
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maxime De Rudder | + 4027 word(s) | 4027 | 2021-08-04 03:51:33 | | | |
| 2 | Maxime De Rudder | -21 word(s) | 4006 | 2021-08-17 10:34:48 | | | | |
| 3 | Peter Tang | + 42 word(s) | 4048 | 2021-08-17 11:14:59 | | |
Video Upload Options
Liver sinusoids are lined by liver sinusoidal endothelial cells (LSEC), which represent approximately 15 to 20% of the liver cells, but only 3% of the total liver volume. LSEC have unique functions, such as fluid filtration, blood vessel tone modulation, blood clotting, inflammatory cell recruitment, and metabolite and hormone trafficking. Different subtypes of liver endothelial cells are also known to control liver zonation and hepatocyte function. The liver has the exceptional ability to regenerate from small remnants. The past decades have seen increasing awareness in the role of non-parenchymal cells in liver regeneration despite not being the most represented population. While a lot of knowledge has emerged, clarification is needed regarding the role of LSEC in sensing shear stress and on their participation in the inductive phase of regeneration by priming the hepatocytes and delivering mitogenic factors. It is also unclear if bone marrow-derived LSEC participate in the proliferative phase of liver regeneration. Similarly, data are scarce as to LSEC having a role in the termination phase of the regeneration process. Here, we review what is known about the interaction between LSEC and other liver cells during the different phases of liver regeneration. We next explain extended hepatectomy and small liver transplantation, which lead to “small for size syndrome” (SFSS), a lethal liver failure. SFSS is linked to endothelial denudation, necrosis, and lobular disturbance. Using the knowledge learned from partial hepatectomy studies on LSEC, we expose several techniques that are, or could be, used to avoid the “small for size syndrome” after extended hepatectomy or small liver transplantation.
1. Introduction
The extraordinary ability of the liver to regenerate has been known since the Antiquity. The cellular and molecular mechanisms supporting regeneration have being intensely studied for decades. Yet, understanding how the process is fine-tuned to maintain an appropriate cell mass, cell composition and cell organization for an efficient function during lifetime homeostasis and wound healing remains a mystery. Hepatocytes, which accomplish numerous metabolic functions, represent 60% of all liver cells and account for 80% of the liver mass [1]. A large bulk of them, mainly midzonal hepatocytes [2], enter the replicative program when liver mass abruptly decreases as after toxic, ischemic, or viral insults or after surgical removal of part of the organ to restore volume and function. Kupffer cells (KC) and other hepatic immune cells, hepatic stellate cells (HSC), cholangiocytes, and LSEC interact with hepatocytes to support hepatocyte regeneration and ensure a functional structure of the lobule [3][4][5][6][7].
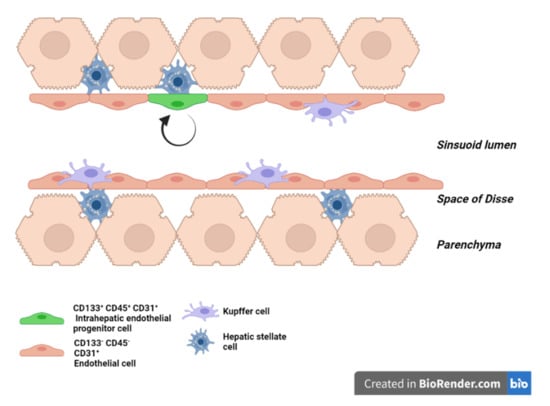
2. LSEC Renewal during Homeostasis
3. LSEC during Regeneration after Partial Hepatectomy
3.1. LSEC during the Inductive Phase of Liver Regeneration
3.1.1. How Do LSEC Modulate Hepatocyte Regeneration
3.1.2. How Do LSEC Interact with Hepatocytes, NPC’s and Circulating Progenitors
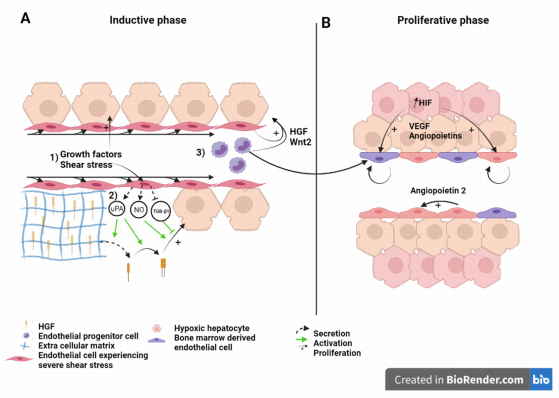
Figure 2. Liver sinusoid and endothelial cells during the (A) inductive phase and (B) angiogenic phase of liver regeneration after partial hepatectomy. Scheme on the role of LSEC during regeneration after partial hepatectomy during the inductive phase (A) where (1) growth factors, (2) increased shear stress, as well as (3) bone marrow endothelial progenitors induce the proliferation of the hepatocytes. This is also overused during the proliferative phase (B), where LSEC upregulate angiopoietin-2 paracrine secretion and proliferating hepatocytes, which experience a relative hypoxia, secrete pro-angiogenic factors to induce the proliferation of LSEC. Recruited endothelial progenitor cells become LSEC during regeneration. HGF: Hepatic growth factor; NO: Nitric oxide; TGB-β1: Tumor growth factor beta 1; Wnt2: Wingless-type MMTV integration site family, member 2; HIF: Hypoxia inducible factor; VEGF: Vascular endothelial growth factor.
3.2. LSEC during the Angiogenic Phase of Liver Regeneration
During the angiogenic phase, LSEC upregulates the expression of angiopoietin-2. This pro-angiogenic factor indirectly stimulates LSEC proliferation in a paracrine manner through upregulation of VEGFR2 [58] (Figure 2). VEGFR2 is the main mediator of VEGF signal during liver regeneration [8]. Hepatocytes engaged in cell cycle or that newly completed cell division also express pro-angiogenic factors, mainly VEGF and angiopoietins, that subsequently stimulate a pro-angiogenic response characterized by DNA synthesis and cell duplication of LSEC [59][60][61]. Hepatocytes, which number has increased upon cell division, experience relative hypoxia that engage the hypoxia-inducible factor (HIF) pathway and the downstream production of pro-angiogenic factors [62] (Figure 2B). Subsequently, endothelial cell proliferation leads to the elongation of the sinusoidal network. While hepatocyte replication reaches its maximum 24 to 48 h after hepatectomy in rats, and mice, respectively, LSEC proliferation peaks at post-surgery day 3 to 4 in rodents.
3.3. LSEC during the Termination Phase of Liver Regeneration
4. Role of LSEC in Extended Hepatectomy
4.1. What Causes Mortality after Extended Hepatectomy?
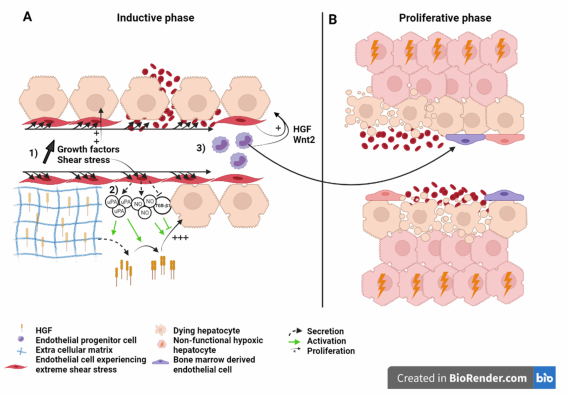
Figure 3. Liver sinusoid and endothelial cells during the (A) inductive phase and (B) angiogenic phase of liver regeneration after extended hepatectomy.Scheme on the role of LSEC during regeneration after extended hepatectomy during the inductive phase (A) where (1) growth factors, (2) severe shear stress, as well as (3) bone marrow endothelial progenitors induce the proliferation of the hepatocytes. Severe shear stress induces sinusoidal denudation and hemorrhage. During the proliferative phase (B), non-functional hypoxic hepatocytes and hemorrhage-induced necrosis lead to organ function insufficiency and PHLF. Recruited endothelial progenitor cells become LSEC during regeneration. HGF: Hepatic growth factor; NO: Nitric oxide; TGB-β1: Tumor growth factor beta 1; Wnt2: Wingless-type MMTV integration site family, member 2; HIF: Hypoxia inducible factor; VEGF: Vascular endothelial growth factor.
4.2. Liver Failure Because of Sinusoid Insufficiency?
4.3. Effect of the Modulation of Portal Hyperflow and Shear Stress after Extended Hepatectomy
5. Conclusions
Due to their location in the liver lobule, interposed between blood stream and hepatocytes, embraced by hepatic stellate cells and in physical contact with Kupffer cells, LSEC interact with and integrate an array of information from the environment. In this review, we presented research supporting the critical role of LSEC during liver regeneration. LSEC are necessary for the proliferation of hepatocyte and for the maintenance of an organized architecture of the lobule. Bone marrow- derived and native LSEC cooperate to play a role in the initiation, proliferative and termination phases of liver regeneration. The process becomes non-operational upon extended hepatectomy. Extreme and brutal increase in portal pressure leads to endothelial denudation with subsequent tissue necrosis and disturbance of the lobule structure. Regenerating hepatocytes do not have an organized vascular network along with which to align. Therefore, their function is compromised and leads to organ failure. The essential role of LSEC in liver regeneration designate them as attractive targets in reducing mortality. Surgical procedures and pharmaceutical treatments that decrease portal pressure also maintain the conventional lobular architecture with great results with respect to survival, both in animal and clinical studies. The need for a competent sinusoidal network to ensure proper function during regeneration supports the major role of LSEC and encourages more research targeting LSEC in liver regeneration.
References
- Stanger, B.Z. Cellular Homeostasis and Repair in the Mammalian Liver. Annu. Rev. Physiol. 2015, 77, 179–200.
- Wei, Y.; Wang, Y.G.; Jia, Y.; Li, L.; Yoon, J.; Zhang, S.; Wang, Z.; Zhang, Y.; Zhu, M.; Sharma, T.; et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes. Science 2021, 371, eabb1625.
- Michalopoulos, G.K. Liver regeneration. J. Cell. Physiol. 2007, 213, 286–300.
- Preziosi, M.E.; Monga, S.P. Update on the Mechanisms of Liver Regeneration. Semin. Liver Dis. 2017, 37, 141–151.
- Michalopoulos, G.K.; DeFrances, M.C.; Cressman, D.E.; Greenbaum, L.E.; DeAngelis, R.A.; Ciliberto, G.; Furth, E.E.; Poli, V.; Taub, R. Liver Regeneration. Science 1997, 276, 60–66.
- Fausto, N. Liver regeneration. J. Hepatol. 2000, 32, 19–31.
- Fausto, N.; Campbell, J.S.; Riehle, K.J. Liver regeneration. Hepatology 2006, 43, S45–S53.
- Poisson, J.; Lemoinne, S.; Boulanger, C.M.; Durand, F.; Moreau, R.; Valla, D.; Rautou, P.-E. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J. Hepatol. 2017, 66, 212–227.
- Wang, L.; Wang, X.; Xie, G.; Wang, L.; Hill, C.K.; Deleve, L.D. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J. Clin. Investig. 2012, 122, 1567–1573.
- Deleve, L.D. Liver sinusoidal endothelial cells and liver regeneration. J. Clin. Investig. 2013, 123, 1861–1866.
- Sakamoto, T.; Liu, Z.; Murase, N.; Ezure, T.; Yokomuro, S.; Poli, V.; Demetris, A.J. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology 1999, 29, 403–411.
- Sato, Y.; Koyama, S.; Tsukada, K.; Hatakeyama, K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg. Today 1997, 27, 518–526.
- Schoen, J.M.; Wangb, H.H.; Minukac, G.Y.; Lautt, W. Shear Stress-Induced Nitric Oxide Release Triggers the Liver Regeneration Cascade. Nitric Oxide 2001, 5, 453–464.
- Albinsson, S.; Hellstrand, P. Integration of signal pathways for stretch-dependent growth and differentiation in vascular smooth muscle. Am. J. Physiol. Cell Physiol. 2007, 293, C772–C782.
- Weinbaum, S.; Zhang, X.; Han, Y.; Vink, H.; Cowin, S.C. Mechanotransduction and flow across the endothelial glycocalyx. Proc. Natl. Acad. Sci. USA 2003, 100, 7988–7995.
- Gracia-Sancho, J.; Russo, L.; García-Calderó, H.; Garcia-Pagan, J.C.; García-Cardeña, G.; Bosch, J. Endothelial expression of transcription factor Kruppel-like factor 2 and its vasoprotective target genes in the normal and cirrhotic rat liver. Gut 2010, 60, 517–524.
- Díaz-Juárez, J.A.; Hernández-Muñoz, R. Rat Liver Enzyme Release Depends on Blood Flow-Bearing Physical Forces Acting in Endothelium Glycocalyx rather than on Liver Damage. Oxidative Med. Cell. Longev. 2017, 2017, 1360565.
- Schoen, J.M.; Lautt, W.W. Nitric Oxide Potentiates C-Fos mRNA Expression after 2/3 Partial Hepatectomy. Proc. West. Pharmacol. Soc. 2002, 45, 47–48.
- Isomura, H.; Sawada, N.; Nakajima, Y.; Sakamoto, H.; Ikeda, T.; Kojima, T.; Enomoto, K.; Mori, M. Increase in portal flow induces c-myc expression in isolated perfused rat liver. J. Cell. Physiol. 1993, 154, 329–332.
- Gan, L.-M.; Doroudi, R.; Hägg, U.; Johansson, A.-M.; Selin-Sjögren, L.; Jern, S. Differential immediate-early gene responses to shear stress and intraluminal pressure in intact human conduit vessels. FEBS Lett. 2000, 477, 89–94.
- Lauber, D.T.; Tihanyi, D.K.; Czigány, Z.; Kovács, T.; Budai, A.; Drozgyik, D.; Fülöp, A.; Szijártó, A. Liver regeneration after different degrees of portal vein ligation. J. Surg. Res. 2016, 203, 451–458.
- Meier, M.; Andersen, K.J.; Knudsen, A.R.; Nyengaard, J.R.; Hamilton-Dutoit, S.; Mortensen, F.V. Liver regeneration is dependent on the extent of hepatectomy. J. Surg. Res. 2016, 205, 76–84.
- Shimazu, M.; Kato, Y.; Kawachi, S.; Tanabe, M.; Hoshino, K.; Wakabayashi, G.; Kitagawa, Y.; Kitajima, M. Impact of Portal Hemodynamic Changes in Partial Liver Grafts on Short-Term Graft Regeneration in Living Donor Liver Transplantation. Transplant. Proc. 2016, 48, 2747–2755.
- Eguchi, S.; Yanaga, K.; Sugiyama, N.; Okudaira, S.; Furui, J.; Kanematsu, T. Relationship between portal venous flow and liver regeneration in patients after living donor right-lobe liver transplantation. Liver Transplant. 2003, 9, 547–551.
- Oyama, T.; Sadamori, H.; Matsukawa, H.; Murata, H.; Umeda, Y.; Watanabe, Y.; Ozaki, M.; Iwagaki, H.; Tanaka, N.; Yagi, T. Small liver graft regenerates through immediate increase of HGF and IL-6--possible involvement of sinusoidal tensile/shear stress in small liver graft. Hepatogastroenterology 2008, 54, 2078–2083.
- Jones, D.E.; Tran-Patterson, R.; Cui, D.M.; Davin, D.; Estell, K.P.; Miller, D.M. Epidermal growth factor secreted from the salivary gland is necessary for liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 1995, 268, G872–G878.
- Rubin, R.A.; O’Keefe, E.J.; Earp, H.S. Alteration of epidermal growth factor-dependent phosphorylation during rat liver regeneration. Proc. Natl. Acad. Sci. USA 1982, 79, 776–780.
- Olsen, P.S.; Boesby, S.; Kirkegaard, P.; Therkelsen, K.; Almdal, T.; Poulsen, S.S.; Nexø, E. Influence of epidermal growth factor on liver regeneration after partial hepatectomy in rats. Hepatology 1988, 8, 992–996.
- Shavandi, A.; Saeedi, P.; Gérard, P.; Jalalvandi, E.; Cannella, D.; Bekhit, A.E. The role of microbiota in tissue repair and regeneration. J. Tissue Eng. Regen. Med. 2020, 14, 539–555.
- Shah, V.; Haddad, F.G.; Garcia-Cardena, G.; Frangos, J.A.; Mennone, A.; Groszmann, R.J.; Sessa, W.C. Liver sinusoidal endothelial cells are responsible for nitric oxide modulation of resistance in the hepatic sinusoids. J. Clin. Investig. 1997, 100, 2923–2930.
- Patijn, G.A.; Lieber, A.; Schowalter, D.B.; Schwall, R.; Kay, M.A. Hepatocyte growth factor induces hepatocyte proliferationin vivo and allows for efficient retroviral-mediated gene transfer in mice. Hepatology 1998, 28, 707–716.
- Sokabe, T.; Yamamoto, K.; Ohura, N.; Nakatsuka, H.; Qin, K.; Obi, S.; Kamiya, A.; Ando, J. Differential regulation of urokinase-type plasminogen activator expression by fluid shear stress in human coronary artery endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2027–H2034.
- Dolan, J.M.; Sim, F.J.; Meng, H.; Kolega, J. Endothelial cells express a unique transcriptional profile under very high wall shear stress known to induce expansive arterial remodeling. Am. J. Physiol. Cell Physiol. 2012, 302, C1109–C1118.
- Kim, T.; Mars, W.M.; Stolz, D.B.; Petersen, B.E.; Michalopoulos, G.K. Extracellular matrix remodeling at the early stages of liver regeneration in the rat. Hepatology 1997, 26, 896–904.
- Nejak-Bowen, K.; Orr, A.; Bowen, W.C., Jr.; Michalopoulos, G.K. Conditional Genetic Elimination of Hepatocyte Growth Factor in Mice Compromises Liver Regeneration after Partial Hepatectomy. PLoS ONE 2013, 8, e59836.
- Mars, W.M.; Zarnegar, R.; Michalopoulos, G.K. Activation of Hepatocyte Growth Factor by the Plasminogen Activators uPA and tPA. Am. J. Pathol. 1993, 143, 949–958.
- Mars, W.M.; Kim, T.H.; Stolz, D.B.; Liu, M.L.; Michalopoulos, G.K. Presence of urokinase in serum-free primary rat hepatocyte cultures and its role in activating hepatocyte growth factor. Cancer Res. 1996, 56, 2837–2843.
- Shanmukhappa, K.; Sabla, G.E.; Degen, J.L.; Bezerra, J.A. Urokinase-type plasminogen activator supports liver repair independent of its cellular receptor. BMC Gastroenterol. 2006, 6, 40.
- Ding, B.-S.; Nolan, D.J.; Butler, J.M.; James, D.; Babazadeh, A.O.; Rosenwaks, Z.; Mittal, V.; Kobayashi, H.; Shido, K.; Lyden, D.; et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nat. Cell Biol. 2010, 468, 310–315.
- Döme, B.; Dobos, J.; Tóvári, J.; Paku, S.; Kovács, G.; Ostoros, G.; Tímár, J. Circulating bone marrow-derived endothelial progenitor cells: Characterization, mobilization, and therapeutic considerations in malignant disease. Cytom. Part A 2008, 73, 186–193.
- Timmermans, F.; Plum, J.; Yoder, M.; Ingram, D.A.; Vandekerckhove, B.; Case, J. Endothelial progenitor cells: Identity defined? J. Cell. Mol. Med. 2008, 13, 87–102.
- Hristov, M.; Erl, W.; Weber, P.C. Endothelial progenitor cells: Mobilization, differentiation, and homing. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1185–1189.
- Fujii, H.; Hirose, T.; Oe, S.; Yasuchika, K.; Azuma, H.; Fujikawa, T.; Nagao, M.; Yamaoka, Y. Contribution of bone marrow cells to liver regeneration after partial hepatectomy in mice. J. Hepatol. 2002, 36, 653–659.
- Li, N.; Hua, J. Immune cells in liver regeneration. Oncotarget 2016, 8, 3628–3639.
- Harb, R.; Xie, G.; Lutzko, C.; Guo, Y.; Wang, X.; Hill, C.K.; Kanel, G.C.; DeLeve, L.D. Bone Marrow Progenitor Cells Repair Rat Hepatic Sinusoidal Endothelial Cells After Liver Injury. Gastroenterology 2009, 137, 704–712.
- Deleve, L.D.; Wang, X.; Wang, L. VEGF-sdf1 recruitment of CXCR7 bone marrow progenitors of liver sinusoidal endothelial cells promotes rat liver regeneration. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, 739–746.
- Odent (Grigorescu), G.; Rosca, A.-M.; Preda, M.B.; Tutuianu, R.; Simionescu, M.; Burlacu, A. Synergic effects of VEGF-A and SDF-1 on the angiogenic properties of endothelial progenitor cells. J. Tissue Eng. Regen. Med. 2016, 11, 3241–3252.
- Moore, M.A.; Hattori, K.; Heissig, B.; Shieh, J.-H.; Dias, S.; Crystal, R.G.; Rafii, S. Mobilization of Endothelial and Hematopoietic Stem and Progenitor Cells by Adenovector-Mediated Elevation of Serum Levels of SDF-1, VEGF, and Angiopoietin-1. Ann. N. Y. Acad. Sci. 2006, 938, 36–47.
- Peled, A.; Grabovsky, V.; Habler, L.; Sandbank, J.; Arenzana-Seisdedos, F.; Petit, I.; Ben-Hur, H.; Lapidot, T.; Alon, R. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J. Clin. Investig. 1999, 104, 1199–1211.
- Silverman, M.D.; Haas, C.S.; Rad, A.M.; Arbab, A.S.; Koch, A.E. The role of vascular cell adhesion molecule 1/very late activation antigen 4 in endothelial progenitor cell recruitment to rheumatoid arthritis synovium. Arthritis Rheum. 2007, 56, 1817–1826.
- Yoon, C.-H.; Hur, J.; Oh, I.-Y.; Park, K.-W.; Kim, T.-Y.; Shin, J.-H.; Kim, J.-H.; Lee, C.-S.; Chung, J.-K.; Park, Y.-B.; et al. Intercellular Adhesion Molecule-1 is Upregulated in Ischemic Muscle, Which Mediates Trafficking of Endothelial Progenitor Cells. Arter. Thromb. Vasc. Biol. 2006, 26, 1066–1072.
- Jin, H.; Aiyer, A.; Su, J.; Borgström, P.; Stupack, D.; Friedlander, M.; Varner, J. A homing mechanism for bone marrow–derived progenitor cell recruitment to the neovasculature. J. Clin. Investig. 2006, 116, 652–662.
- Chavakis, E.; Aicher, A.; Heeschen, C.; Sasaki, K.-I.; Kaiser, R.; El Makhfi, N.; Urbich, C.; Peters, T.; Scharffetter-Kochanek, K.; Zeiher, A.M.; et al. Role of β2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J. Exp. Med. 2004, 201, 63–72.
- Tilling, L.; Chowienczyk, P.; Clapp, B. Progenitors in motion: Mechanisms of mobilization of endothelial progenitor cells. Br. J. Clin. Pharmacol. 2009, 68, 484–492.
- Myronovych, A.; Murata, S.; Chiba, M.; Matsuo, R.; Ikeda, O.; Watanabe, M.; Hisakura, K.; Nakano, Y.; Kohno, K.; Kawasaki, T.; et al. Role of platelets on liver regeneration after 90% hepatectomy in mice. J. Hepatol. 2008, 49, 363–372.
- Meyer, J.; Lejmi, E.; Fontana, P.; Morel, P.; Gonelle-Gispert, C.; Bühler, L. A focus on the role of platelets in liver regeneration: Do platelet-endothelial cell interactions initiate the regenerative process? J. Hepatol. 2015, 63, 1263–1271.
- Melgar-Lesmes, P.; Edelman, E. Monocyte-endothelial cell interactions in the regulation of vascular sprouting and liver regeneration in mouse. J. Hepatol. 2015, 63, 917–925.
- Hu, J.; Srivastava, K.; Wieland, M.; Runge, A.; Mogler, C.; Besemfelder, E.; Terhardt, D.; Vogel, M.J.; Cao, L.; Korn, C.; et al. Endothelial Cell-Derived Angiopoietin-2 Controls Liver Regeneration as a Spatiotemporal Rheostat. Science 2014, 343, 416–419.
- Taniguchi, E.; Sakisaka, S.; Matsuo, K.; Tanikawa, K.; Sata, M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J. Histochem. Cytochem. 2001, 49, 121–129.
- Shimizu, H.; Miyazaki, M.; Wakabayashi, Y.; Mitsuhashi, N.; Kato, A.; Ito, H.; Nakagawa, K.; Yoshidome, H.; Kataoka, M.; Nakajima, N. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J. Hepatol. 2001, 34, 683–689.
- Furnus, C.; Inda, A.; Andrini, L.; García, M.; García, A.; Badrán, A.; Errecalde, A. Chronobiology of the proliferative events related to angiogenesis in mice liver regeneration after partial hepatectomy. Cell Biol. Int. 2003, 27, 383–386.
- Kron, P.; Linecker, M.; Limani, P.; Schlegel, A.; Kambakamba, P.; Lehn, J.-M.; Nicolau, C.; Graf, R.; Humar, B.; Clavien, P.-A. Hypoxia-driven Hif2α coordinates mouse liver regeneration by coupling parenchymal growth to vascular expansion. Hepatology 2016, 64, 2198–2209.
- Braun, L.; Mead, J.E.; Panzica, M.; Mikumo, R.; Bell, G.I.; Fausto, N. Transforming growth factor beta mRNA increases during liver regeneration: A possible paracrine mechanism of growth regulation. Proc. Natl. Acad. Sci. USA 1988, 85, 1539–1543.
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55.
- Poon, R.T.; Fan, S.T.; Lo, C.M.; Liu, C.L.; Lam, C.M.; Yuen, W.K.; Yeung, C.; Wong, J.; Nagorney, D.M.; Henderson, J.M.; et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: Analysis of 1222 consecutive patients from a prospective database. Ann. Surg. 2004, 240, 698–710.
- Imamura, H.; Seyama, Y.; Kokudo, N.; Aoki, T.; Sano, K.; Minagawa, M.; Sugawara, Y.; Makuuchi, M. Single and multiple resections of multiple hepatic metastases of colorectal origin. Surgery 2004, 135, 508–517.
- Dahm, F.; Georgiev, P.; Clavien, P.-A. Small-for-Size Syndrome after Partial Liver Transplantation: Definition, Mechanisms of Disease and Clinical Implications. Arab. Archaeol. Epigr. 2005, 5, 2605–2610.
- Yagi, S.; Iida, T.; Hori, T.; Taniguchi, K.; Yamamoto, C.; Yamagiwa, K.; Uemoto, S. Optimal Portal Venous Circulation for Liver Graft Function after Living-Donor Liver Transplantation. Transplantation 2006, 81, 373–378.
- Guglielmi, A.; Ruzzenente, A.; Conci, S.; Valdegamberi, A.; Iacono, C. How Much Remnant Is Enough in Liver Resection? Dig. Surg. 2012, 29, 6–17.
- Lehmann, K.; Tschuor, C.; Rickenbacher, A.; Jang, J.; Oberkofler, C.E.; Tschopp, O.; Schultze, S.M.; Raptis, D.A.; Weber, A.; Graf, R.; et al. Liver Failure After Extended Hepatectomy in Mice Is Mediated by a p21-Dependent Barrier to Liver Regeneration. Gastroenterology 2012, 143, 1609–1619.e4.
- Zieve, L.; Anderson, W.R.; Lindblad, S. Course of hepatic regeneration after 80% to 90% resection of normal rat liver. Comparison with two-lobe and one-lobe hepatectomy. J. Lab. Clin. Med. 1985, 105, 331–336.
- Moser, M.J.; Gong, Y.; Zhang, M.N.; Johnston, J.; Lipschitz, J.; Minuk, G.Y. Immediate-Early Protooncogene Expression and Liver Function Following Various Extents of Partial Hepatectomy in the Rat. Dig. Dis. Sci. 2001, 46, 907–914.
- Ninomiya, M.; Shirabe, K.; Terashi, T.; Ijichi, H.; Yonemura, Y.; Harada, N.; Soejima, Y.; Taketomi, A.; Shimada, M.; Maehara, Y. Deceleration of Regenerative Response Improves the Outcome of Rat with Massive Hepatectomy. Arab. Archaeol. Epigr. 2010, 10, 1580–1587.
- Gridelli, B.G.; Gruttadauria, S.; Pagano, D.; Liotta, R.; Tropea, A.; Tuzzolino, F.; Marrone, G.; Mamone, G.; Marsh, J.W.; Miraglia, R.; et al. Liver Volume Restoration and Hepatic Microarchitecture in Small-for-Size Syndrome. Ann. Transplant. 2015, 20, 381–389.
- Byun, S.H.; Yang, H.S.; Kim, J.H. Liver graft hyperperfusion in the early postoperative period promotes hepatic regeneration 2 weeks after living donor liver transplantation. Medicine 2016, 95, e5404.
- Demetris, A.J.; Kelly, D.M.; Eghtesad, B.; Fontes, P.; Marsh, J.W.; Tom, K.; Tan, H.P.; Shaw-Stiffel, T.; Boig, L.; Novelli, P.; et al. Pathophysiologic Observations and Histopathologic Recognition of the Portal Hyperperfusion or Small-for-Size Syndrome. Am. J. Surg. Pathol. 2006, 30, 986–993.
- Vasavada, B.; Chen, C.L.; Zakaria, M. Portal flow is the main predictor of early graft dysfunction regardless of the GRWR status in living donor liver transplantation—A retrospective analysis of 134 patients. Int. J. Surg. 2014, 12, 177–180.
- Asencio, J.; Vaquero, J.; Olmedilla, L.; Sabrido, J.G. “Small-for-flow” syndrome: Shifting the “size” paradigm. Med. Hypotheses 2013, 80, 573–577.
- Allard, M.-A.; Adam, R.; Bucur, P.-O.; Termos, S.; Cunha, A.S.; Bismuth, H.; Castaing, D.; Vibert, E. Posthepatectomy Portal Vein Pressure Predicts Liver Failure and Mortality after Major Liver Resection on Noncirrhotic Liver. Ann. Surg. 2013, 258, 822–830.
- Brown, R.S. Live Donors in Liver Transplantation. Gastroenterology 2008, 134, 1802–1813.
- Golse, N.; Bucur, P.O.; Adam, R.; Castaing, D.; Cunha, A.S.; Vibert, E. New Paradigms in Post-hepatectomy Liver Failure. J. Gastrointest. Surg. 2012, 17, 593–605.
- Umeda, Y.; Yagi, T.; Sadamori, H.; Fujiwara, T. Small-for-Size Syndrome after Living Donor Liver Transplantation. In Liver Transplantation: Technical Issues and Complications; Abdeldayem, H., Ed.; IntechOpen: London, UK, 2012; ISBN 978-953-51-0015-7.
- Troisi, R.; Ricciardi, S.; Smeets, P.; Petrovic, M.; Van Maele, G.; Colle, I.; Van Vlierberghe, H.; De Hemptinne, B. Effects of Hemi-Portocaval Shunts for Inflow Modulation on the Outcome of Small-for-Size Grafts in Living Donor Liver Transplantation. Arab. Archaeol. Epigr. 2005, 5, 1397–1404.
- Sato, Y.; Yamamoto, S.; Oya, H.; Nakatsuka, H.; Tsukahara, A.; Kobayashi, T.; Watanabe, T.; Hatakeyama, K. Sple-nectomy for reduction of excessive portal hypertension after adult living-related donor liver transplantation. Hepatogastroenterology 2002, 49, 1652–1655.
- Yamada, T.; Tanaka, K.; Uryuhara, K.; Ito, K.; Takada, Y.; Uemoto, S. Selective Hemi-Portocaval Shunt Based on Portal Vein Pressure for Small-for-Size Graft in Adult Living Donor Liver Transplantation. Arab. Archaeol. Epigr. 2008, 8, 847–853.
- Boillot, O. Portomesenteric disconnection for small-for-size grafts in liver transplantation: Preclinical studies in pigs. Liver Transplant. 2003, 9, S42–S46.
- Yamanaka, K.; Hatano, E.; Narita, M.; Kitamura, K.; Yanagida, A.; Asechi, H.; Nagata, H.; Taura, K.; Nitta, T.; Uemoto, S. Olprinone attenuates excessive shear stress through up-regulation of endothelial nitric oxide synthase in a rat excessive hepatectomy model. Liver Transplant. 2010, 17, 60–69.
- Morioka, D.; Matsuo, K.; Endo, I.; Togo, S.; Kubota, T.; Sekido, H.; Saito, S.; Ichikawa, Y.; Shimada, H. Prostaglandin E1 improved the function of transplanted fatty liver in a rat reduced-size-liver transplantation model under conditions of permissible cold preservation. Liver Transplant. 2003, 9, 79–86.
- Man, K.; Lee, K.W.; Liang, T.B.; Lo, C.M.; Fung, P.C.-W.; Tsui, S.H.; Li, X.L.; Ng, K.T.-P.; Fan, S.T. FK 409 Ameliorates Small-for-Size Liver Graft Injury by Attenuation of Portal Hypertension and Down-Regulation of Egr-1 Pathway. Ann. Surg. 2004, 240, 159–168.
- Xu, X.; Man, K.; Zheng, S.S.; Liang, T.B.; Lee, K.W.; Ng, K.T.-P.; Fan, S.T.; Lo, C.M. Attenuation of acute phase shear stress by somatostatin improves small-for-size liver graft survival. Liver Transplant. 2006, 12, 621–627.
- Mohkam, K.; Darnis, B.; Schmitt, Z.; Duperret, S.; Ducerf, C.; Mabrut, J.-Y. Successful modulation of portal inflow by somatostatin in a porcine model of small-for-size syndrome. Am. J. Surg. 2016, 212, 321–326.
- Dili, A.; Bertrand, C.; Lebrun, V.; Pirlot, B.; Leclercq, I.A. Hypoxia protects the liver from Small for Size Syndrome: A lesson learned from the associated liver partition and portal vein ligation for staged hepatectomy (ALPPS) procedure in rats. Arab. Archaeol. Epigr. 2019, 19, 2979–2990.




