
Video Upload Options
Since the clear demonstration of lysophosphatidic acid (LPA)’s pathological roles in cancer in the mid-1990s, more than 1000 papers relating LPA to various types of cancer were published. Through these studies, LPA was established as a target for cancer. Although LPA-related inhibitors entered clinical trials for fibrosis, the concept of targeting LPA is yet to be moved to clinical cancer treatment. The major challenges that we are facing in moving LPA application from bench to bedside include the intrinsic and complicated metabolic, functional, and signaling properties of LPA, as well as technical issues, which are discussed in this entry. Potential strategies and perspectives to improve the translational progress are suggested. Despite these challenges, we are optimistic that LPA blockage, particularly in combination with other agents, is on the horizon to be incorporated into clinical applications.
1. Introduction
2. LPA: From Bench to Bedside
2.1. A Brief History and Milestones of LPA Research
2.1.1. Before the Identification of LPA Receptors
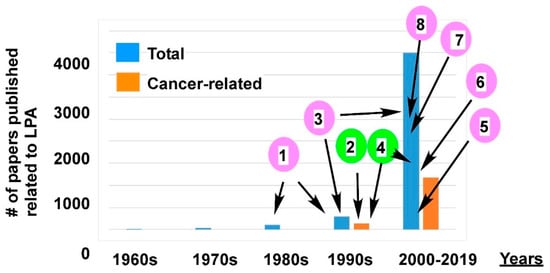
2.1.2. Post Identification of LPA Receptors
2.2. Challenges and Obstacles of LPA Clinical Applications in Cancer
2.2.1. The Issues with LPA as a Marker for Cancer
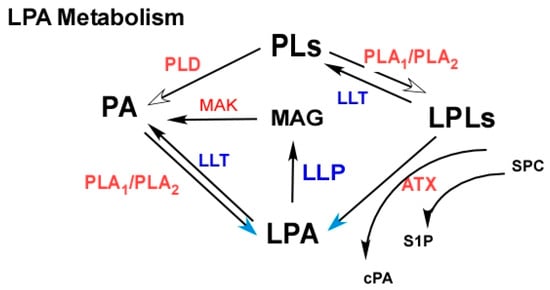
2.2.2. Targeting LPA Metabolism
2.2.3. Targeting LPA Receptors
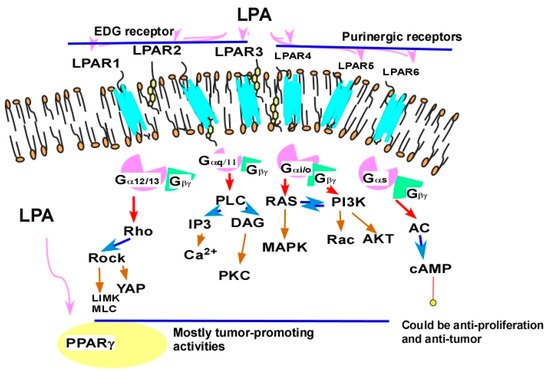
2.2.4. Targeting LPA Cross-Talk
2.2.4.1. Cross-Talk between LPA Signaling and Other Cell Signaling Receptors
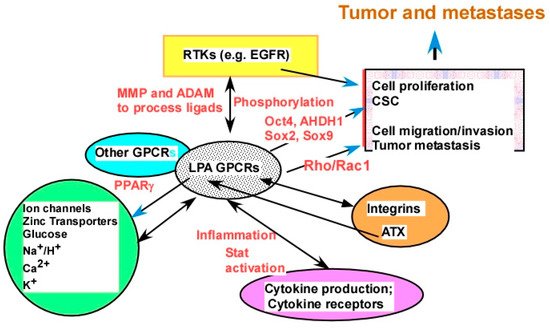
2.2.4.2. The Molecular Mechanisms of LPA Cross-Talks
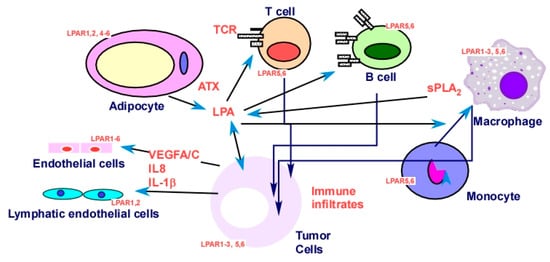
2.2.5. Targeting Tumor–Stromal Interactions in the TME
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70.
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674.
- Mills, G.B.; Moolenaar, W.H. The emerging role of lysophosphatidic acid in cancer. Nat. Rev. Cancer 2003, 3, 582–591.
- Sengupta, S.; Wang, Z.; Tipps, R.; Xu, Y. Biology of LPA in health and disease. Semin. Cell Dev. Biol. 2004, 15, 503–512.
- Leblanc, R.; Peyruchaud, O. New insights into the autotaxin/LPA axis in cancer development and metastasis. Exp. Cell Res. 2015, 333, 183–189.
- Jesionowska, A.; Cecerska-Heryc, E.; Marczuk, N.; Safranow, K.; Dolegowska, B. Lysophosphatidic acid and malignant neoplasms. Postepy Biochem. 2015, 61, 381–387.
- Xu, Y. Lysophospholipid Signaling in the Epithelial Ovarian Cancer Tumor Microenvironment. Cancers 2018, 10, 227.
- Xu, Y.; Gaudette, D.C.; Boynton, J.D.; Frankel, A.; Fang, X.J.; Sharma, A.; Hurteau, J.; Casey, G.; Goodbody, A.; Mellors, A.; et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin. Cancer Res. 1995, 1, 1223–1232.
- Xu, Y.; Casey, G.; Mills, G.B. Effect of lysophospholipids on signaling in the human Jurkat T cell line. J. Cell. Physiol. 1995, 163, 441–450.
- Xu, Y.; Fang, X.J.; Casey, G.; Mills, G.B. Lysophospholipids activate ovarian and breast cancer cells. Biochem. J. 1995, 309, 933–940.
- Deng, W.; Wang, D.A.; Gosmanova, E.; Johnson, L.R.; Tigyi, G. LPA protects intestinal epithelial cells from apoptosis by inhibiting the mitochondrial pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, 821–829.
- Sui, Y.; Yang, Y.; Wang, J.; Li, Y.; Ma, H.; Cai, H.; Liu, X.; Zhang, Y.; Wang, S.; Li, Z.; et al. Lysophosphatidic acid Inhibits apoptosis induced by cisplatin in cervical cancer cells. Biomed. Res. Int. 2015, 2015, e598386.
- Bermudez, Y.; Yang, H.; Saunders, B.O.; Cheng, J.Q.; Nicosia, S.V.; Kruk, P.A. VEGF- and LPA-induced telomerase in human ovarian cancer cells is Sp1-dependent. Gynecol. Oncol. 2007, 106, 526–537.
- Sako, A.; Kitayama, J.; Shida, D.; Suzuki, R.; Sakai, T.; Ohta, H.; Nagawa, H. Lysophosphatidic acid (LPA)-induced vascular endothelial growth factor (VEGF) by mesothelial cells and quantification of host-derived VEGF in malignant ascites. J. Surg. Res. 2006, 130, 94–101.
- Lin, C.I.; Chen, C.N.; Huang, M.T.; Lee, S.J.; Lin, C.H.; Chang, C.C.; Lee, H. Lysophosphatidic acid upregulates vascular endothelial growth factor-C and tube formation in human endothelial cells through LPA(1/3), COX-2, and NF-kappaB activation- and EGFR transactivation-dependent mechanisms. Cell. Signal. 2008, 20, 1804–1814.
- Lin, C.H.; Lu, J.; Lee, H. Interleukin-1beta expression is required for lysophosphatidic Acid-induced lymphangiogenesis in human umbilical vein endothelial cells. Int. J. Inflam. 2010, 2011, e351010.
- Lin, Y.C.; Chen, C.C.; Chen, W.M.; Lu, K.Y.; Shen, T.L.; Jou, Y.C.; Shen, C.H.; Ohbayashi, N.; Kanaho, Y.; Huang, Y.L.; et al. LPA1/3 signaling mediates tumor lymphangiogenesis through promoting CRT expression in prostate cancer. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1305–1315.
- Wu, P.Y.; Lin, Y.C.; Huang, Y.L.; Chen, W.-M.; Chen, C.-C.; Lee, H.L. Mechanisms of lysophosphatidic acid-mediated lymphangiogenesis in prostate cancer. Cancers 2018, 10, 413.
- Schwartz, B.M.; Hong, G.; Morrison, B.H.; Wu, W.; Baudhuin, L.M.; Xiao, Y.J.; Mok, S.C.; Xu, Y. Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecol. Oncol. 2001, 81, 291–300.
- Fang, X.; Yu, S.; Bast, R.C.; Liu, S.; Xu, H.J.; Hu, S.X.; LaPushin, R.; Claret, F.X.; Aggarwal, B.B.; Lu, Y.; et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J. Biol. Chem. 2004, 279, 9653–9661.
- Hisano, Y.; Hla, T. Bioactive lysolipids in cancer and angiogenesis. Pharmacol. Ther. 2019, 193, 91–98.
- Sutphen, R.; Xu, Y.; Wilbanks, G.D.; Fiorica, J.; Grendys, E.C., Jr.; LaPolla, J.P.; Arango, H.; Hoffman, M.S.; Martino, M.; Wakeley, K.; et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1185–1191.
- Kim, K.S.; Sengupta, S.; Berk, M.; Kwak, Y.G.; Escobar, P.F.; Belinson, J.; Mok, S.C.; Xu, Y. Hypoxia enhances lysophosphatidic acid responsiveness in ovarian cancer cells and lysophosphatidic acid induces ovarian tumor metastasis in vivo. Cancer Res. 2006, 66, 7983–7990.
- Ren, J.; Xiao, Y.J.; Singh, L.S.; Zhao, X.; Zhao, Z.; Feng, L.; Rose, T.M.; Prestwich, G.D.; Xu, Y. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006, 66, 3006–3014.
- Sengupta, S.; Kim, K.S.; Berk, M.P.; Oates, R.; Escobar, P.; Belinson, J.; Li, W.; Lindner, D.J.; Williams, B.; Xu, Y. Lysophosphatidic acid downregulates tissue inhibitor of metalloproteinases, which are negatively involved in lysophosphatidic acid-induced cell invasion. Oncogene 2007, 26, 2894–2901.
- Liu, S.; Umezu-Goto, M.; Murph, M.; Lu, Y.; Liu, W.; Zhang, F.; Yu, S.; Stephens, L.C.; Cui, X.; Murrow, G.; et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell 2009, 15, 539–550.
- Cholia, R.P.; Dhiman, M.; Kumar, R.; Mantha, A.K. Oxidative stress stimulates invasive potential in rat C6 and human U-87 MG glioblastoma cells via activation and cross-talk between PKM2, ENPP2 and APE1 enzymes. Metab. Brain Dis. 2018, 33, 1307–1326.
- Balogh, A.; Shimizu, Y.; Lee, S.C.; Norman, D.D.; Gangwar, R.; Bavaria, M.; Moon, C.; Shukla, P.; Rao, R.; Ray, R.; et al. The autotaxin-LPA2 GPCR axis is modulated by gamma-irradiation and facilitates DNA damage repair. Cell. Signal. 2015, 27, 1751–1762.
- Barekzi, E.; Roman, J.; Hise, K.; Georas, S.; Steinke, J.W. Lysophosphatidic acid stimulates inflammatory cascade in airway epithelial cells. Prostaglandins Leukot. Essent. Fatty Acids 2006, 74, 357–363.
- Spangelo, B.L.; Jarvis, W.D. Lysophosphatidylcholine stimulates interleukin-6 release from rat anterior pituitary cells in vitro. Endocrinology 1996, 137, 4419–4426.
- Jesionowska, A.; Cecerska-Heryc, E.; Matoszka, N.; Dolegowska, B. Lysophosphatidic acid signaling in ovarian cancer. J. Recept. Signal. Transduct. Res. 2015, 35, 578–584.
- D’Souza, K.; Nzirorera, C.; Cowie, A.M.; Varghese, G.P.; Trivedi, P.; Eichmann, T.O.; Biswas, D.; Touaibia, M.; Morris, A.J.; Aidinis, V.; et al. Autotaxin-LPA signaling contributes to obesity-induced insulin resistance in muscle and impairs mitochondrial metabolism. J. Lipid Res. 2018, 59, 1805–1817.
- Ha, J.H.; Radhakrishnan, R.; Jayaraman, M.; Yan, M.; Ward, J.D.; Fung, K.M.; Moxley, K.; Sood, A.K.; Isidoro, C.; Mukherjee, P.; et al. LPA Induces Metabolic Reprogramming in Ovarian Cancer via a Pseudohypoxic Response. Cancer Res. 2018, 78, 1923–1934.
- Meshcheryakova, A.; Svoboda, M.; Jaritz, M.; Mungenast, F.; Salzmann, M.; Pils, D.; Cacsire Castillo-Tong, D.; Hager, G.; Wolf, A.; Braicu, E.I.; et al. Interrelations of Sphingolipid and Lysophosphatidate Signaling with Immune System in Ovarian Cancer. Comput. Struct. Biotechnol. J. 2019, 17, 537–560.
- Moolenaar, W.H. LPA: A novel lipid mediator with diverse biological actions. Trends Cell Biol. 1994, 4, 213–219.
- Contos, J.J.; Ishii, I.; Chun, J. Lysophosphatidic acid receptors. Mol. Pharmacol. 2000, 58, 1188–1196.
- Fang, X.; Schummer, M.; Mao, M.; Yu, S.; Tabassam, F.H.; Swaby, R.; Hasegawa, Y.; Tanyi, J.L.; LaPushin, R.; Eder, A.; et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim. Biophys. Acta 2002, 1582, 257–264.
- Goetzl, E.J.; Graeler, M.; Huang, M.C.; Shankar, G. Lysophospholipid growth factors and their G protein-coupled receptors in immunity, coronary artery disease, and cancer. Sci. World J. 2002, 2, 324–338.
- Mills, G.B.; Eder, A.; Fang, X.; Hasegawa, Y.; Mao, M.; Lu, Y.; Tanyi, J.; Tabassam, F.H.; Wiener, J.; Lapushin, R.; et al. Critical role of lysophospholipids in the pathophysiology, diagnosis, and management of ovarian cancer. Cancer Treat. Res. 2002, 107, 259–283.
- Tokumura, A. Physiological and pathophysiological roles of lysophosphatidic acids produced by secretory lysophospholipase D in body fluids. Biochim. Biophys. Acta 2002, 1582, 18–25.
- Xu, Y.; Xiao, Y.J.; Zhu, K.; Baudhuin, L.M.; Lu, J.; Hong, G.; Kim, K.S.; Cristina, K.L.; Song, L.; Williams, F.S. Unfolding the pathophysiological role of bioactive lysophospholipids. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2003, 3, 23–32.
- Aoki, J. Mechanisms of lysophosphatidic acid production. Semin. Cell Dev. Biol. 2004, 15, 477–489.
- Pua, T.L.; Wang, F.Q.; Fishman, D.A. Roles of LPA in ovarian cancer development and progression. Future Oncol. 2009, 5, 1659–1673.
- Tabuchi, S. The autotaxin-lysophosphatidic acid-lysophosphatidic acid receptor cascade: Proposal of a novel potential therapeutic target for treating glioblastoma multiforme. Lipids Health Dis. 2015, 14, e56.
- Bar-Shavit, R.; Maoz, M.; Kancharla, A.; Nag, J.K.; Agranovich, D.; Grisaru-Granovsky, S.; Uziely, B. G Protein-Coupled Receptors in Cancer. Int. J. Mol. Sci. 2016, 17, 1320.
- Benesch, M.G.; Tang, X.; Venkatraman, G.; Bekele, R.T.; Brindley, D.N. Recent advances in targeting the autotaxin-lysophosphatidate-lipid phosphate phosphatase axis in vivo. J. Biomed. Res. 2016, 30, 272–284.
- Benesch, M.G.K.; MacIntyre, I.T.K.; McMullen, T.P.W.; Brindley, D.N. Coming of Age for Autotaxin and Lysophosphatidate Signaling: Clinical Applications for Preventing, Detecting and Targeting Tumor-Promoting Inflammation. Cancers 2018, 10, e73.
- Lee, D.; Suh, D.S.; Lee, S.C.; Tigyi, G.J.; Kim, J.H. Role of autotaxin in cancer stem cells. Cancer Metastasis Rev. 2018, 37, 509–518.
- Yun, C.C. Lysophosphatidic Acid and Autotaxin-associated Effects on the Initiation and Progression of Colorectal Cancer. Cancers 2019, 11, 958.
- Kirschner, H.; Vogt, W. Pharmacologically active lipidsoluble acids in brain extracts: Isolation of lysophosphatidic acid and ganglioside. Biochem. Pharmacol. 1961, 8, 224–234.
- Chan, L.C.; Peters, W.; Xu, Y.; Chun, J.; Farese, R.V., Jr.; Cases, S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA). J. Leukoc. Biol. 2007, 82, 1193–1200.
- Liu, S.; Murph, M.; Panupinthu, N.; Mills, G.B. ATX-LPA receptor axis in inflammation and cancer. Cell Cycle 2009, 8, 3695–3701.
- Van Corven, E.J.; Groenink, A.; Jalink, K.; Eichholtz, T.; Moolenaar, W.H. Lysophosphatidate-induced cell proliferation: Identification and dissection of signaling pathways mediated by G proteins. Cell 1989, 59, 45–54.
- Jalink, K.; van Corven, E.J.; Moolenaar, W.H. Lysophosphatidic acid, but not phosphatidic acid, is a potent Ca2(+)-mobilizing stimulus for fibroblasts. Evidence for an extracellular site of action. J. Biol. Chem. 1990, 265, 12232–12239.
- Moolenaar, W.H.; van Corven, E.J. Growth factor-like action of lysophosphatidic acid: Mitogenic signalling mediated by G proteins. Ciba Found. Symp. 1990, 72, e99.
- Moolenaar, W.H. Mitogenic action of lysophosphatidic acid. Adv. Cancer Res. 1991, 57, 87–102.
- Jalink, K.; Eichholtz, T.; Postma, F.R.; van Corven, E.J.; Moolenaar, W.H. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: Similarity to thrombin action. Cell Growth Differ. 1993, 4, 247–255.
- Jalink, K.; Moolenaar, W.H.; Van Duijn, B. Lysophosphatidic acid is a chemoattractant for Dictyostelium discoideum amoebae. Proc. Natl. Acad Sci. USA 1993, 90, 1857–1861.
- Van Corven, E.J.; Hordijk, P.L.; Medema, R.H.; Bos, J.L.; Moolenaar, W.H. Pertussis toxin-sensitive activation of p21ras by G protein-coupled receptor agonists in fibroblasts. Proc. Natl. Acad. Sci. USA 1993, 90, 1257–1261.
- Weiner, J.A.; Hecht, J.H.; Chun, J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J. Comp. Neurol. 1998, 398, 587–598.
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214.
- Chun, J.; Goetzl, E.J.; Hla, T.; Igarashi, Y.; Lynch, K.R.; Moolenaar, W.; Pyne, S.; Tigyi, G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2002, 54, 265–269.
- An, S.; Bleu, T.; Huang, W.; Hallmark, O.G.; Coughlin, S.R.; Goetzl, E.J. Identification of cDNAs encoding two G protein-coupled receptors for lysosphingolipids. FEBS Lett. 1997, 417, 279–282.
- An, S.; Bleu, T.; Hallmark, O.G.; Goetzl, E.J. Characterization of a novel subtype of human G protein-coupled receptor for lysophosphatidic acid. J. Biol. Chem. 1998, 273, 7906–7910.
- Bandoh, K.; Aoki, J.; Hosono, H.; Kobayashi, S.; Kobayashi, T.; Murakami-Murofushi, K.; Tsujimoto, M.; Arai, H.; Inoue, K. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J. Biol. Chem. 1999, 274, 27776–27785.
- Yanagida, K.; Ishii, S.; Hamano, F.; Noguchi, K.; Shimizu, T. LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. J. Biol. Chem. 2007, 282, 5814–5824.
- Kotarsky, K.; Boketoft, A.; Bristulf, J.; Nilsson, N.E.; Norberg, A.; Hansson, S.; Owman, C.; Sillard, R.; Leeb-Lundberg, L.M.; Olde, B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J. Pharmacol. Exp. Ther. 2006, 318, 619–628.
- Lee, C.W.; Rivera, R.; Gardell, S.; Dubin, A.E.; Chun, J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 2006, 281, 23589–23597.
- Pasternack, S.M.; von Kugelgen, I.; Al Aboud, K.; Lee, Y.A.; Ruschendorf, F.; Voss, K.; Hillmer, A.M.; Molderings, G.J.; Franz, T.; Ramirez, A.; et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat. Genet. 2008, 40, 329–334.
- Yanagida, K.; Masago, K.; Nakanishi, H.; Kihara, Y.; Hamano, F.; Tajima, Y.; Taguchi, R.; Shimizu, T.; Ishii, S. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J. Biol. Chem. 2009, 284, 17731–17741.
- Kihara, Y.; Maceyka, M.; Spiegel, S.; Chun, J. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol. 2014, 171, 3575–3594.
- Xu, Y.; Shen, Z.; Wiper, D.W.; Wu, M.; Morton, R.E.; Elson, P.; Kennedy, A.W.; Belinson, J.; Markman, M.; Casey, G. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA 1998, 280, 719–723.
- Xiao, Y.; Chen, Y.; Kennedy, A.W.; Belinson, J.; Xu, Y. Evaluation of plasma lysophospholipids for diagnostic significance using electrospray ionization mass spectrometry (ESI-MS) analyses. Ann. N. Y. Acad. Sci. 2000, 905, 242–259.
- Sedlakova, I.; Vavrova, J.; Tosner, J.; Hanousek, L. Lysophosphatidic acid in ovarian cancer patients. Ceska Gynekol. 2006, 71, 312–317.
- Meleh, M.; Pozlep, B.; Mlakar, A.; Meden-Vrtovec, H.; Zupancic-Kralj, L. Determination of serum lysophosphatidic acid as a potential biomarker for ovarian cancer. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 858, 287–291.
- Sedlakova, I.; Vavrova, J.; Tosner, J.; Hanousek, L. Lysophosphatidic acid: An ovarian cancer marker. Eur. J. Gynaecol. Oncol. 2008, 29, 511–514.
- Nakamura, K.; Igarashi, K.; Ohkawa, R.; Yokota, H.; Masuda, A.; Nakagawa, S.; Yano, T.; Ikeda, H.; Aoki, J.; Yatomi, Y. Serum autotaxin is not a useful biomarker for ovarian cancer. Lipids 2012, 47, 927–930.
- Lu, Z.; Chen, Y.; Hu, Z.; Hu, C. Diagnostic value of total plasma lysophosphatidic acid in ovarian cancer: A meta-analysis. Int. J. Gynecol Cancer 2015, 25, 18–23.
- Zhang, Y.J.; Cao, L.Y.; Fu, Z.Z.; Wang, Y.J.; Wang, G.X.; Gu, T. Clinical significance of plasma lysophosphatidic acid levels in the differential diagnosis of ovarian cancer. J. Cancer Res. Ther. 2015, 11, 375–380.
- Li, Y.Y.; Zhang, W.C.; Zhang, J.L.; Zheng, C.J.; Zhu, H.; Yu, H.M.; Fan, L.M. Plasma levels of lysophosphatidic acid in ovarian cancer versus controls: A meta-analysis. Lipids Health Dis. 2015, 14, e72.
- Moore, R.G.; Blackman, A.; Miller, M.C.; Robison, K.; DiSilvestro, P.A.; Eklund, E.E.; Strongin, R.; Messerlian, G. Multiple biomarker algorithms to predict epithelial ovarian cancer in women with a pelvic mass: Can additional makers improve performance? Gynecol. Oncol. 2019, 154, 150–155.
- Zeng, R.; Li, B.; Huang, J.; Zhong, M.; Li, L.; Duan, C.; Zeng, S.; Huang, J.; Liu, W.; Lu, J.; et al. Lysophosphatidic Acid is a Biomarker for Peritoneal Carcinomatosis of Gastric Cancer and Correlates with Poor Prognosis. Genet. Test. Mol. Biomark. 2017, 21, 641–648.
- Xiao, Y.J.; Schwartz, B.; Washington, M.; Kennedy, A.; Webster, K.; Belinson, J.; Xu, Y. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: Comparison of the lysophospholipid contents in malignant vs nonmalignant ascitic fluids. Anal. Biochem. 2001, 290, 302–313.
- Liebisch, G.; Scherer, M. Quantification of bioactive sphingo- and glycerophospholipid species by electrospray ionization tandem mass spectrometry in blood. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 883–884, 141–146.
- Zhao, Z.; Xu, Y. Measurement of endogenous lysophosphatidic acid by ESI-MS/MS in plasma samples requires pre-separation of lysophosphatidylcholine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 3739–3742.
- Zhao, Z.; Xu, Y. An extremely simple method for extraction of lysophospholipids and phospholipids from blood samples. J. Lipid Res. 2010, 51, 652–659.
- Jesionowska, A.; Cecerska, E.; Dolegowska, B. Methods for quantifying lysophosphatidic acid in body fluids: A review. Anal. Biochem. 2014, 453, 38–43.
- Umezu-Goto, M.; Kishi, Y.; Taira, A.; Hama, K.; Dohmae, N.; Takio, K.; Yamori, T.; Mills, G.B.; Inoue, K.; Aoki, J.; et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol. 2002, 158, 227–233.
- Tokumura, A.; Majima, E.; Kariya, Y.; Tominaga, K.; Kogure, K.; Yasuda, K.; Fukuzawa, K. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem. 2002, 277, 39436–39442.
- Inoue, K.; Tanaka, N.; Haga, A.; Yamasaki, K.; Umeda, T.; Kusakabe, Y.; Sakamoto, Y.; Nonaka, T.; Deyashiki, Y.; Nakamura, K.T. Crystallization and preliminary X-ray crystallographic analysis of human autotaxin. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 450–453.
- Hausmann, J.; Kamtekar, S.; Christodoulou, E.; Day, J.E.; Wu, T.; Fulkerson, Z.; Albers, H.M.; van Meeteren, L.A.; Houben, A.J.; van Zeijl, L.; et al. Structural basis of substrate discrimination and integrin binding by autotaxin. Nat. Struct. Mol. Biol. 2011, 18, 198–204.
- Nishimasu, H.; Okudaira, S.; Hama, K.; Mihara, E.; Dohmae, N.; Inoue, A.; Ishitani, R.; Takagi, J.; Aoki, J.; Nureki, O. Crystal structure of autotaxin and insight into GPCR activation by lipid mediators. Nat. Struct. Mol. Biol. 2011, 18, 205–212.
- Chrencik, J.E.; Roth, C.B.; Terakado, M.; Kurata, H.; Omi, R.; Kihara, Y.; Warshaviak, D.; Nakade, S.; Asmar-Rovira, G.; Mileni, M.; et al. Crystal Structure of Antagonist Bound Human Lysophosphatidic Acid Receptor 1. Cell 2015, 161, 1633–1643.
- Taniguchi, R.; Inoue, A.; Sayama, M.; Uwamizu, A.; Yamashita, K.; Hirata, K.; Yoshida, M.; Tanaka, Y.; Kato, H.E.; Nakada-Nakura, Y.; et al. Structural insights into ligand recognition by the lysophosphatidic acid receptor LPA6. Nature 2017, 548, 356–360.
- Benesch, M.G.K.; Yang, Z.; Tang, X.; Meng, G.; Brindley, D.N. Lysophosphatidate Signaling: The Tumor Microenvironment’s New Nemesis. Trends Cancer 2017, 3, 748–752.
- Tokumura, A.; Fukuzawa, K.; Isobe, J.; Tsukatani, H. Lysophosphatidic acid-induced aggregation of human and feline platelets: Structure-activity relationship. Biochem. Biophys. Res. Commun. 1981, 99, 391–398.
- Tokumura, A.; Kume, T.; Fukuzawa, K.; Tsukatani, H. Cardiovascular effects of lysophosphatidic acid and its structural analogs in rats. J. Pharmacol. Exp. Ther. 1981, 219, 219–224.
- Tokumura, A.; Mostafa, M.H.; Nelson, D.R.; Hanahan, D.J. Stimulation of (Ca2+ + Mg2+)-ATPase activity in human erythrocyte membranes by synthetic lysophosphatidic acids and lysophosphatidylcholines. Effects of chain length and degree of unsaturation of the fatty acid groups. Biochim. Biophys. Acta 1985, 812, 568–574.
- Nugent, D.; Xu, Y. Sphingosine-1-phosphate: Characterization of its inhibition of platelet aggregation. Platelets 2000, 11, 226–232.
- Sato, A.; Nakazawa, K.; Sugawara, A.; Yamazaki, Y.; Ebina, K. The interaction of beta2-glycoprotein I with lysophosphatidic acid in platelet aggregation and blood clotting. Biochim. Biophys. Acta Proteins Proteom. 2018, 1866, 1232–1241.
- Gerrard, J.M.; Kindom, S.E.; Peterson, D.A.; Peller, J.; Krantz, K.E.; White, J.G. Lysophosphatidic acids. Influence on platelet aggregation and intracellular calcium flux. Am. J. Pathol. 1979, 96, 423–438.
- Mauco, G.; Chap, H.; Simon, M.F.; Douste-Blazy, L. Phosphatidic and lysophosphatidic acid production in phospholipase C-and thrombin-treated platelets. Possible involvement of a platelet lipase. Biochimie 1978, 60, 653–661.
- Lapetina, E.G.; Billah, M.M.; Cuatrecasas, P. Lysophosphatidic acid potentiates the thrombin-induced production of arachidonate metabolites in platelets. J. Biol. Chem. 1981, 256, 11984–11987.
- Oyesanya, R.A.; Lee, Z.P.; Wu, J.; Chen, J.; Song, Y.; Mukherjee, A.; Dent, P.; Kordula, T.; Zhou, H.; Fang, X. Transcriptional and post-transcriptional mechanisms for lysophosphatidic acid-induced cyclooxygenase-2 expression in ovarian cancer cells. FASEB J. 2008, 22, 2639–2651.
- Watsky, M.A. Lysophosphatidic acid, serum, and hyposmolarity activate Cl- currents in corneal keratocytes. Am. J. Physiol. 1995, 269, 1385–1393.
- Yang, L.; Andrews, D.A.; Low, P.S. Lysophosphatidic acid opens a Ca(++) channel in human erythrocytes. Blood 2000, 95, 2420–2425.
- Juarez-Contreras, R.; Rosenbaum, T.; Morales-Lazaro, S.L. Lysophosphatidic Acid and Ion Channels as Molecular Mediators of Pain. Front. Mol. Neurosci. 2018, 11, e462.
- MacIntyre, D.E.; Shaw, A.M. Phospholipid-induced human platelet activation: Effects of calcium channel blockers and calcium chelators. Thromb. Res. 1983, 31, 833–844.
- Petrou, S.; Ordway, R.W.; Hamilton, J.A.; Walsh, J.V., Jr.; Singer, J.J. Structural requirements for charged lipid molecules to directly increase or suppress K+ channel activity in smooth muscle cells. Effects of fatty acids, lysophosphatidate, acyl coenzyme A and sphingosine. J. Gen. Physiol. 1994, 103, 471–486.
- Hernandez-Araiza, I.; Morales-Lazaro, S.L.; Canul-Sanchez, J.A.; Islas, L.D.; Rosenbaum, T. Role of lysophosphatidic acid in ion channel function and disease. J. Neurophysiol. 2018, 120, 1198–1211.
- Tokumura, A.; Fukuzawa, K.; Tsukatani, H. Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids 1978, 13, 572–574.
- Mark, K.; Bragg, B.; Chawla, K.; Hladky, K. Medical abortion in women with large uterine fibroids: A case series. Contraception 2016, 94, 572–574.
- Xu, Y.J.; Aziz, O.A.; Bhugra, P.; Arneja, A.S.; Mendis, M.R.; Dhalla, N.S. Potential role of lysophosphatidic acid in hypertension and atherosclerosis. Can. J. Cardiol. 2003, 19, 1525–1536.
- Tokumura, A.; Yube, N.; Fujimoto, H.; Tsukatani, H. Lysophosphatidic acids induce contraction of rat isolated colon by two different mechanisms. J. Pharm. Pharmacol. 1991, 43, 774–778.
- Koschel, K.; Tas, P.W. Lysophosphatidic acid reverts the beta-adrenergic agonist-induced morphological response in C6 rat glioma cells. Exp. Cell Res. 1993, 206, 162–166.
- Chettibi, S.; Lawrence, A.J.; Stevenson, R.D.; Young, J.D. Effect of lysophosphatidic acid on motility, polarisation and metabolic burst of human neutrophils. FEMS Immunol. Med. Microbiol. 1994, 8, 271–281.
- Hill, C.S.; Oh, S.Y.; Schmidt, S.A.; Clark, K.J.; Murray, A.W. Lysophosphatidic acid inhibits gap-junctional communication and stimulates phosphorylation of connexin-43 in WB cells: Possible involvement of the mitogen-activated protein kinase cascade. Biochem. J. 1994, 303 Pt 2, 475–479.
- Zhang, C.; Lambert, M.P.; Bunch, C.; Barber, K.; Wade, W.S.; Krafft, G.A.; Klein, W.L. Focal adhesion kinase expressed by nerve cell lines shows increased tyrosine phosphorylation in response to Alzheimer’s A beta peptide. J. Biol. Chem. 1994, 269, 25247–25250.
- Seufferlein, T.; Rozengurt, E. Lysophosphatidic acid stimulates tyrosine phosphorylation of focal adhesion kinase, paxillin, and p130. Signaling pathways and cross-talk with platelet-derived growth factor. J. Biol. Chem. 1994, 269, 9345–9351.
- Schimmel, R.J.; Honeyman, T.W.; McMahon, K.K.; Serio, R.; Clark, R.B. Inhibition of cyclic AMP accumulation in hamster adipocytes with phosphatidic acid: Differences and similarities with alpha adrenergic effects. J. Cycl. Nucleotide Res. 1980, 6, 437–449.
- Gerrard, J.M.; Beattie, L.L.; McCrae, J.M.; Singhroy, S. The influence of lysophosphatidic acid on platelet protein phosphorylation. Biochem. Cell Biol. 1987, 65, 642–650.
- Ridley, A.J.; Hall, A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992, 70, 389–399.
- Howard, A.D.; McAllister, G.; Feighner, S.D.; Liu, Q.; Nargund, R.P.; Van der Ploeg, L.H.; Patchett, A.A. Orphan G-protein-coupled receptors and natural ligand discovery. Trends Pharmacol. Sci. 2001, 22, 132–140.
- Tabata, K.; Baba, K.; Shiraishi, A.; Ito, M.; Fujita, N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2007, 363, 861–866.
- Ochiai, S.; Furuta, D.; Sugita, K.; Taniura, H.; Fujita, N. GPR87 mediates lysophosphatidic acid-induced colony dispersal in A431 cells. Eur. J. Pharmacol. 2013, 715, 15–20.
- Zhao, P.; Abood, M.E. GPR55 and GPR35 and their relationship to cannabinoid and lysophospholipid receptors. Life Sci. 2013, 92, 453–457.
- Murakami, M.; Shiraishi, A.; Tabata, K.; Fujita, N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem. Biophys. Res. Commun. 2008, 371, 707–712.
- McIntyre, T.M.; Pontsler, A.V.; Silva, A.R.; St Hilaire, A.; Xu, Y.; Hinshaw, J.C.; Zimmerman, G.A.; Hama, K.; Aoki, J.; Arai, H.; et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc. Natl. Acad. Sci. USA 2003, 100, 131–136.
- Tsukahara, T. The Role of PPARgamma in the Transcriptional Control by Agonists and Antagonists. PPAR Res. 2012, 2012, e362361.
- Tsukahara, T. PPAR gamma Networks in Cell Signaling: Update and Impact of Cyclic Phosphatidic Acid. J. Lipids 2013, 2013, e246597.
- Crowder, M.K.; Seacrist, C.D.; Blind, R.D. Phospholipid regulation of the nuclear receptor superfamily. Adv. Biol. Regul. 2017, 63, 6–14.
- Yousefnia, S.; Momenzadeh, S.; Seyed Forootan, F.; Ghaedi, K.; Nasr Esfahani, M.H. The influence of peroxisome proliferator-activated receptor gamma (PPARgamma) ligands on cancer cell tumorigenicity. Gene 2018, 649, 14–22.
- Fan, Q.; Cai, Q.; Li, P.; Wang, W.; Wang, J.; Gerry, E.; Wang, T.L.; Shih, I.M.; Nephew, K.P.; Xu, Y. The novel ZIP4 regulation and its role in ovarian cancer. Oncotarget 2017, 8, 90090–90107.
- Tokumura, A.; Harada, K.; Fukuzawa, K.; Tsukatani, H. Involvement of lysophospholipase D in the production of lysophosphatidic acid in rat plasma. Biochim. Biophys. Acta 1986, 875, 31–38.
- Tigyi, G.J.; Yue, J.; Norman, D.D.; Szabo, E.; Balogh, A.; Balazs, L.; Zhao, G.; Lee, S.C. Regulation of tumor cell—Microenvironment interaction by the autotaxin-lysophosphatidic acid receptor axis. Adv. Biol. Regul. 2019, 71, 183–193.
- Blaho, V.A.; Chun, J. ’Crystal’ clear? lysophospholipid receptor structure insights and controversies. Trends Pharmacol Sci. 2018, 39, 953–966.
- Chun, J. Lysophospholipid receptors: Implications for neural signaling. Crit. Rev. Neurobiol. 1999, 13, 151–168.
- Moolenaar, W.H.; Houben, A.J.; Lee, S.J.; van Meeteren, L.A. Autotaxin in embryonic development. Biochim. Biophys. Acta 2013, 1831, 13–19.
- Ye, X.; Chun, J. Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol. Metab. 2010, 21, 17–24.
- Murph, M.; Tanaka, T.; Pang, J.; Felix, E.; Liu, S.; Trost, R.; Godwin, A.K.; Newman, R.; Mills, G. Liquid chromatography mass spectrometry for quantifying plasma lysophospholipids: Potential biomarkers for cancer diagnosis. Methods Enzymol. 2007, 433, 1–25.
- Yagi, T.; Shoaib, M.; Kuschner, C.; Nishikimi, M.; Becker, L.B.; Lee, A.T.; Kim, J. Challenges and Inconsistencies in Using Lysophosphatidic Acid as a Biomarker for Ovarian Cancer. Cancers 2019, 11, 520.
- Kennerly, D.A. Molecular species analysis of lysophospholipids using high-performance liquid chromatography and argentation thin-layer chromatography. J. Chromatogr. 1987, 409, 291–297.
- Schmidt, R.; Kunze, D.; Egger, E. Two-dimensional thin layer chromatographic separation of phosphatidic acid and lysophosphatidic acid in lipid mixtures. Z. Med. Lab. Diagn. 1978, 19, 306–311.
- Fleming, J.K.; Glass, T.R.; Lackie, S.J.; Wojciak, J.M. A novel approach for measuring sphingosine-1-phosphate and lysophosphatidic acid binding to carrier proteins using monoclonal antibodies and the Kinetic Exclusion Assay. J. Lipid Res. 2016, 57, 1737–1747.
- Eisenried, A.; Meidahl, A.C.N.; Klukinov, M.; Tzabazis, A.Z.; Sabbadini, R.A.; Clark, J.D.; Yeomans, D.C. Nervous system delivery of antilysophosphatidic acid antibody by nasal application attenuates mechanical allodynia after traumatic brain injury in rats. Pain 2017, 158, 2181–2188.
- Shao, Y.; Yu, Y.; He, Y.; Chen, Q.; Liu, H. Serum ATX as a novel biomarker for breast cancer. Medicine (Baltimore) 2019, 98, e14973.
- Masuda, A.; Nakamura, K.; Izutsu, K.; Igarashi, K.; Ohkawa, R.; Jona, M.; Higashi, K.; Yokota, H.; Okudaira, S.; Kishimoto, T.; et al. Serum autotaxin measurement in haematological malignancies: A promising marker for follicular lymphoma. Br. J. Haematol. 2008, 143, 60–70.
- Tokumura, A.; Tominaga, K.; Yasuda, K.; Kanzaki, H.; Kogure, K.; Fukuzawa, K. Lack of significant differences in the corrected activity of lysophospholipase D, producer of phospholipid mediator lysophosphatidic acid, in incubated serum from women with and without ovarian tumors. Cancer 2002, 94, 141–151.
- Yanagida, K.; Ishii, S. Non-Edg family LPA receptors: The cutting edge of LPA research. J. Biochem. 2011, 150, 223–232.
- Zheng, X.; Li, W.; Ren, L.; Liu, J.; Pang, X.; Chen, X.; Kang, D.; Wang, J.; Du, G. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacol. Ther. 2019, 195, 85–99.
- Geffken, K.; Spiegel, S. Sphingosine kinase 1 in breast cancer. Adv. Biol. Regul. 2018, 67, 59–65.
- Friedman, P.; Haimovitz, R.; Markman, O.; Roberts, M.F.; Shinitzky, M. Conversion of lysophospholipids to cyclic lysophosphatidic acid by phospholipase D. J. Biol. Chem. 1996, 271, 953–957.
- Grzelczyk, A.; Koziolkiewicz, M. Cyclic phosphatidic acids and their analogues--unique lipid mediators. Postepy Biochem. 2012, 58, 327–343.
- Murakami-Murofushi, K.; Uchiyama, A.; Fujiwara, Y.; Kobayashi, T.; Kobayashi, S.; Mukai, M.; Murofushi, H.; Tigyi, G. Biological functions of a novel lipid mediator, cyclic phosphatidic acid. Biochim. Biophys. Acta 2002, 1582, 1–7.
- Tsukahara, T.; Matsuda, Y.; Haniu, H. Lysophospholipid-Related Diseases and PPARgamma Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 2730.
- Wykle, R.L.; Malone, B.; Snyder, F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J. Biol. Chem. 1980, 255, 10256–10260.
- Jancar, S.; Chammas, R. PAF receptor and tumor growth. Curr. Drug Targets 2014, 15, 982–987.
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The implication of platelet activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399.
- Aoki, J.; Inoue, A.; Okudaira, S. Two pathways for lysophosphatidic acid production. Biochim. Biophys. Acta 2008, 1781, 513–518.
- Scott, K.F.; Sajinovic, M.; Hein, J.; Nixdorf, S.; Galettis, P.; Liauw, W.; de Souza, P.; Dong, Q.; Graham, G.G.; Russell, P.J. Emerging roles for phospholipase A2 enzymes in cancer. Biochimie 2010, 92, 601–610.
- Liu, N.K.; Deng, L.X.; Zhang, Y.P.; Lu, Q.B.; Wang, X.F.; Hu, J.G.; Oakes, E.; Bonventre, J.V.; Shields, C.B.; Xu, X.M. Cytosolic phospholipase A2 protein as a novel therapeutic target for spinal cord injury. Ann. Neurol. 2014, 75, 644–658.
- Cai, H.; Chiorean, E.G.; Chiorean, M.V.; Rex, D.K.; Robb, B.W.; Hahn, N.M.; Liu, Z.; Loehrer, P.J.; Harrison, M.L.; Xu, Y. Elevated phospholipase A2 activities in plasma samples from multiple cancers. PLoS ONE 2013, 8, e57081.
- Liu, N.K.; Byers, J.S.; Lam, T.; Lu, Q.B.; Sengelaub, D.R.; Xu, X.M. Inhibition of cPLA2 has neuroprotective effects on motoneuron and muscle atrophy following spinal cord injury. J. Neurotrauma 2014.
- Sengupta, S.; Xiao, Y.J.; Xu, Y. A novel laminin-induced LPA autocrine loop in the migration of ovarian cancer cells. FASEB J. 2003, 17, 1570–1572.
- Liu, N.K.; Zhang, Y.P.; Titsworth, W.L.; Jiang, X.; Han, S.; Lu, P.H.; Shields, C.B.; Xu, X.M. A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann. Neurol. 2006, 59, 606–619.
- Shen, Z.; Belinson, J.; Morton, R.E.; Xu, Y.; Xu, Y. Phorbol 12-myristate 13-acetate stimulates lysophosphatidic acid secretion from ovarian and cervical cancer cells but not from breast or leukemia cells. Gynecol. Oncol. 1998, 71, 364–368.
- Leblanc, R.; Houssin, A.; Peyruchaud, O. Platelets, autotaxin and lysophosphatidic acid signalling: Win-win factors for cancer metastasis. Br. J. Pharmacol. 2018, 175, 3100–3110.
- Xu, J.; Zhang, Q.X.; Pilquil, C.; Berthiaume, L.G.; Waggoner, D.W.; Brindley, D.N. Lipid phosphate phosphatase-1 in the regulation of lysophosphatidate signaling. Ann. N. Y. Acad. Sci. 2000, 905, 81–90.
- Tania, M.; Khan, M.A.; Zhang, H.; Li, J.; Song, Y. Autotaxin: A protein with two faces. Biochem. Biophys. Res. Commun. 2010, 401, 493–497.
- Sakane, F.; Mizuno, S.; Takahashi, D.; Sakai, H. Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways. Adv. Biol. Regul. 2018, 67, 101–108.
- Sato, Y.; Murakami, C.; Yamaki, A.; Mizuno, S.; Sakai, H.; Sakane, F. Distinct 1-monoacylglycerol and 2-monoacylglycerol kinase activities of diacylglycerol kinase isozymes. Biochim. Biophys. Acta 2016, 1864, 1170–1176.
- Angkawijaya, A.E.; Nguyen, V.C.; Nakamura, Y. Lysophosphatidic acid acyltransferases 4 and 5 are involved in glycerolipid metabolism and nitrogen starvation response in Arabidopsis. New Phytol. 2019, 224, 336–351.
- Sugimoto, H.; Yamashita, S. Purification, characterization, and inhibition by phosphatidic acid of lysophospholipase transacylase from rat liver. J. Biol. Chem. 1994, 269, 6252–6258.
- Thompson, F.J.; Clark, M.A. Purification of a lysophosphatidic acid-hydrolysing lysophospholipase from rat brain. Biochem. J. 1994, 300, 457–461.
- Nakayama, J.; Raines, T.A.; Lynch, K.R.; Slack-Davis, J.K. Decreased peritoneal ovarian cancer growth in mice lacking expression of lipid phosphate phosphohydrolase 1. PLoS ONE 2015, 10, e0120071.
- Gendaszewska-Darmach, E. Lysophosphatidic acids, cyclic phosphatidic acids and autotaxin as promising targets in therapies of cancer and other diseases. Acta Biochim. Pol. 2008, 55, 227–240.
- Sun, S.; Wang, R.; Song, J.; Guan, M.; Li, N.; Zhang, X.; Zhao, Z.; Zhang, J. Blocking gp130 signaling suppresses autotaxin expression in adipocytes and improves insulin sensitivity in diet-induced obesity. J. Lipid Res. 2017, 58, 2102–2113.
- Surgand, J.S.; Rodrigo, J.; Kellenberger, E.; Rognan, D. A chemogenomic analysis of the transmembrane binding cavity of human G-protein-coupled receptors. Proteins 2006, 62, 509–538.
- Im, D.S. Orphan G protein-coupled receptors and beyond. Jpn. J. Pharmacol. 2002, 90, 101–106.
- Dalesio, N.M.; Barreto Ortiz, S.F.; Pluznick, J.L.; Berkowitz, D.E. Olfactory, Taste, and Photo Sensory Receptors in Non-sensory Organs: It Just Makes Sense. Front. Physiol. 2018, 9, e1673.
- Wise, A.; Jupe, S.C.; Rees, S. The identification of ligands at orphan G-protein coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 43–66.
- Zurawski, Z.; Yim, Y.Y.; Alford, S.; Hamm, H.E. The expanding roles and mechanisms of G protein-mediated presynaptic inhibition. J. Biol. Chem. 2019, 294, 1661–1670.
- McDonald, P.H.; Lefkowitz, R.J. Beta-Arrestins: New roles in regulating heptahelical receptors’ functions. Cell. Signal. 2001, 13, 683–689.
- Svoboda, P.; Teisinger, J.; Novotny, J.; Bourova, L.; Drmota, T.; Hejnova, L.; Moravcova, Z.; Lisy, V.; Rudajev, V.; Stohr, J.; et al. Biochemistry of transmembrane signaling mediated by trimeric G proteins. Physiol. Res. 2004, 53, 141–152.
- Masago, K.; Kihara, Y.; Yanagida, K.; Hamano, F.; Nakagawa, S.; Niwa, M.; Shimizu, T. Lysophosphatidic acid receptor, LPA6, regulates endothelial blood-brain barrier function: Implication for hepatic encephalopathy. Biochem. Biophys. Res. Commun. 2018, 501, 1048–1054.
- Kano, K.; Matsumoto, H.; Inoue, A.; Yukiura, H.; Kanai, M.; Chun, J.; Ishii, S.; Shimizu, T.; Aoki, J. Molecular mechanism of lysophosphatidic acid-induced hypertensive response. Sci. Rep. 2019, 9, e2662.
- Lee, M.; Choi, S.; Hallden, G.; Yo, S.J.; Schichnes, D.; Aponte, G.W. P2Y5 is a G(alpha)i, G(alpha)12/13 G protein-coupled receptor activated by lysophosphatidic acid that reduces intestinal cell adhesion. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, 641–654.
- Fan, Q.; Cai, Q.; Xu, Y. FOXM1 is a downstream target of LPA and YAP oncogenic signaling pathways in high grade serous ovarian cancer. Oncotarget 2015, 6, 27688–27699.
- Cai, H.; Xu, Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun. Signal. 2013, 11, e31.
- Gurevich, E.V.; Gainetdinov, R.R.; Gurevich, V.V. G protein-coupled receptor kinases as regulators of dopamine receptor functions. Pharmacol. Res. 2016, 111, 1–16.
- Spiegel, A.M. Hormone resistance caused by mutations in G proteins and G protein-coupled receptors. J. Pediatr. Endocrinol. Metab. 1999, 12, 303–309.
- Gardella, T.J.; Vilardaga, J.P. International Union of Basic and Clinical Pharmacology. XCIII. The parathyroid hormone receptors--family B G protein-coupled receptors. Pharmacol. Rev. 2015, 67, 310–337.
- Filardo, E.J.; Thomas, P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: Its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 2012, 153, 2953–2962.
- Wolf Horrell, E.M.; Boulanger, M.C.; D’Orazio, J.A. Melanocortin 1 Receptor: Structure, Function, and Regulation. Front. Genet. 2016, 7, e95.
- Marangos, P.J.; Boulenger, J.P. Basic and clinical aspects of adenosinergic neuromodulation. Neurosci. Biobehav. Rev. 1985, 9, 421–430.
- Hamblin, M.W.; Guthrie, C.R.; Kohen, R.; Heidmann, D.E. Gs protein-coupled serotonin receptors: Receptor isoforms and functional differences. Ann. N. Y. Acad. Sci. 1998, 861, 31–37.
- Srinivasan, S.; Vaisse, C.; Conklin, B.R. Engineering the melanocortin-4 receptor to control G(s) signaling in vivo. Ann. N. Y. Acad Sci. 2003, 994, 225–232.
- Malnic, B.; Godfrey, P.A.; Buck, L.B. The human olfactory receptor gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2584–2589.
- Takahashi, K.; Fukushima, K.; Otagaki, S.; Ishimoto, K.; Minami, K.; Fukushima, N.; Honoki, K.; Tsujiuchi, T. Effects of LPA1 and LPA6 on the regulation of colony formation activity in colon cancer cells treated with anticancer drugs. J. Recept. Signal. Transduct. Res. 2018, 38, 71–75.
- Matayoshi, S.; Chiba, S.; Lin, Y.; Arakaki, K.; Matsumoto, H.; Nakanishi, T.; Suzuki, M.; Kato, S. Lysophosphatidic acid receptor 4 signaling potentially modulates malignant behavior in human head and neck squamous cell carcinoma cells. Int. J. Oncol. 2013, 42, 1560–1568.
- Ishii, S.; Hirane, M.; Fukushima, K.; Tomimatsu, A.; Fukushima, N.; Tsujiuchi, T. Diverse effects of LPA4, LPA5 and LPA6 on the activation of tumor progression in pancreatic cancer cells. Biochem. Biophys. Res. Commun. 2015, 461, 59–64.
- Araki, M.; Kitayoshi, M.; Dong, Y.; Hirane, M.; Ozaki, S.; Mori, S.; Fukushima, N.; Honoki, K.; Tsujiuchi, T. Inhibitory effects of lysophosphatidic acid receptor-5 on cellular functions of sarcoma cells. Growth Factors 2014, 32, 117–122.
- Okabe, K.; Hayashi, M.; Yamawaki, Y.; Teranishi, M.; Honoki, K.; Mori, T.; Fukushima, N.; Tsujiuchi, T. Possible involvement of lysophosphatidic acid receptor-5 gene in the acquisition of growth advantage of rat tumor cells. Mol. Carcinog. 2011, 50, 635–642.
- Mathew, D.; Kremer, K.N.; Strauch, P.; Tigyi, G.; Pelanda, R.; Torres, R.M. LPA5 Is an Inhibitory Receptor That Suppresses CD8 T-Cell Cytotoxic Function via Disruption of Early TCR Signaling. Front. Immunol. 2019, 10, e1159.
- Bandoh, K.; Aoki, J.; Taira, A.; Tsujimoto, M.; Arai, H.; Inoue, K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000, 478, 159–165.
- Okudaira, S.; Yukiura, H.; Aoki, J. Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie 2010, 92, 698–706.
- Tsujiuchi, T.; Araki, M.; Hirane, M.; Dong, Y.; Fukushima, N. Lysophosphatidic acid receptors in cancer pathobiology. Histol. Histopathol. 2014, 29, 313–321.
- Yung, Y.C.; Stoddard, N.C.; Mirendil, H.; Chun, J. Lysophosphatidic Acid signaling in the nervous system. Neuron 2015, 85, 669–682.
- Zhou, Y.; Little, P.J.; Ta, H.T.; Xu, S.; Kamato, D. Lysophosphatidic acid and its receptors: Pharmacology and therapeutic potential in atherosclerosis and vascular disease. Pharmacol. Ther. 2019.
- No, Y.R.; He, P.; Yoo, B.K.; Yun, C.C. Regulation of NHE3 by lysophosphatidic acid is mediated by phosphorylation of NHE3 by RSK2. Am. J. Physiol. Cell Physiol. 2015, 309, 14–21.
- Kouba, S.; Ouldamer, L.; Garcia, C.; Fontaine, D.; Chantome, A.; Vandier, C.; Goupille, C.; Potier-Cartereau, M. Lipid metabolism and Calcium signaling in epithelial ovarian cancer. Cell Calcium. 2019, 81, 38–50.
- Song, X.; Zheng, X.; Malbon, C.C.; Wang, H. Galpha i2 enhances in vivo activation of and insulin signaling to GLUT4. J. Biol. Chem. 2001, 276, 34651–34658.
- Pyne, N.J.; Waters, C.; Moughal, N.A.; Sambi, B.S.; Pyne, S. Receptor tyrosine kinase-GPCR signal complexes. Biochem. Soc. Trans. 2003, 31, 1220–1225.
- Boerner, J.L.; Biscardi, J.S.; Silva, C.M.; Parsons, S.J. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol. Carcinog. 2005, 44, 262–273.
- Obara, Y.; Okano, Y.; Ono, S.; Yamauchi, A.; Hoshino, T.; Kurose, H.; Nakahata, N. Betagamma subunits of G(i/o) suppress EGF-induced ERK5 phosphorylation, whereas ERK1/2 phosphorylation is enhanced. Cell. Signal. 2008, 20, 1275–1283.
- Wang, Z. Transactivation of Epidermal Growth Factor Receptor by G Protein-Coupled Receptors: Recent Progress, Challenges and Future Research. Int. J. Mol. Sci. 2016, 17, 95.
- Hopkins, M.M.; Liu, Z.; Meier, K.E. Positive and Negative Cross-Talk between Lysophosphatidic Acid Receptor 1, Free Fatty Acid Receptor 4, and Epidermal Growth Factor Receptor in Human Prostate Cancer Cells. J. Pharmacol. Exp. Ther. 2016, 359, 124–133.
- Harper, K.; Lavoie, R.R.; Charbonneau, M.; Brochu-Gaudreau, K.; Dubois, C.M. The Hypoxic Tumor Microenvironment Promotes Invadopodia Formation and Metastasis through LPA1 Receptor and EGFR Cooperation. Mol. Cancer Res. 2018, 16, 1601–1613.
- Gschwind, A.; Prenzel, N.; Ullrich, A. Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res. 2002, 62, 6329–6336.
- Baudhuin, L.M.; Jiang, Y.; Zaslavsky, A.; Ishii, I.; Chun, J.; Xu, Y. S1P3-mediated Akt activation and cross-talk with platelet-derived growth factor receptor (PDGFR). FASEB J. 2004, 18, 341–343.
- Hellberg, C.; Schmees, C.; Karlsson, S.; Ahgren, A.; Heldin, C.H. Activation of protein kinase C alpha is necessary for sorting the PDGF beta-receptor to Rab4a-dependent recycling. Mol. Biol. Cell 2009, 20, 2856–2863.
- Goppelt-Struebe, M.; Fickel, S.; Reiser, C.O. The platelet-derived-growth-factor receptor, not the epidermal-growth-factor receptor, is used by lysophosphatidic acid to activate p42/44 mitogen-activated protein kinase and to induce prostaglandin G/H synthase-2 in mesangial cells. Biochem. J. 2000, 345, 217–224.
- Herrlich, A.; Daub, H.; Knebel, A.; Herrlich, P.; Ullrich, A.; Schultz, G.; Gudermann, T. Ligand-independent activation of platelet-derived growth factor receptor is a necessary intermediate in lysophosphatidic, acid-stimulated mitogenic activity in L cells. Proc. Natl. Acad. Sci. USA 1998, 95, 8985–8990.
- Wang, L.; Cummings, R.; Zhao, Y.; Kazlauskas, A.; Sham, J.K.; Morris, A.; Georas, S.; Brindley, D.N.; Natarajan, V. Involvement of phospholipase D2 in lysophosphatidate-induced transactivation of platelet-derived growth factor receptor-beta in human bronchial epithelial cells. J. Biol. Chem. 2003, 278, 39931–39940.
- Nan, L.; Wei, J.; Jacko, A.M.; Culley, M.K.; Zhao, J.; Natarajan, V.; Ma, H.; Zhao, Y. Cross-talk between lysophosphatidic acid receptor 1 and tropomyosin receptor kinase A promotes lung epithelial cell migration. Biochim. Biophys. Acta 2016, 1863, 229–235.
- Abdulkhalek, S.; Guo, M.; Amith, S.R.; Jayanth, P.; Szewczuk, M.R. G-protein coupled receptor agonists mediate Neu1 sialidase and matrix metalloproteinase-9 cross-talk to induce transactivation of TOLL-like receptors and cellular signaling. Cell. Signal. 2012, 24, 2035–2042.
- Zhao, Y.; He, D.; Stern, R.; Usatyuk, P.V.; Spannhake, E.W.; Salgia, R.; Natarajan, V. Lysophosphatidic acid modulates c-Met redistribution and hepatocyte growth factor/c-Met signaling in human bronchial epithelial cells through PKC delta and E-cadherin. Cell. Signal. 2007, 19, 2329–2338.
- Madhusoodanan, K.S.; Guo, D.; McGarrigle, D.K.; Maack, T.; Huang, X.Y. Csk mediates G-protein-coupled lysophosphatidic acid receptor-induced inhibition of membrane-bound guanylyl cyclase activity. Biochemistry 2006, 45, 3396–3403.
- Muller, D.; Cortes-Dericks, L.; Budnik, L.T.; Brunswig-Spickenheier, B.; Pancratius, M.; Speth, R.C.; Mukhopadhyay, A.K.; Middendorff, R. Homologous and lysophosphatidic acid-induced desensitization of the atrial natriuretic peptide receptor, guanylyl cyclase-A, in MA-10 leydig cells. Endocrinology 2006, 147, 2974–2985.
- Abbey, S.E.; Potter, L.R. Lysophosphatidic acid inhibits C-type natriuretic peptide activation of guanylyl cyclase-B. Endocrinology 2003, 144, 240–246.
- Zhao, Y.; He, D.; Saatian, B.; Watkins, T.; Spannhake, E.W.; Pyne, N.J.; Natarajan, V. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J. Biol. Chem. 2006, 281, 19501–19511.
- Dikic, I.; Tokiwa, G.; Lev, S.; Courtneidge, S.A.; Schlessinger, J. A role for Pyk2 and Src in linking G-protein-coupled receptors with MAP kinase activation. Nature 1996, 383, 547–550.
- Jia, W.; Tran, S.K.; Ruddick, C.A.; Murph, M.M. The Src homology 3 binding domain is required for lysophosphatidic acid 3 receptor-mediated cellular viability in melanoma cells. Cancer Lett. 2015, 356, 589–596.
- Ha, J.H.; Ward, J.D.; Radhakrishnan, R.; Jayaraman, M.; Song, Y.S.; Dhanasekaran, D.N. Lysophosphatidic acid stimulates epithelial to mesenchymal transition marker Slug/Snail2 in ovarian cancer cells via Galphai2, Src, and HIF1alpha signaling nexus. Oncotarget 2016, 7, 37664–37679.
- Seo, J.H.; Jeong, K.J.; Oh, W.J.; Sul, H.J.; Sohn, J.S.; Kim, Y.K.; Cho, D.Y.; Kang, J.K.; Park, C.G.; Lee, H.Y. Lysophosphatidic acid induces STAT3 phosphorylation and ovarian cancer cell motility: Their inhibition by curcumin. Cancer Lett. 2010, 288, 50–56.
- Hopkins, M.M.; Zhang, Z.; Liu, Z.; Meier, K.E. Eicosopentaneoic Acid and Other Free Fatty Acid Receptor Agonists Inhibit Lysophosphatidic Acid- and Epidermal Growth Factor-Induced Proliferation of Human Breast Cancer Cells. J. Clin. Med. 2016, 5, 16.
- Ren, Y.; Pan, C.; Wu, Q.; Pang, Y.; Tang, C.; Qi, Y. [Involvement of endothelin in the proliferative effect of lysophosphatidic acid on vascular smooth muscle cells in rats]. Beijing Da Xue Xue Bao Yi Xue Ban 2003, 35, 508–511.
- Shumay, E.; Tao, J.; Wang, H.Y.; Malbon, C.C. Lysophosphatidic acid regulates trafficking of beta2-adrenergic receptors: The Galpha13/p115RhoGEF/JNK pathway stimulates receptor internalization. J. Biol. Chem. 2007, 282, 21529–21541.
- Garcia-Sainz, J.A.; Vazquez-Cuevas, F.G.; Romero-Avila, M.T. Phosphorylation and desensitization of alpha1d-adrenergic receptors. Biochem. J. 2001, 353, 603–610.
- Apaydin, S.; Oktem, H.A. Modulatory role of lysophosphatidic acid on opioid receptor binding. Neurobiology (Bp.) 1998, 6, 421–427.
- Callihan, P.; Ali, M.W.; Salazar, H.; Quach, N.; Wu, X.; Stice, S.L.; Hooks, S.B. Convergent regulation of neuronal differentiation and Erk and Akt kinases in human neural progenitor cells by lysophosphatidic acid, sphingosine 1-phosphate, and LIF: Specific roles for the LPA1 receptor. ASN Neuro 2014, 6, e1759091414558416.
- Shida, D.; Fang, X.; Kordula, T.; Takabe, K.; Lepine, S.; Alvarez, S.E.; Milstien, S.; Spiegel, S. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 2008, 68, 6569–6577.
- Nakanaga, K.; Hama, K.; Kano, K.; Sato, T.; Yukiura, H.; Inoue, A.; Saigusa, D.; Tokuyama, H.; Tomioka, Y.; Nishina, H.; et al. Overexpression of autotaxin, a lysophosphatidic acid-producing enzyme, enhances cardia bifida induced by hypo-sphingosine-1-phosphate signaling in zebrafish embryo. J. Biochem. 2014, 155, 235–241.
- Watterson, K.R.; Lanning, D.A.; Diegelmann, R.F.; Spiegel, S. Regulation of fibroblast functions by lysophospholipid mediators: Potential roles in wound healing. Wound Repair Regen. 2007, 15, 607–616.
- Wu, J.; Mukherjee, A.; Lebman, D.A.; Fang, X. Lysophosphatidic acid-induced p21Waf1 expression mediates the cytostatic response of breast and ovarian cancer cells to TGFbeta. Mol. Cancer Res. 2011, 9, 1562–1570.
- Rodriguez Perez, C.E.; Nie, W.; Sinnett-Smith, J.; Rozengurt, E.; Yoo, J. TNF-alpha potentiates lysophosphatidic acid-induced COX-2 expression via PKD in human colonic myofibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, 637–646.
- Hisano, Y.; Kono, M.; Cartier, A.; Engelbrecht, E.; Kano, K.; Kawakami, K.; Xiong, Y.; Piao, W.; Galvani, S.; Yanagida, K.; et al. Lysolipid receptor cross-talk regulates lymphatic endothelial junctions in lymph nodes. J. Exp. Med. 2019, 216, 1582–1598.
- Sakai, T.; Peyruchaud, O.; Fassler, R.; Mosher, D.F. Restoration of beta1A integrins is required for lysophosphatidic acid-induced migration of beta1-null mouse fibroblastic cells. J. Biol. Chem. 1998, 273, 19378–19382.
- Xu, M.Y.; Porte, J.; Knox, A.J.; Weinreb, P.H.; Maher, T.M.; Violette, S.M.; McAnulty, R.J.; Sheppard, D.; Jenkins, G. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q). Am. J. Pathol. 2009, 174, 1264–1279.
- Xu, M.; Yin, H.; Cai, Y.; Huang, W.; Ji, Q.; Liu, F.; Shi, S.; Deng, X. Lysophosphatidic acid induces integrin beta6 expression in human oral squamous cell carcinomas cells via LPAR1 coupling to Galphai and downstream SMAD3 and ETS-1 activation. Cell. Signal. 2019, 60, 81–90.
- Valenick, L.V.; Schwarzbauer, J.E. Ligand density and integrin repertoire regulate cellular response to LPA. Matrix Biol. 2006, 25, 223–231.
- Waters, C.M.; Saatian, B.; Moughal, N.A.; Zhao, Y.; Tigyi, G.; Natarajan, V.; Pyne, S.; Pyne, N.J. Integrin signalling regulates the nuclear localization and function of the lysophosphatidic acid receptor-1 (LPA1) in mammalian cells. Biochem. J. 2006, 398, 55–62.
- Walsh, C.T.; Stupack, D.; Brown, J.H. G protein-coupled receptors go extracellular: RhoA integrates the integrins. Mol. Interv. 2008, 8, 165–173.
- Chen, M.; O’Connor, K.L. Integrin alpha6beta4 promotes expression of autotaxin/ENPP2 autocrine motility factor in breast carcinoma cells. Oncogene 2005, 24, 5125–5130.
- Perrakis, A.; Moolenaar, W.H. Autotaxin: Structure-function and signaling. J. Lipid Res. 2014, 55, 1010–1018.
- Zhao, Y.; Hasse, S.; Zhao, C.; Bourgoin, S.G. Targeting the autotaxin—Lysophosphatidic acid receptor axis in cardiovascular diseases. Biochem. Pharmacol. 2019, 164, 74–81.
- Hu, J.; Oda, S.K.; Shotts, K.; Donovan, E.E.; Strauch, P.; Pujanauski, L.M.; Victorino, F.; Al-Shami, A.; Fujiwara, Y.; Tigyi, G.; et al. Lysophosphatidic acid receptor 5 inhibits B cell antigen receptor signaling and antibody response. J. Immunol. 2014, 193, 85–95.
- Rubenfeld, J.; Guo, J.; Sookrung, N.; Chen, R.; Chaicumpa, W.; Casolaro, V.; Zhao, Y.; Natarajan, V.; Georas, S. Lysophosphatidic acid enhances interleukin-13 gene expression and promoter activity in T cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, 66–74.
- Snider, A.J.; Zhang, Z.; Xie, Y.; Meier, K.E. Epidermal growth factor increases lysophosphatidic acid production in human ovarian cancer cells: Roles for phospholipase D2 and receptor transactivation. Am. J. Physiol. Cell Physiol. 2010, 298, 163–170.
- Moughal, N.A.; Waters, C.; Sambi, B.; Pyne, S.; Pyne, N.J. Nerve growth factor signaling involves interaction between the Trk A receptor and lysophosphatidate receptor 1 systems: Nuclear translocation of the lysophosphatidate receptor 1 and Trk A receptors in pheochromocytoma 12 cells. Cell. Signal. 2004, 16, 127–136.
- Lidgerwood, G.E.; Pitson, S.M.; Bonder, C.; Pebay, A. Roles of lysophosphatidic acid and sphingosine-1-phosphate in stem cell biology. Prog. Lipid Res. 2018, 72, 42–54.
- Wang, X.; Huai, G.; Wang, H.; Liu, Y.; Qi, P.; Shi, W.; Peng, J.; Yang, H.; Deng, S.; Wang, Y. Mutual regulation of the Hippo/Wnt/LPA/TGFbeta signaling pathways and their roles in glaucoma (Review). Int. J. Mol. Med. 2018, 41, 1201–1212.
- Yasuda, D.; Kobayashi, D.; Akahoshi, N.; Ohto-Nakanishi, T.; Yoshioka, K.; Takuwa, Y.; Mizuno, S.; Takahashi, S.; Ishii, S. Lysophosphatidic acid-induced YAP/TAZ activation promotes developmental angiogenesis by repressing Notch ligand Dll4. J. Clin. Investig. 2019, 129, 4332–4349.
- Ren, Z.; Zhang, C.; Ma, L.; Zhang, X.; Shi, S.; Tang, D.; Xu, J.; Hu, Y.; Wang, B.; Zhang, F.; et al. Lysophosphatidic acid induces the migration and invasion of SGC-7901 gastric cancer cells through the LPA2 and Notch signaling pathways. Int. J. Mol. Med. 2019, 44, 67–78.
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791.
- Zaslavsky, A.; Singh, L.S.; Tan, H.; Ding, H.; Liang, Z.; Xu, Y. Homo- and hetero-dimerization of LPA/S1P receptors, OGR1 and GPR4. Biochim. Biophys. Acta 2006, 1761, 1200–1212.
- Liu, J.P.; Komachi, M.; Tomura, H.; Mogi, C.; Damirin, A.; Tobo, M.; Takano, M.; Nochi, H.; Tamoto, K.; Sato, K.; et al. Ovarian cancer G protein-coupled receptor 1-dependent and -independent vascular actions to acidic pH in human aortic smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, 731–742.
- Colin-Santana, C.C.; Avendano-Vazquez, S.E.; Alcantara-Hernandez, R.; Garcia-Sainz, J.A. EGF and angiotensin II modulate lysophosphatidic acid LPA(1) receptor function and phosphorylation state. Biochim. Biophys. Acta 2011, 1810, 1170–1177.
- Rodland, K.D.; Bollinger, N.; Ippolito, D.; Opresko, L.K.; Coffey, R.J.; Zangar, R.; Wiley, H.S. Multiple mechanisms are responsible for transactivation of the epidermal growth factor receptor in mammary epithelial cells. J. Biol. Chem. 2008, 283, 31477–31487.
- Lu, J.; Xiao Yj, Y.J.; Baudhuin, L.M.; Hong, G.; Xu, Y. Role of ether-linked lysophosphatidic acids in ovarian cancer cells. J. Lipid Res. 2002, 43, 463–476.
- Chen, S.U.; Chou, C.H.; Chao, K.H.; Lee, H.; Lin, C.W.; Lu, H.F.; Yang, Y.S. Lysophosphatidic acid up-regulates expression of growth-regulated oncogene-alpha, interleukin-8, and monocyte chemoattractant protein-1 in human first-trimester trophoblasts: Possible roles in angiogenesis and immune regulation. Endocrinology 2010, 151, 369–379.
- Sivashanmugam, P.; Tang, L.; Daaka, Y. Interleukin 6 mediates the lysophosphatidic acid-regulated cross-talk between stromal and epithelial prostate cancer cells. J. Biol. Chem. 2004, 279, 21154–21159.
- Sun, B.; Nishihira, J.; Suzuki, M.; Fukushima, N.; Ishibashi, T.; Kondo, M.; Sato, Y.; Todo, S. Induction of macrophage migration inhibitory factor by lysophosphatidic acid: Relevance to tumor growth and angiogenesis. Int. J. Mol. Med. 2003, 12, 633–641.
- Mihara, M.; Hashizume, M.; Yoshida, H.; Suzuki, M.; Shiina, M. IL-6/IL-6 receptor system and its role in physiological and pathological conditions. Clin. Sci. (Lond.) 2012, 122, 143–159.
- Seo, E.J.; Kwon, Y.W.; Jang, I.H.; Kim, D.K.; Lee, S.I.; Choi, E.J.; Kim, K.H.; Suh, D.S.; Lee, J.H.; Choi, K.U.; et al. Autotaxin Regulates Maintenance of Ovarian Cancer Stem Cells through Lysophosphatidic Acid-Mediated Autocrine Mechanism. Stem Cells 2016, 34, 551–564.
- Fan, Q.; Cai, Q.; Xu, Y. LPA Regulates SOX9 in Ovarian Cancer Cells; Gavin Publishers: Lisle, IL, USA, 2017.
- Yart, A.; Chap, H.; Raynal, P. Phosphoinositide 3-kinases in lysophosphatidic acid signaling: Regulation and cross-talk with the Ras/mitogen-activated protein kinase pathway. Biochim. Biophys. Acta 2002, 1582, 107–111.
- Daub, H.; Wallasch, C.; Lankenau, A.; Herrlich, A.; Ullrich, A. Signal characteristics of G protein-transactivated EGF receptor. EMBO J. 1997, 16, 7032–7044.
- Castelino, F.V.; Varga, J. Emerging cellular and molecular targets in fibrosis: Implications for scleroderma pathogenesis and targeted therapy. Curr. Opin. Rheumatol. 2014, 26, 607–614.
- Li, L.; Tam, L.; Liu, L.; Jin, T.; Ng, D.S. Wnt-signaling mediates the anti-adipogenic action of lysophosphatidic acid through cross talking with the Rho/Rho associated kinase (ROCK) pathway. Biochem. Cell Biol. 2011, 89, 515–521.
- Chen, Y.; Wang, Y.; Yu, H.; Wang, F.; Xu, W. The cross talk between protein kinase A- and RhoA-mediated signaling in cancer cells. Exp. Biol. Med. (Maywood) 2005, 230, 731–741.
- Farooqui, A.A.; Ong, W.Y.; Farooqui, T. Lipid mediators in the nucleus: Their potential contribution to Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 906–916.
- Bektas, M.; Payne, S.G.; Liu, H.; Goparaju, S.; Milstien, S.; Spiegel, S. A novel acylglycerol kinase that produces lysophosphatidic acid modulates cross talk with EGFR in prostate cancer cells. J. Cell Biol. 2005, 169, 801–811.
- Wong, J.L.; Obermajer, N.; Odunsi, K.; Edwards, R.P.; Kalinski, P. Synergistic COX2 Induction by IFNgamma and TNFalpha Self-Limits Type-1 Immunity in the Human Tumor Microenvironment. Cancer Immunol. Res. 2016, 4, 303–311.
- Reinartz, S.; Finkernagel, F.; Adhikary, T.; Rohnalter, V.; Schumann, T.; Schober, Y.; Nockher, W.A.; Nist, A.; Stiewe, T.; Jansen, J.M.; et al. A transcriptome-based global map of signaling pathways in the ovarian cancer microenvironment associated with clinical outcome. Genome Biol. 2016, 17, e108.
- Brown, A.; Hossain, I.; Perez, L.J.; Nzirorera, C.; Tozer, K.; D’Souza, K.; Trivedi, P.C.; Aguiar, C.; Yip, A.M.; Shea, J.; et al. Lysophosphatidic acid receptor mRNA levels in heart and white adipose tissue are associated with obesity in mice and humans. PLoS ONE 2017, 12, e0189402.
- Takara, K.; Eino, D.; Ando, K.; Yasuda, D.; Naito, H.; Tsukada, Y.; Iba, T.; Wakabayashi, T.; Muramatsu, F.; Kidoya, H.; et al. Lysophosphatidic Acid Receptor 4 Activation Augments Drug Delivery in Tumors by Tightening Endothelial Cell-Cell Contact. Cell Rep. 2017, 20, 2072–2086.
- Sordelli, M.S.; Beltrame, J.S.; Zotta, E.; Gomez, N.; Dmytrenko, G.; Sales, M.E.; Blois, S.M.; Davio, C.; Martinez, S.P.; Franchi, A.M.; et al. Endogenous lysophosphatidic acid participates in vascularisation and decidualisation at the maternal-fetal interface in the rat. Reprod. Fertil. Dev. 2017, 29, 2112–2126.
- Cai, J.; Wei, J.; Li, S.; Suber, T.; Zhao, J. AM966, an Antagonist of Lysophosphatidic Acid Receptor 1, Increases Lung Microvascular Endothelial Permeability through Activation of Rho Signaling Pathway and Phosphorylation of VE-Cadherin. Mediat. Inflamm. 2017, 2017, e6893560.
- Ptaszynska, M.M.; Pendrak, M.L.; Stracke, M.L.; Roberts, D.D. Autotaxin signaling via lysophosphatidic acid receptors contributes to vascular endothelial growth factor-induced endothelial cell migration. Mol. Cancer Res. 2010, 8, 309–321.
- Mu, H.; Calderone, T.L.; Davies, M.A.; Prieto, V.G.; Wang, H.; Mills, G.B.; Bar-Eli, M.; Gershenwald, J.E. Lysophosphatidic acid induces lymphangiogenesis and IL-8 production in vitro in human lymphatic endothelial cells. Am. J. Pathol. 2012, 180, 2170–2181.
- Ray, R.; Rai, V. Lysophosphatidic acid converts monocytes into macrophages in both mice and humans. Blood 2017, 129, 1177–1183.
- Clair, T.; Aoki, J.; Koh, E.; Bandle, R.W.; Nam, S.W.; Ptaszynska, M.M.; Mills, G.B.; Schiffmann, E.; Liotta, L.A.; Stracke, M.L. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 2003, 63, 5446–5453.
- Susanto, O.; Koh, Y.W.H.; Morrice, N.; Tumanov, S.; Thomason, P.A.; Nielson, M.; Tweedy, L.; Muinonen-Martin, A.J.; Kamphorst, J.J.; Mackay, G.M.; et al. LPP3 mediates self-generation of chemotactic LPA gradients by melanoma cells. J. Cell Sci. 2017, 130, 3455–3466.




