
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Oliver Faust | + 1641 word(s) | 1641 | 2020-05-09 03:39:48 | | | |
| 2 | Nicole Yin | -63 word(s) | 1578 | 2020-10-30 07:28:51 | | |
Video Upload Options
Atrial Fibrillation (AF) is a heart rhythm disorder. Hence, AF diagnosis is based on measurements which reflect the activity of the heart. Detectable symptoms of the disease might not be present all the time. Therefore, the measurement duration is positively correlated with the AF detection rate. Suitable measurements include photoplethysmogram, electrocardiogram, and heart rate. The data volume, produced through long term measurement, mandates computer support to automate the AF detection task.
1. Introduction
In this entry, we discuss the different measurements that can be used to support AF diagnosis. From a service perspective, patient-led data acquisition is an important requirement. Imaging methods, such as echocardiogram[1][2][3][4] and chest X-ray[5][6], are unsuitable for measurements in the patient environment. Blood test biomarkers can be useful for risk prediction[7]. However, it is not yet feasible to carry out either measurement or analysis in the home environment.
ECG and RR interval signals result from passive non-invasive measurements. These measurements can be carried out in the patient environment. ECG results from measurement of the electric field emitted by heart[8]. The resulting signals provide a good base for automated AF detection[9][10][11]. This is because AF symptoms, such as poorly coordinated atrial activation and turbulent cardiac beating[12], alter the signal morphology[13][14][15]. RR interval signals can be extracted from the ECG, and these signals are useful for detecting heart rhythm disorders, such as AF[16]. The following sections provide further details concerning both ECG- and RR interval-based AF detection. Wearable Photoplethysmogram (PPG) might provide an alternative for measuring the RR intervals[17].
2. Photoplethysmogram
PPG is an optical measurement that detects variations in light reflected from human tissue[18]. The main feature of the resulting signal is the peripheral pulse, which is synchronized with the R peaks in the ECG. Once the PPG signal is captured, the beat-to-beat interval is measured and saved as an RR interval signal[19]. Hence, the AF detection methods are similar to those that process RR intervals extracted from ECG. The measurement can be done with mobile devices, such as wrist watches[20]. As such, the PPG measurement becomes an add-on feature for a mobile device, which keeps the cost down. Widespread availability, combined with little extra cost for the sensor, might lead to a significant increase in data volume. Given the right detection algorithms, that data can help to establish the presence of AF in the wider population.
For user comfort, these devices can move around, which is likely to cause activity specific artifacts in the measurements[21]. These artifacts mandate preprocessing algorithms that clean up the signal for the AF detector. In 2017, Sološenko et al. addressed the lack of annotated public PPG databases by generating a photoplethysmogram model from the well-known MIT–BIHAF database[22]. This model attempts to reflect the cardiovascular system; hence, there might be variation in how well that model fits for a particular patient. That uncertainty also affects the AF detection methods that were developed with the photoplethysmogram model. Furthermore, detectors with a high computational complexity might exploit some of the hidden assumptions that went into the photoplethysmogram model design. In a practical scenario, these assumptions might not hold, which reduces the detector performance. Another approach is to record PPG alongside ECG and transfer the labels, established by a cardiologist, from ECG to PPG[17][23]. Such double recording is necessary, because PPG signals cannot be used for diagnosis. In other words, cardiologists diagnose AF based on ECG signals. Until that changes, PPG-based AF detection can only be used in gadgets that can raise the suspicion that AF symptoms are present. The medical diagnosis will require additional measurements.
3. Electrocardiogram
Twelve-lead ECG is the reference standard for AF detection[24]. In order to establish a diagnosis, the signal is analyzed by a trained clinician[25]. Figure 1 shows two ECG signal segments from PhysioNet’s AF database[26][27]. A normal ECG cycle is referred to as Normal Sinus Rhythm (NSR). It consists of a P wave, QRScomplex, and T wave. The first plot in Figure 1 provides an example of NSR. In that plot, the first P wave is marked and labeled. Pathologically, AF can be described as a breakdown of organized atrial electrical activity[28]. This breakdown manifests itself in the absence of a P wave in the ECG signal and the R peak appearing at irregular intervals[29]. The second plot in Row 1 of Figure 1 provides an example of an ECG signal that shows signs of AF. The R peaks appear at irregular time intervals, and the P wave is muted or absent. Table 1 provides a summary of studies that investigated computer support for automated AF detection in ECG signals.
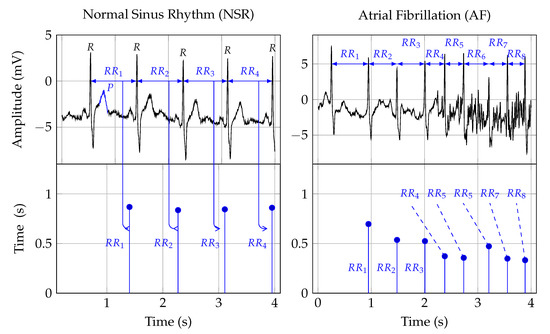
Figure 1. The first row depicts two ECG signals and the second row the corresponding RR interval traces. The two plots in the first column show NSR, and the plots in the second row show AF symptoms. The Rpeak, in the ECG plots, indicates ventricular depolarization, i.e., the time of the heart beat. The time between two heart beats, say a and b, is indicated as the RRinterval. That time duration forms the amplitude, and the time location of the second beat b is the time location of an RR interval sample. The Pwave, labeled in the NSR ECG plot, indicates atrial depolarization.
Table 1. A summary of automated AF detection in ECG signals. A “+” in the Salient features column indicates a positive point. Conversely, a “−” indicates a negative point.
| Authors | Data | Digital Biomarkers | AI | Performance in % | Salient Features | ||
|---|---|---|---|---|---|---|---|
| Acc | Sen | Spe | |||||
| Wang et al., 2020[30] | MIT-BIHAFDB | WPD followed by multivariate statistical features | ANN | 98.8 | 98.7 | 98.9 |
|
| Cao et al., 2020[31] | CinCchallenge 2017 | Data augmentation | DNN | 78.35 | - | - |
|
| Marsili et al., 2019[32] | MIT-BIH AFDB and measurements | Shannon entropy | Threshold | 98.1 | 99.2 | 97.3 |
|
| Yao et al., 2019[33] | CinC challenge 2017 | DWT | Multi-scale CNN | 98.18 | 98.22 | 98.11 |
|
| Lui et al., 2018[34] | MIT-BIH AFDB/NSR/Arrhythmia Database | Normalized fuzzy entropy | Threshold | - | - | - |
|
| Xia et al., 2018[35] | MIT-BIH AFIB | STFT, SWT | CNN | 98.63 | 98.79 | 97.87 |
|
| Kora et al., 2017[36] | MIT-BIH AFIB | CS-SCHT | LMNN | 99.30 | 96.97 | 99.43 |
|
| Tripathy et al., 2017[37] | MIT-BIH AFIB | Sample entropy, VMD | DBN | 98.27 | 98.80 | 97.77 |
|
| Annavarapu and Padmavathi, 2016[38] | MIT-BIH Arrhythmia Database | CS-SCHT | LMNN | 99.50 | 99.97 | 98.70 |
|
| Abdul-Kadir et al., 2016[39] | MIT-BIH NSR/AFDB | Dynamic system | ANN, SVM | 95.00 |
|
||
| Yuan et al., 2016[40] | MIT-BIH NSR/AFDB/LTAFDB | - | Autoencoder DL | 98.31 | 96.56 | 99.04 |
|
| Asgari et al., 2015[41] | MIT-BIH AFDB | SWT, Log-energy entropy, peak-to-average power ratio | SVM | 97.10 | 97.00 | 97.10 |
|
| Daqrouq et al., 2014[42] | MIT-BIH AFIB | WPD | PNN | 97.92 | - | - |
|
| Martis et al., 2013[43] | MIT-BIH AFDB/Arrhythmia Database | DWT | NB | 99.33 | 99.32 | 99.33 |
|
| Majia et al., 2013[44] | MIT-BIH Arrhythmia Database | HOS and EMD | Thresholded | - | 96 | - |
|
| Rincón et al., 2012[45] | MIT-BIH AFIB | Statistical measures | Fuzzy classifier | - | 98.09 | 91.66 |
|
| Lee et al., 2012[46] | MIT-BIH NSR/AFDB | RMSSD, sample entropy, Shannon entropy | Threshold | 98.44 | 97.63 | 99.61 |
|
| Fukunami et al., 1991[47] | Measurement data | Frequency-domain | Statistical analysis | - | 91 | 76 |
|
| Parvaresh and Ayatollahi, 2011[48] | MIT-BIH AFIB | Autoregressive model | Statistical classifier | - | 96.14 | 93.20 |
|
The morphology of normal ECG signals differs from person-to-person[49]. ECG-based AF diagnosis means to detect disease-related morphology changes. The main challenge is that these morphology changes may not be unique, which can lead to reduced specificity in identifying the disease that causes these changes. Extending the observation time might help to solve the sensitivity problem[50][51]. Furthermore, the relationship between atrial activity, measured by surface ECGs, and AF mechanisms, is not yet well understood[52]. This ambiguity translates into inaccurate detection algorithms[53]. Detecting AF based on the symptomatic heart rhythm change could be a helpful way forward.
4. Heart Rate
Heart Rate (HR) can be used to detect AF episodes[54]. The second row in Figure 1 shows the RR intervals that correspond to the ECG signals shown in the first row. As such, RR interval signals are constructed from RR intervals. These RR intervals represent the time from one R peak in the ECG signal to the next. In Figure 1, this is indicated by the arrows from the ECG to the RR interval signal.
For a human expert, it might be difficult to detect the subtle linear and nonlinear changes in the RR interval trace that indicate AF. Therefore, manual analysis of RR interval measurements may result in inter- and intra-observer variability. Digital biomarkers and algorithmic decision support can help to avoid these problems and hence improve diagnostic quality[55][56].
The occurrence of symptomatic episodes in paroxysmal AF is uncertain[57][58]. Some episodes may last more than 48 h[57]. Statistical analysis shows that during AF, RR intervals have a larger standard deviation and a shorter correlation length than those during NSR. Hence, these measures can be used as digital biomarkers for AF detection. However, they are not specific enough when it comes to differentiating AF from other arrhythmias[59][60].
References
- Oh, S.L.; Ng, E.Y.; San Tan, R.; Acharya, U.R. Automated beat-wise arrhythmia diagnosis using modified U-net on extended electrocardiographic recordings with heterogeneous arrhythmia types. Comput. Biol. Med. 2019, 105, 92–101.
- Sanfilippo, A.J.; Abascal, V.M.; Sheehan, M.; Oertel, L.B.; Harrigan, P.; Hughes, R.A.; Weyman, A.E. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation 1990, 82, 792–797.
- Michałkiewicz, D.; Dziuk, M.; Kamiński, G.; Olszewski, R.; Cholewa, M.; Cwetsch, A.; Markuszewski, L. Detection of patients at risk for paroxysmal atrial fibrillation (PAF) by signal averaged P wave, standard ECG and echocardiography. Polski merkuriusz lekarski: Organ Polskiego Towarzystwa Lekarskiego 2006, 20, 69–72.
- Klein, A.L.; Grimm, R.A.; Murray, R.D.; Apperson-Hansen, C.; Asinger, R.W.; Black, I.W.; Davidoff, R.; Erbel, R.; Halperin, J.L.; Orsinelli, D.A.; et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N. Engl. J. Med. 2001, 344, 1411–1420.
- Fonseca, C.; Mota, T.; Morais, H.; Matias, F.; Costa, C.; Oliveira, A.G.; Ceia, F.; Investigators, E. The value of the electrocardiogram and chest X-ray for confirming or refuting a suspected diagnosis of heart failure in the community. Eur. J. Heart Fail. 2004, 6, 807–812.
- Thomas, J.T.; Kelly, R.F.; Thomas, S.J.; Stamos, T.D.; Albasha, K.; Parrillo, J.E.; Calvin, J.E. Utility of history, physical examination, electrocardiogram, and chest radiograph for differentiating normal from decreased systolic function in patients with heart failure. Am. J. Med. 2002, 112, 437–445.
- Renate B. Schnabel; Martin G. Larson; Jennifer F. Yamamoto; Lisa M. Sullivan; Michael J. Pencina; James B. Meigs; Geoffrey H. Tofler; Jacob Selhub; Paul F. Jacques; Philip A. Wolf; et al.Jared W. MagnaniPatrick T. EllinorThomas J. WangDaniel LevyRamachandran S. VasanEmelia J. Benjamin Relations of Biomarkers of Distinct Pathophysiological Pathways and Atrial Fibrillation Incidence in the Community. Circulation 2010, 121, 200-207, 10.1161/circulationaha.109.882241.
- Polychronis E. Dilaveris; Elias J. Gialafos; Skevos K. Sideris; Artemis M. Theopistou; George K. Andrikopoulos; Michael Kyriakidis; John E. Gialafos; Pavlos K. Toutouzas; Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. American Heart Journal 1998, 135, 733-738, 10.1016/s0002-8703(98)70030-4.
- Dash, S.; Chon, K.; Lu, S.; Raeder, E. Automatic real time detection of atrial fibrillation. Ann. Biomed. Eng. 2009, 37, 1701–1709.
- Babaeizadeh, S.; Gregg, R.E.; Helfenbein, E.D.; Lindauer, J.M.; Zhou, S.H. Improvements in atrial fibrillation detection for real-time monitoring. J. Electrocardiol. 2009, 42, 522–526.
- Park, J.; Lee, S.; Jeon, M. Atrial fibrillation detection by heart rate variability in Poincare plot. Biomed. Eng. Online 2009, 8, 38.
- Mari A Watanabe; Heart Rate Turbulence: A Review. Indian Pacing and Electrophysiology Journal 2003, 3, 10-22.
- Yaghouby, F.; Ayatollahi, A.; Bahramali, R.; Yaghouby, M.; Alavi, A.H. Towards automatic detection of atrial fibrillation: A hybrid computational approach. Comput. Biol. Med. 2010, 40, 919–930.
- Lian, J.; Wang, L.; Muessig, D. A simple method to detect atrial fibrillation using RR intervals. Am. J. Cardiol. 2011, 107, 1494–1497.
- Lake, D.E.; Moorman, J.R. Accurate estimation of entropy in very short physiological time series: The problem of atrial fibrillation detection in implanted ventricular devices. Am. J. Physiol.-Heart Circulatory Physiol. 2010, 300, H319–H325.
- Larburu, N.; Lopetegi, T.; Romero, I. Comparative study of algorithms for atrial fibrillation detection. In Proceedings of the 2011 Computing in Cardiology, Hangzhou, China, 18–21 September 2011; pp. 265–268.
- Aviram Hochstadt; Ehud Chorin; Sami Viskin; Arie Lorin Schwartz; Natan Lubman; Raphael Rosso; Continuous heart rate monitoring for automatic detection of atrial fibrillation with novel bio-sensing technology. Journal of Electrocardiology 2019, 52, 23-27, 10.1016/j.jelectrocard.2018.10.096.
- John Allen; Photoplethysmography and its application in clinical physiological measurement. Physiological Measurement 2007, 28, R1-R39, 10.1088/0967-3334/28/3/r01.
- Bonomi, A.G.; Schipper, F.; Eerikäinen, L.M.; Margarito, J.; Aarts, R.M.; Babaeizadeh, S.; de Morree, H.M.; Dekker, L. Atrial fibrillation detection using photo-plethysmography and acceleration data at the wrist. In Proceedings of the 2016 Computing in Cardiology Conference (CinC), Vancouver, BC, Canada, 11–14 September 2016; pp. 277–280.
- Chengming Yang; Cesar Veiga; Juan J. Rodriguez-Andina; Jose Farina; Andres Iniguez; Shen Yin; Using PPG Signals and Wearable Devices for Atrial Fibrillation Screening. IEEE Transactions on Industrial Electronics 2019, 66, 8832-8842, 10.1109/tie.2018.2889614.
- Nemati, S.; Ghassemi, M.M.; Ambai, V.; Isakadze, N.; Levantsevych, O.; Shah, A.; Clifford, G.D. Monitoring and detecting atrial fibrillation using wearable technology. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 3394–3397.
- Andrius Sološenko; Andrius Petrėnas; Vaidotas Marozas; Leif Sörnmo; Modeling of the photoplethysmogram during atrial fibrillation. Computers in Biology and Medicine 2017, 81, 130-138, 10.1016/j.compbiomed.2016.12.016.
- Aliamiri, A.; Shen, Y. Deep learning based atrial fibrillation detection using wearable photoplethysmography sensor. In Proceedings of the 2018 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018; pp. 442–445.
- Tommaso Sanna; H. C. Diener; Rod Passman; Vincenzo Di Lazzaro; Richard A. Bernstein; Carlos A. Morillo; Marilyn Mollman Rymer; Vincent Thijs; Tyson Rogers; Frank Beckers; et al.Kate LindborgJohannes Brachmann Cryptogenic Stroke and Underlying Atrial Fibrillation. New England Journal of Medicine 2014, 370, 2478-2486, 10.1056/nejmoa1313600.
- K Harris; D Edwards; Jonathan Mant; How can we best detect atrial fibrillation?. The Journal of the Royal College of Physicians of Edinburgh 2012, 42, 5–22, 10.4997/JRCPE.2012.S02.
- Moody, G.B.; G, M.R. A new method for detecting atrial fibrillation using R-R intervals. Comput. Cardiol. 1983, 10, 227–230.
- Goldberger, A.L.; Amaral, L.A.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220.
- Developed with the special contribution of the European Heart Rhythm Association (EHRA); A. John Camm; Paulus Kirchhof; Gregory Y.H. Lip; Ulrich Schotten; Irene Savelieva; Sabine Ernst; Isabelle C. Van Gelder; Nawwar Al-Attar; Gerhard Hindricks; et al.Bernard PrendergastHein HeidbuchelOttavio AlfieriAnnalisa AngeliniDan AtarPaolo ColonnaRaffaele De CaterinaJohan De SutterAndreas GoetteBulent GorenekMagnus HeldalStefan H. HohloserPhilippe KolhJean-Yves Le HeuzeyPiotr PonikowskiFrans H. RuttenAlec VahanianAngelo AuricchioJeroen J BaxClaudio CeconiVeronica DeanGerasimos FilippatosChristian Funck-BrentanoRichard HobbsPeter KearneyTheresa McDonaghBogdan A. PopescuŽeljko ReinerUdo SechtemPer Anton SirnesMichal TenderaPanos E. VardasPetr WidimskyVazha AgladzeEtienne AliotTosho BalabanskiCarina Blomstrom-LundqvistAlessandro CapucciHarry J G M CrijnsBjörn DahlöfThierry FolliguetMichael GliksonMarnix GoethalsDietrich C. GulbaSiew Yen HoRobert J. M. KlautzSedat KoseJohn J V McMurrayPasquale Perrone FilardiPekka RaatikainenMaria Jesus SalvadorMartin J. SchalijAlexander ShpektorJoão SousaJanina StepinskaHasso UuetoaJose Luis ZamoranoIgor ZupanEndorsed by the European Association for Cardio-Thoracic Surgery (EACTS)ESC Committee for Practice Guidelines (CPG)Document Reviewers Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). European Heart Journal 2010, 31, 2369-2429, 10.1093/eurheartj/ehq278.
- Marco Budeus; Markus Hennersdorf; Christian Perings; Bodo E. Strauer; Der Nachweis atrialer Sp�tpotentiale mittels P-Wellen-Signalmittelungs-EKG bei Patienten mit paroxysmalem Vorhofflimmern. Zeitschrift für Kardiologie 2003, 92, 362-369, 10.1007/s00392-003-0921-8.
- Jibin Wang; Ping Wang; Automated detection of atrial fibrillation in ECG signals based on wavelet packet transform and correlation function of random process. Biomedical Signal Processing and Control 2020, 55, 101662, 10.1016/j.bspc.2019.101662.
- Ping Cao; Xinyi Li; Kedong Mao; Fei Lu; Gangmin Ning; Luping Fang; Qing Pan; A novel data augmentation method to enhance deep neural networks for detection of atrial fibrillation. Biomedical Signal Processing and Control 2020, 56, 101675, 10.1016/j.bspc.2019.101675.
- Italo Agustin Marsili; Luca Biasiolli; Michela Masè; Alberto Adami; Alberto Oliver Andrighetti; Flavia Ravelli; Giandomenico Nollo; Marsili Ia; Biasiolli L; Michela Masè; et al.AdamI AAndrighetti AoRavelli FNollo G Implementation and validation of real-time algorithms for atrial fibrillation detection on a wearable ECG device. Computers in Biology and Medicine 2020, 116, 103540, 10.1016/j.compbiomed.2019.103540.
- Xin-Cheng Cao; Bin Yao; Bin-Qiang Chen; Atrial Fibrillation Detection Using an Improved Multi-Scale Decomposition Enhanced Residual Convolutional Neural Network. IEEE Access 2019, 7, 89152-89161, 10.1109/access.2019.2926749.
- Chengyu Liu; Julien Oster; Erik Reinertsen; Qiao Li; Lina Zhao; Shamim Nemati; G. D. Clifford; A comparison of entropy approaches for AF discrimination. Physiological Measurement 2018, 39, 074002, 10.1088/1361-6579/aacc48.
- Yong Xia; Naren Wulan; Kuanquan Wang; H. Zhang; Detecting atrial fibrillation by deep convolutional neural networks. Computers in Biology and Medicine 2018, 93, 84-92, 10.1016/j.compbiomed.2017.12.007.
- Padmavathi Kora; Ambika Annavarapu; Priyanka Yadlapalli; K. Sri Rama Krishna; Viswanadharaju Somalaraju; ECG based Atrial Fibrillation detection using Sequency Ordered Complex Hadamard Transform and Hybrid Firefly Algorithm. Engineering Science and Technology, an International Journal 2017, 20, 1084-1091, 10.1016/j.jestch.2017.02.002.
- R.K. Tripathy; Mario R. Arrieta Paternina; Juan G. Arrieta; P. Pattanaik; AUTOMATED DETECTION OF ATRIAL FIBRILLATION ECG SIGNALS USING TWO STAGE VMD AND ATRIAL FIBRILLATION DIAGNOSIS INDEX. Journal of Mechanics in Medicine and Biology 2017, 17, 1740044, 10.1142/s0219519417400449.
- Ambika Annavarapu; Padmavathi Kora; ECG-based atrial fibrillation detection using different orderings of Conjugate Symmetric–Complex Hadamard Transform. International Journal of the Cardiovascular Academy 2016, 2, 151-154, 10.1016/j.ijcac.2016.08.001.
- Nurul Ashikin Abdul-Kadir; Norlaili Mat Safri; Mohd Afzan Othman; Dynamic ECG features for atrial fibrillation recognition. Computer Methods and Programs in Biomedicine 2016, 136, 143-150, 10.1016/j.cmpb.2016.08.021.
- Yuan, C.; Yan, Y.; Zhou, L.; Bai, J.; Wang, L. Automated atrial fibrillation detection based on deep learning network. In Proceedings of the 2016 IEEE International Conference on Information and Automation (ICIA), Ningbo, China, 1–3 August 2016; pp. 1159–1164.
- Shadnaz Asgari; Alireza Mehrnia; Maryam Moussavi; Automatic detection of atrial fibrillation using stationary wavelet transform and support vector machine. Computers in Biology and Medicine 2015, 60, 132-142, 10.1016/j.compbiomed.2015.03.005.
- K. Daqrouq; A. Alkhateeb; M.N. Ajour; A. Morfeq; Neural network and wavelet average framing percentage energy for atrial fibrillation classification. Computer Methods and Programs in Biomedicine 2014, 113, 919-926, 10.1016/j.cmpb.2013.12.002.
- Roshan Joy Martis; U.Rajendra Acharya; Hari Prasad; Chua Kuang Chua; Choo Min Lim; Automated detection of atrial fibrillation using Bayesian paradigm. Knowledge-Based Systems 2013, 54, 269-275, 10.1016/j.knosys.2013.09.016.
- U. Maji; Madhuchhanda Mitra; Saikat Pal; Automatic Detection of Atrial Fibrillation Using Empirical Mode Decomposition and Statistical Approach. Procedia Technology 2013, 10, 45-52, 10.1016/j.protcy.2013.12.335.
- Rincón, F.; Grassi, P.R.; Khaled, N.; Atienza, D.; Sciuto, D. Automated real-time atrial fibrillation detection on a wearable wireless sensor platform. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2472–2475.
- Jinseok Lee; Bersain A. Reyes; David D. McManus; Oscar Maitas; Ki H. Chon; Atrial Fibrillation Detection Using an iPhone 4S. IEEE Transactions on Biomedical Engineering 2012, 60, 203-206, 10.1109/tbme.2012.2208112.
- M Fukunami; T Yamada; M Ohmori; K Kumagai; K Umemoto; A Sakai; N Kondoh; T Minamino; N Hoki; Detection of patients at risk for paroxysmal atrial fibrillation during sinus rhythm by P wave-triggered signal-averaged electrocardiogram.. Circulation 1991, 83, 162–169.
- Parvaresh, S.; Ayatollahi, A. Automatic atrial fibrillation detection using autoregressive modeling. In Proceedings of the 2011 International Conference on Biomedical Engineering and Technology, APCBEES, Kuala Lumpur, Malaysia, 4–5 June 2011; Volume 4.
- Hamid Khorrami; Majid Moavenian; A comparative study of DWT, CWT and DCT transformations in ECG arrhythmias classification. Expert Systems with Applications 2010, 37, 5751-5757, 10.1016/j.eswa.2010.02.033.
- Censi, F.; Calcagnini, G.; Mattei, E.; Gargaro, A.; Biancalana, G.; Capucci, A. Simulation of monitoring strategies for atrial arrhythmia detection. Annali dell’Istituto Superiore di Sanita 2013, 49, 176–182.
- Capucci, A.; Calcagnini, G.; Mattei, E.; Triventi, M.; Bartolini, P.; Biancalana, G.; Gargaro, A.; Puglisi, A.; Censi, F. Daily distribution of atrial arrhythmic episodes in sick sinus syndrome patients: Implications for atrial arrhythmia monitoring. Europace 2012, 14, 1117–1124.
- Simona Petrutiu; Jason Ng; Grace M. Nijm; Haitham Al-Angari; Steven Swiryn; Alan V. Sahakian; Atrial fibrillation and waveform characterization. IEEE Engineering in Medicine and Biology Magazine 2006, 25, 24-30, 10.1109/emb-m.2006.250505.
- Chao Huang; Shuming Ye; Hang Chen; Dingli Li; Fangtian He; Yuewen Tu; A Novel Method for Detection of the Transition Between Atrial Fibrillation and Sinus Rhythm. IEEE Transactions on Biomedical Engineering 2010, 58, 1113-1119, 10.1109/tbme.2010.2096506.
- Tateno, K.; Glass, L. A method for detection of atrial fibrillation using RR intervals. In Proceedings of the 2000 Annual International IEEE Conference Computers in Cardiology, Cambridge, MA, USA, 24–27 September 2008; pp. 391–394.
- Hadhoud, M.M.; Eladawy, M.I.; Farag, A. Computer aided diagnosis of cardiac arrhythmias. In Proceedings of the 2006 International Conference on Computer Engineering and Systems, Cairo, Egypt, 5–7 November 2006; pp. 262–265.
- Hagiwara, Y.; Fujita, H.; Oh, S.L.; Tan, J.H.; San Tan, R.; Ciaccio, E.J.; Acharya, U.R. Computer-aided diagnosis of atrial fibrillation based on ECG signals: A review. Inf. Sci. 2018, 467, 99–114.
- C.W. Israel; G. Grönefeld; J.R. Ehrlich; Y.G. Li; S.H. Hohnloser; Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device. Implications for optimal patient care. ACC Current Journal Review 2004, 13, 49-50, 10.1016/j.accreview.2004.03.016.
- R L Page; W E Wilkinson; W K Clair; E A McCarthy; Edward L.C Pritchett; Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia.. Circulation 1994, 89, 224-227, 10.1161/01.cir.89.1.224.
- Andresen, D.; Brüggemann, T. Heart rate variability preceding onset of atrial fibrillation. J. Cardiovasc. Electrophysiol. 1998, 9, S26-9.
- Murgatroyd, F.D.; Xie, B.; Copie, X.; Blankoff, I.; Camm, A.J.; Malik, M. Identification of Atrial Fibrillation Episodes in Ambulatory Electrocardiographic Recordings: Validation of a Method for Obtaining Labeled R-R Interval Files. Pacing Clin. Electrophysiol. 1995, 18, 1315–1320.




