
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jean-François Beaulieu | + 2787 word(s) | 2787 | 2021-08-05 04:42:31 |
Video Upload Options
Integrin α6β4 is one of the main laminin receptors and is primarily expressed by epithelial cells as an active component of hemidesmosomes. In this article, after a brief summary about integrins in the gut epithelium in general, I review the knowledge and clinical potential of this receptor in human colorectal cancer (CRC) cells. Most CRC cells overexpress both α6 and β4 subunits, in situ in primary tumours as well as in established CRC cell lines. The mechanisms that lead to overexpression have not yet been elucidated but clearly involve specific transcription factors such as MYC. From a functional point of view, one key element affecting CRC cell behaviour is the relocalization of α6β4 to the actin cytoskeleton, favouring a more migratory and anoikis-resistant phenotype.
1. Introduction
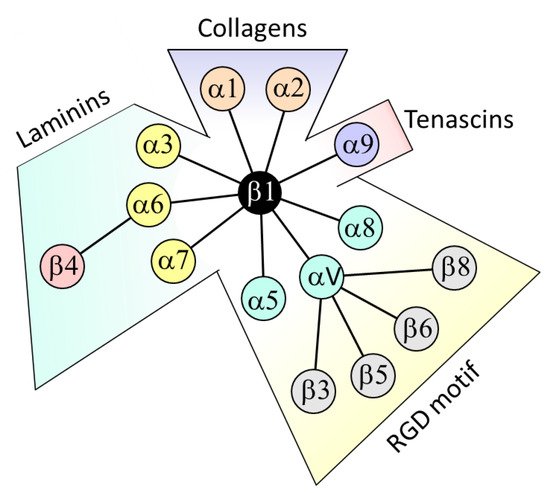
2. β4 Integrin Subunit (ITGB4) in CRC
2.1. Expression
2.2. Regulation of Expression
2.3. Change in Functionality
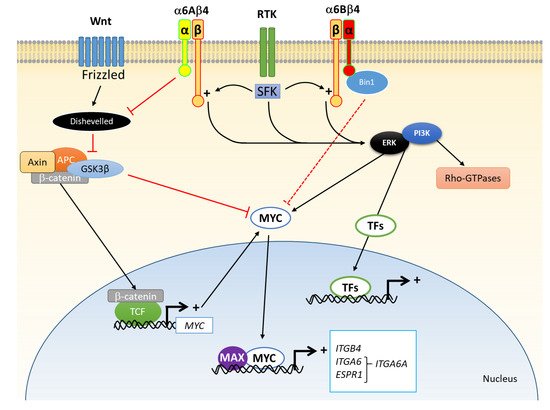
3. α6 Integrin Subunit (ITGA6) in CRC
3.1. Expression
3.2. Regulation of Expression
3.3. Change in Functionality
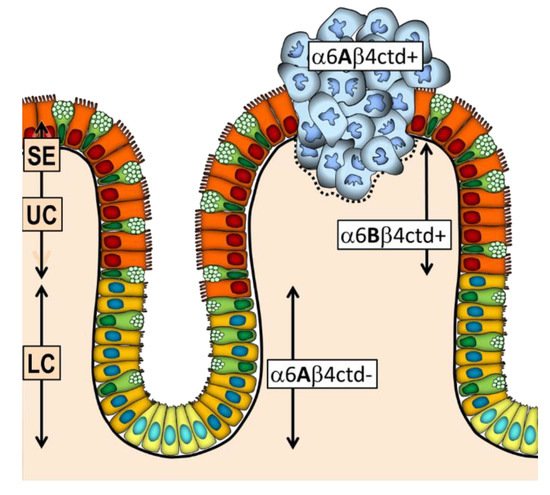
4. The Integrin α6β4 in CRC
References
- Cloutier, G.; Sallenbach-Morrissette, A.; Beaulieu, J.F. Non-integrin laminin receptors in epithelia. Tissue Cell 2019, 56, 71–78.
- Beaulieu, J.F. Integrins and human intestinal cell functions. Front. Biosci. 1999, 4, 310–321.
- Beaulieu, J.F. Extracellular matrix components and integrins in relationship to human intestinal epithelial cell differentiation. Prog. Histochem. Cytochem. 1997, 31, 1–76.
- Humphries, J.D.; Chastney, M.R.; Askari, J.A.; Humphries, M.J. Signal transduction via integrin adhesion complexes. Curr. Opin. Cell Biol. 2019, 56, 14–21.
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473.
- Hussey, G.S.; Keane, T.J.; Badylak, S.F. The extracellular matrix of the gastrointestinal tract: A regenerative medicine platform. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 540–552.
- Boudjadi, S.; Carrier, J.C.; Beaulieu, J.F. Integrin alpha1 subunit is up-regulated in colorectal cancer. Biomark. Res. 2013, 1, 16.
- Boudjadi, S.; Carrier, J.C.; Groulx, J.F.; Beaulieu, J.F. Integrin alpha1beta1 expression is controlled by c-MYC in colorectal cancer cells. Oncogene 2016, 35, 1671–1678.
- Boudjadi, S.; Bernatchez, G.; Senicourt, B.; Beausejour, M.; Vachon, P.H.; Carrier, J.C.; Beaulieu, J.F. Involvement of the Integrin alpha1beta1 in the Progression of Colorectal Cancer. Cancers 2017, 9, 96.
- Basora, N.; Desloges, N.; Chang, Q.; Bouatrouss, Y.; Gosselin, J.; Poisson, J.; Sheppard, D.; Beaulieu, J.F. Expression of the alpha9beta1 integrin in human colonic epithelial cells: Resurgence of the fetal phenotype in a subset of colon cancers and adenocarcinoma cell lines. Int. J. Cancer 1998, 75, 738–743.
- Desloges, N.; Basora, N.; Perreault, N.; Bouatrouss, Y.; Sheppard, D.; Beaulieu, J.F. Regulated expression of the integrin alpha9beta1 in the epithelium of the developing human gut and in intestinal cell lines: Relation with cell proliferation. J. Cell. Biochem. 1998, 71, 536–545.
- Cantor, D.I.; Cheruku, H.R.; Nice, E.C.; Baker, M.S. Integrin alphavbeta6 sets the stage for colorectal cancer metastasis. Cancer Metastasis Rev. 2015, 34, 715–734.
- Pelillo, C.; Bergamo, A.; Mollica, H.; Bestagno, M.; Sava, G. Colorectal Cancer Metastases Settle in the Hepatic Microenvironment Through alpha5beta1 Integrin. J. Cell. Biochem. 2015, 116, 2385–2396.
- Benoit, Y.D.; Lussier, C.; Ducharme, P.A.; Sivret, S.; Schnapp, L.M.; Basora, N.; Beaulieu, J.F. Integrin alpha8beta1 regulates adhesion, migration and proliferation of human intestinal crypt cells via a predominant RhoA/ROCK-dependent mechanism. Biol. Cell 2009, 101, 695–708.
- Benoit, Y.D.; Larrivee, J.F.; Groulx, J.F.; Stankova, J.; Vachon, P.H.; Beaulieu, J.F. Integrin alpha8beta1 confers anoikis susceptibility to human intestinal epithelial crypt cells. Biochem. Biophys. Res. Commun. 2010, 399, 434–439.
- Kozlova, N.I.; Morozevich, G.E.; Chubukina, A.N.; Berman, A.E. Integrin alphavbeta3 promotes anchorage-dependent apoptosis in human intestinal carcinoma cells. Oncogene 2001, 20, 4710–4717.
- Morozevich, G.E.; Kozlova, N.I.; Chubukina, A.N.; Berman, A.E. Role of integrin alphavbeta3 in substrate-dependent apoptosis of human intestinal carcinoma cells. Biochemistry (Moscow) 2003, 68, 416–423.
- Ramovs, V.; Te Molder, L.; Sonnenberg, A. The opposing roles of laminin-binding integrins in cancer. Matrix Biol. 2017, 57–58, 213–243.
- Pouliot, N.; Kusuma, N. Laminin-511: A multi-functional adhesion protein regulating cell migration, tumor invasion and metastasis. Cell Adhes. Migr. 2013, 7, 142–149.
- de Melker, A.A.; Sonnenberg, A. Integrins: Alternative splicing as a mechanism to regulate ligand binding and integrin signaling events. Bioessays 1999, 21, 499–509.
- Stallmach, A.; von Lampe, B.; Matthes, H.; Bornhoft, G.; Riecken, E.O. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut 1992, 33, 342–346.
- Lohi, J.; Oivula, J.; Kivilaakso, E.; Kiviluoto, T.; Frojdman, K.; Yamada, Y.; Burgeson, R.E.; Leivo, I.; Virtanen, I. Basement membrane laminin-5 is deposited in colorectal adenomas and carcinomas and serves as a ligand for alpha3beta1 integrin. Apmis 2000, 108, 161–172.
- Falcioni, R.; Turchi, V.; Vitullo, P.; Navarra, G.; Ficari, F.; Cavaliere, F.; Sacchi, A.; Marianicostantini, R. Integrin beta-4 expression in colorectal-cancer. Int. J. Oncol. 1994, 5, 573–578.
- Sordat, I.; Bosman, F.T.; Dorta, G.; Rousselle, P.; Aberdam, D.; Blum, A.L.; Sordat, B. Differential expression of laminin-5 subunits and integrin receptors in human colorectal neoplasia. J. Pathol. 1998, 185, 44–52.
- Ni, H.; Dydensborg, A.B.; Herring, F.E.; Basora, N.; Gagne, D.; Vachon, P.H.; Beaulieu, J.F. Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene 2005, 24, 6820–6829.
- Basora, N.; Herring-Gillam, F.E.; Boudreau, F.; Perreault, N.; Pageot, L.P.; Simoneau, M.; Bouatrouss, Y.; Beaulieu, J.F. Expression of functionally distinct variants of the beta4A integrin subunit in relation to the differentiation state in human intestinal cells. J. Biol. Chem. 1999, 274, 29819–29825.
- Takaoka, A.S.; Yamada, T.; Gotoh, M.; Kanai, Y.; Imai, K.; Hirohashi, S. Cloning and characterization of the human beta4-integrin gene promoter and enhancers. J. Biol. Chem. 1998, 273, 33848–33855.
- Phillips, J.L.; Taberlay, P.C.; Woodworth, A.M.; Hardy, K.; Brettingham-Moore, K.H.; Dickinson, J.L.; Holloway, A.F. Distinct mechanisms of regulation of the ITGA6 and ITGB4 genes by RUNX1 in myeloid cells. J. Cell. Physiol. 2018, 233, 3439–3453.
- An, X.Z.; Zhao, Z.G.; Luo, Y.X.; Zhang, R.; Tang, X.Q.; Hao, D.; Zhao, X.; Lv, X.; Liu, D. Netrin-1 suppresses the MEK/ERK pathway and ITGB4 in pancreatic cancer. Oncotarget 2016, 7, 24719–24733.
- Ma, B.; Zhang, L.; Zou, Y.; He, R.; Wu, Q.; Han, C.; Zhang, B. Reciprocal regulation of integrin beta4 and KLF4 promotes gliomagenesis through maintaining cancer stem cell traits. J. Exp. Clin. Cancer Res. 2019, 38, 23.
- Yang, L.; Zhang, L.; Wu, Q.; Boyd, D.D. Unbiased screening for transcriptional targets of ZKSCAN3 identifies integrin beta 4 and vascular endothelial growth factor as downstream targets. J. Biol. Chem. 2008, 283, 35295–35304.
- Li, M.; Jiang, X.; Wang, G.; Zhai, C.; Liu, Y.; Li, H.; Zhang, Y.; Yu, W.; Zhao, Z. ITGB4 is a novel prognostic factor in colon cancer. J. Cancer 2019, 10, 5223–5233.
- Ferraro, A.; Kontos, C.K.; Boni, T.; Bantounas, I.; Siakouli, D.; Kosmidou, V.; Vlassi, M.; Spyridakis, Y.; Tsipras, I.; Zografos, G.; et al. Epigenetic regulation of miR-21 in colorectal cancer: ITGB4 as a novel miR-21 target and a three-gene network (miR-21-ITGBeta4-PDCD4) as predictor of metastatic tumor potential. Epigenetics 2014, 9, 129–141.
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367.
- Walko, G.; Castanon, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 529–544.
- Stewart, R.L.; O’Connor, K.L. Clinical significance of the integrin alpha6beta4 in human malignancies. Lab. Investig. 2015, 95, 976–986.
- Benoit, Y.D.; Groulx, J.F.; Gagne, D.; Beaulieu, J.F. RGD-Dependent Epithelial Cell-Matrix Interactions in the Human Intestinal Crypt. J. Signal Transduct. 2012, 2012.
- Shaw, L.M.; Rabinovitz, I.; Wang, H.H.; Toker, A.; Mercurio, A.M. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell 1997, 91, 949–960.
- Beausejour, M.; Boutin, A.; Vachon, P.H. Anoikis regulation—Complexities, distinction, and cell differentiation. In Apoptosis and Beyond: The Many Ways Cells Die; Radosevich, J.A., Ed.; Wiley: Hoboken, NJ, USA, 2017; pp. 145–182.
- Boudjadi, S.; Beaulieu, J.F. MYC and integrins interplay in colorectal cancer. Oncoscience 2016, 3, 50–51.
- Hogervorst, F.; Admiraal, L.G.; Niessen, C.; Kuikman, I.; Janssen, H.; Daams, H.; Sonnenberg, A. Biochemical characterization and tissue distribution of the A and B variants of the integrin alpha 6 subunit. J. Cell. Biol. 1993, 121, 179–191.
- Dydensborg, A.B.; Teller, I.C.; Basora, N.; Groulx, J.F.; Auclair, J.; Francoeur, C.; Escaffit, F.; Pare, F.; Herring, E.; Menard, D.; et al. Differential expression of the integrins alpha6Abeta4 and alpha6Bbeta4 along the crypt-villus axis in the human small intestine. Histochem. Cell Biol. 2009, 131, 531–536.
- Dydensborg, A.B.; Teller, I.C.; Groulx, J.F.; Basora, N.; Pare, F.; Herring, E.; Gauthier, R.; Jean, D.; Beaulieu, J.F. Integrin alpha6Bbeta4 inhibits colon cancer cell proliferation and c-Myc activity. BMC Cancer 2009, 9, 223.
- Groulx, J.F.; Giroux, V.; Beausejour, M.; Boudjadi, S.; Basora, N.; Carrier, J.C.; Beaulieu, J.F. Integrin alpha6A splice variant regulates proliferation and the Wnt/beta-catenin pathway in human colorectal cancer cells. Carcinogenesis 2014, 35, 1217–1227.
- Nishida, K.; Kitazawa, R.; Mizuno, K.; Maeda, S.; Kitazawa, S. Identification of regulatory elements of human alpha 6 integrin subunit gene. Biochem. Biophys. Res. Commun. 1997, 241, 258–263.
- Gaudreault, M.; Vigneault, F.; Leclerc, S.; Guerin, S.L. Laminin reduces expression of the human alpha6 integrin subunit gene by altering the level of the transcription factors Sp1 and Sp3. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3490–3505.
- Sikora, K.; Chan, S.; Evan, G.; Gabra, H.; Markham, N.; Stewart, J.; Watson, J. c-myc oncogene expression in colorectal cancer. Cancer 1987, 59, 1289–1295.
- Erisman, M.D.; Rothberg, P.G.; Diehl, R.E.; Morse, C.C.; Spandorfer, J.M.; Astrin, S.M. Deregulation of c-myc gene expression in human colon carcinoma is not accompanied by amplification or rearrangement of the gene. Mol. Cell. Biol. 1985, 5, 1969–1976.
- Groulx, J.F.; Boudjadi, S.; Beaulieu, J.F. MYC Regulates alpha6 Integrin Subunit Expression and Splicing Under Its Pro-Proliferative ITGA6A form in Colorectal Cancer Cells. Cancers 2018, 10, 42.
- Goel, H.L.; Gritsko, T.; Pursell, B.; Chang, C.; Shultz, L.D.; Greiner, D.L.; Norum, J.H.; Toftgard, R.; Shaw, L.M.; Mercurio, A.M. Regulated splicing of the alpha6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep. 2014, 7, 747–761.
- Kretzschmar, K.; Clevers, H. Wnt/beta-catenin signaling in adult mammalian epithelial stem cells. Dev. Biol. 2017, 428, 273–282.
- Beaulieu, J.F. Integrin alpha6beta4 in colorectal cancer. World J. Gastrointest. Pathophysiol. 2010, 1, 3–11.




