Campbell, P.T. Type II diabetes mellitus and the incidence of epithelial ovarian cancer in the cancer prevention study-II nutrition cohort. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2000–2005.
- La Vecchia, C.; Negri, E.; Franceschi, S.; D’Avanzo, B.; Boyle, P. A case-control study of diabetes mellitus and cancer risk. Br. J. Cancer 1994, 70, 950–953.
- Inoue, M.; Iwasaki, M.; Otani, T.; Sasazuki, S.; Noda, M.; Tsugane, S. Diabetes mellitus and the risk of cancer: Results from a large-scale population-based cohort study in Japan. Arch. Intern. Med. 2006, 166, 1871–1877.
- Lee, J.Y.; Jeon, I.; Kim, J.W.; Song, Y.S.; Yoon, J.M.; Park, S.M. Diabetes mellitus and ovarian cancer risk: A systematic review and meta-analysis of observational studies. Int. J. Gynecol. Cancer 2013, 23, 402–412.
- Wang, L.; Wang, L.; Zhang, J.; Wang, B.; Liu, H. Association between diabetes mellitus and subsequent ovarian cancer in women: A systematic review and meta-analysis of cohort studies. Medicine 2017, 96, e6396.
- Lauf, P.K.; Adragna, N.C. K-Cl cotransport: Properties and molecular mechanism. Cell Physiol. Biochem. 2000, 10, 341–354.
- Shen, M.R.; Lin, A.C.; Hsu, Y.M.; Chang, T.J.; Tang, M.J.; Alper, S.L.; Ellory, J.C.; Chou, C.Y. Insulin-like growth factor 1 stimulates KCl cotransport, which is necessary for invasion and proliferation of cervical cancer and ovarian cancer cells. J. Biol. Chem. 2004, 279, 40017–40025.
- Shen, M.R.; Chou, C.Y.; Hsu, K.F.; Hsu, Y.M.; Chiu, W.T.; Tang, M.J.; Alper, S.L.; Ellory, J.C. KCl cotransport is an important modulator of human cervical cancer growth and invasion. J. Biol. Chem. 2003, 278, 39941–39950.
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183.
- Chen, Y.F.; Chou, C.Y.; Wilkins, R.J.; Ellory, J.C.; Mount, D.B.; Shen, M.R. Motor protein-dependent membrane trafficking of KCl cotransporter-4 is important for cancer cell invasion. Cancer Res. 2009, 69, 8585–8593.
- Evans, J.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005, 330, 1304–1305.
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585.
- Ikhlas, S.; Ahmad, M. Metformin: Insights into its anticancer potential with special reference to AMPK dependent and independent pathways. Life Sci. 2017, 185, 53–62.
- Li, H.; Zeng, J.; Shen, K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch. Gynecol. Obstet. 2014, 290, 1067–1078.
- Gasparri, M.L.; Bardhi, E.; Ruscito, I.; Papadia, A.; Farooqi, A.A.; Marchetti, C.; Bogani, G.; Ceccacci, I.; Mueller, M.D.; Benedetti Panici, P. PI3K/AKT/mTOR Pathway in Ovarian Cancer Treatment: Are We on the Right Track? Geburtshilfe Frauenheilkd 2017, 77, 1095–1103.
- Zhang, F.; Chen, H.; Du, J.; Wang, B.; Yang, L. Anticancer Activity of Metformin, an Antidiabetic Drug, Against Ovarian Cancer Cells Involves Inhibition of Cysteine-Rich 61 (Cyr61)/Akt/Mammalian Target of Rapamycin (mTOR) Signaling Pathway. Med. Sci. Monit. 2018, 24, 6093–6101.
- Lee, K.B.; Byun, H.J.; Park, S.H.; Park, C.Y.; Lee, S.H.; Rho, S.B. CYR61 controls p53 and NF-κB expression through PI3K/Akt/mTOR pathways in carboplatin-induced ovarian cancer cells. Cancer Lett. 2012, 315, 86–95.
- Fu, Y.L.; Zhang, Q.H.; Wang, X.W.; He, H. Antidiabetic drug metformin mitigates ovarian cancer SKOV3 cell growth by triggering G2/M cell cycle arrest and inhibition of m-TOR/PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1169–1175.
- Xu, S.; Yang, Z.; Jin, P.; Yang, X.; Li, X.; Wei, X.; Wang, Y.; Long, S.; Zhang, T.; Chen, G.; et al. Metformin Suppresses Tumor Progression by Inactivating Stromal Fibroblasts in Ovarian Cancer. Mol. Cancer Ther. 2018, 17, 1291–1302.
- Zheng, Y.; Zhu, J.; Zhang, H.; Liu, Y.; Sun, H. Metformin inhibits ovarian cancer growth and migration in vitro and in vivo by enhancing cisplatin cytotoxicity. Am. J. Transl. Res. 2018, 10, 3086–3098.
- Lengyel, E.; Litchfield, L.M.; Mitra, A.K.; Nieman, K.M.; Mukherjee, A.; Zhang, Y.; Johnson, A.; Bradaric, M.; Lee, W.; Romero, I.L. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am. J. Obstet. Gynecol. 2015, 212, 479.e1–479.e10.
- Mert, I.; Chhina, J.; Allo, G.; Dai, J.; Seward, S.; Carey, M.S.; Llaurado, M.; Giri, S.; Rattan, R.; Munkarah, A.R. Synergistic effect of MEK inhibitor and metformin combination in low grade serous ovarian cancer. Gynecol. Oncol. 2017, 146, 319–326.
- Hamedi, B.; Khalili, A.; Roozmeh, S.; Namazi, G.; Saraf, Z. Combination of Metformin and Chemotherapy Decreases the Recurrence Rates of Epithelial Ovarian Cancers: A Randomized Clinical Trial. Int. J. Cancer Manag. 2018, 11, e11621.
- Facchinetti, F.; Orru, B.; Grandi, G.; Unfer, V. Short-term effects of metformin and myo-inositol in women with polycystic ovarian syndrome (PCOS): A meta-analysis of randomized clinical trials. Gynecol. Endocrinol. 2019, 35, 198–206.
- Hameed, M.; Khan, K.; Salman, S.; Mehmood, N. Dose Comparison and Side Effect Profile of Metformin Extended Release Versus Metformin Immediate Release. J. Ayub Med. Coll. Abbottabad 2017, 29, 225–229.
- Haikala, H.M.; Anttila, J.M.; Marques, E.; Raatikainen, T.; Ilander, M.; Hakanen, H.; Ala-Hongisto, H.; Savelius, M.; Balboa, D.; Von Eyss, B.; et al. Pharmacological reactivation of MYC-dependent apoptosis induces susceptibility to anti-PD-1 immunotherapy. Nat. Commun. 2019, 10, 1–17.
- Scharping, N.E.; Menk, A.V.; Whetstone, R.D.; Zeng, X.; Delgoffe, G.M. Efficacy of PD-1 Blockade Is Potentiated by Metformin-Induced Reduction of Tumor Hypoxia. Cancer Immunol. Res. 2017, 5, 9–16.
- Liu, W.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Pleiotropic Effects of Metformin on the Antitumor Efficiency of Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 3724.
- Xue, J.; Li, L.; Li, N.; Li, F.; Qin, X.; Li, T.; Liu, M. Metformin suppresses cancer cell growth in endometrial carcinoma by inhibiting PD-L1. Eur. J. Pharmacol. 2019, 859, 172541.




