
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sadanand Pandey | + 7045 word(s) | 7045 | 2021-07-14 11:43:59 | | | |
| 2 | Conner Chen | Meta information modification | 7045 | 2021-07-26 02:57:08 | | |
Video Upload Options
The rapid development of multidrug co-delivery and nano-medicines has made spontaneous progress in tumor treatment and diagnosis. DNA is a unique biological molecule that can be tailored and molded into various nanostructures. The addition of ligands or stimuli-responsive elements enables DNA nanostructures to mediate highly targeted drug delivery to the cancer cells. Smart DNA nanostructures, owing to their various shapes, sizes, geometry, sequences, and characteristics, have various modes of cellular internalization and final disposition. On the other hand, functionalized DNA nanocarriers have specific receptor-mediated uptake, and most of these ligand anchored nanostructures able to escape lysosomal degradation.
1. Classification and Applications of Smart Nanocarrier System in Cancer Targeting
DNA is a novel and smart biomaterials that can be implied to synthesize the various types of nanocarriers system based on its key GC/AT complementary base pairing. The data from numerous studies revealed that the DNA nanostructure is an effective tool for addressing major issues in cancer care, such as toxicity and drug efficacy. Therefore, some significant improvements have been made in recent years [1]. One of the advancements to these intelligent nanocarrier systems is multidrug co-delivery, which increases the targetability with the help of various ligands and adaptations of active target strategies. Different methods are adopted for the preparation of DNA based nanocarrier systems. Basically, these nanocarrier systems consist of functional DNA sequences, biomloecules, that are bound using physical, chemical, or biological engineering tools. Back into the history regarding the evolution of DNA based nanocarrier system, first static four-arm structure of DNA was designed by Nadrain Seeman in 1983, consisting of four strands of DNA. Each strand has a different base sequence to make a junction point at specific loci [2]. These static DNA joints are basic blocks to design a stable and rigid DNA nanostructures. With further advancement in this field more arms including three, five, six, eight, twelve were generated for the production of various DNA nanostructures. Structural DNA nanotechnology has become significantly important in the field of nanoscience since the 1980s [3]. The various dynamic and static data DNA devices with various dimensions and structures have been introduced and developed. The pure DNA consisted of nanostructure have been divided into various types as shown in schematic representation (Figure 1) of different classification of DNA based nanosystem [4].
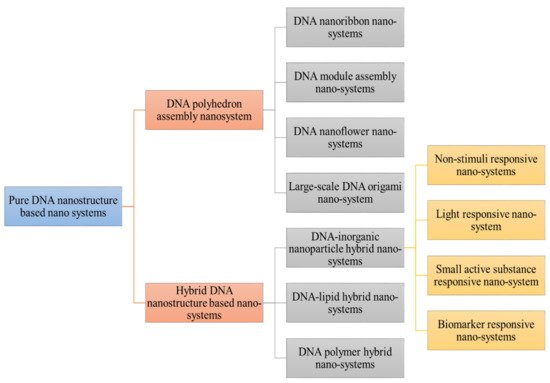
Figure 1. Demonstrated different classification of DNA nanostructure. Pure DNA nanostructure is divided into DNA polyhedron assembly nanosystem (DNA nanoribbon, DNA module assembly nanosystem, DNA nanoflower system) and hybrid DNA nanosystem (DNA-inorganic nanoparticle hybrid nanosystems (non-stimuli responsive, small active responsive, biomarker responsive) DNA lipid hybrid and DNA polymer hybrid nanocargo.
The DNA polyhedron nanosystems have been designed from tetrahedron and DNA octahedron to DNA icosahedron that served as simple carriers in anti-cancer drug delivery. Tuberfield and his colleagues developed classic DNA tetrahedron for the first time. Thereafter, it has been used as an efficient cargo for anti-cancer drugs including photosensitizer, DOX, siRNA, and other drugs concurrently. The anti-neoplastic drugs (Dox, doxorubicin) encapsulated into DNA tetrahedron can kill the circulating tumor cells (CTC) [5][6]. Furthermore, the light will cause the photosensitizer marked on the DNA tetrahedron, resulting in enhanced cytotoxic effects. There are several abilities of DNA nanodevices to increase the endocytotic uptake of anti-neoplastic agents and also increased the drug loading capacity with greater efficacy. Most of the present literature study data emphasize the progress in modification to increase the drug ability and to decrease its adverse effects [7]. One research group created a DNA tetrahedron to encapsulate DOX with available conjugation sites for attaching cetuximab antibodies that target the epidermal growth factor receptor specifically. The findings of the following study showed that this nanosystem have greater targeting ability and better killing efficacy of malignant cells. Chen et al. developed biotins conjugated to DNA tetrahedron (ruthenium polypyridyl complexes). The DNA cage also increases its specific cellular uptake along with drug cytotoxicity and retention against HepG2 cells [8][9].
Lo’s group has produced a DNA nanocage for the first time for mitochondrial delivery of DOX by conjugating lipids. In contrast to DOX localization in lysosomes, DOX retention in mitochondria causes major cytotoxicity and cellular apoptosis in MCF-7 cells, according to the findings. However, with the introduction of stimuli responsive DNA tetrahedrons and switchable DNA nanosuitcases, more stimuli responsive DNA polyhedron drug delivery strategies will be established and used in advanced nanotechnology cancer treatment [10][11].
Aside from hybridization, catalytic hairpin conjugation may generate DNA nanoribbons. Rigid and programmable DNA tiles have previously been used to cause significant one-dimensional (1D) nanoribbons, nanotubes, two-dimensional (2D) arrays, and even three-dimensional (3D) crystals [12]. By use of different technologies number of researchers design different nanoribbons to deliver the siRNA, DOX, photosensitizer, and so on [13]. Weizmann et al. developed DNA nanoribbon by a modified DNA origami strategy. Furthermore, various studies proved that the DNA nanoribbons was an efficient siRNA delivery cargo in human cells cancer. The functionalized DNA nanoribbon structures and devices show extraordinary performance in cancer diagnosis and treatment because of their small sizes, morphology, and greater biocompatibility. Several research groups collaborated to develop various types of DNA nanoribbons, for example, Liang et al. developed DNA nanoribbon with two compartments, one was loaded with -GC- base pairs for DOX delivery. Another component was the AS1411 aptamer, which is a DNA aptamer. The following system helped to increase the tolerability of human breast cancer cells to the DOX with inhibition of tumor cell proliferation. Self-assembled DNA nanocentipede was developed by Roh et al. to deliver multivalent aptamers to functionalize in cancer targeting [14][15]. Chu’s and his colleagues developed an aptamer probe to target the cancer cells via structure switching. Hybridization chain reaction (HCR) accumulated higher encapsulated prodrugs from a drug labeled probe and induced their conversion and uptake into cisplatin in cells for selective tumor targeting using this strategy [16]. Another type of DNA assembly nanosystems was designed by a group of researchers. They classified these materials into two groups: DNA nanohydrogels and DNA dendrimers [17]. DNA dendrimers are basically hybridized layer by layer self-assembled functional branched DNA [18]. DNA nanohydrogels, on the other hand, are made from functional building blocks by base-pairing hybridization or liquid crystallization and dense packaging. Since they can be configured into and provide further docking sites to encapsulate drugs or other functional elements, these DNA nanosystems are denser. Yang et al. developed DNA dendrimers and encapsulate DOX. Other researchers groups developed nanohydrogels from hybridization of different building blocks to synergistic cancer therapy with Dox [19][20]. Different researchers have applied different methods for the development of DNA nano-hydrogels for targeting DOX delivery by using building blocks and liquid crystallization without base-pairing hybridization. The DNA nanohydrogel is comprised of three building blocks unit including functional moiety, DNAzyme, and aptamer. Each of these parts have different functionalization. These three parts are self-assembled into nanohydrogels by hybridization between sticky ends [21].
DNA nanoflower system in comparison to self-assembled, form long DNA strands via rolling circle replication along with liquid crystallization and dense packaging. Despite the drawbacks of large nanostructures, the above type of nanostructure seems to have its own set of characteristics. To deliver anti-cancer drugs, this form of structure is very light in sequence design and its size can be tuned by varying the assembly time and template sequence. A group of researchers had developed series of nanoflowers to encapsulate the anti-cancer agents (CpG, DOX). Furthermore, the researchers modify the nanoflowers to encapsulate different types of agents for multigene therapy [22][23].
Since DNA origami is large and dense, it has a high ability to target tumors without the need for targeted modification. The first DNA origami design was complicated because it relied on the hybridization of a long ssDNA from the M13 phage genome with hundreds of short-staple strands. However, further improvements in this design were implied by many researchers to simplify the method of its development by using an RCP-amplified scaffold in replacement to ssDNA from the M13 phage [24]. Likewise, with other DNA-based nanostructures, it is efficient for DOX, CpG, photosensitizer, etc. More advanced DNA origami structures include DNA rod/tube and DNA triangle to encapsulate a high load of drugs. Another study used DOX encapsulated DNA origami delivery systems that can induce remarkable cytotoxicity in cancer targeting. Bachelet et al. designed a hollow hexagonal barrel-shaped DNA origami as a wonderful logic gated nanorobot to handle the release of encapsulated molecules while identifying specific receptor proteins [25]. Following that, they build more complex nanorobots that can interact with one another and generate logical outputs to turn molecular payloads on or off [26].
DNA structure is further classified into hybrid DNA nanostructured system that is subdivided into DNA-inorganic nanoparticle (non-stimuli responsive, light-responsive, small molecule, DNA lipid hybrid, DNA polymer hybrid nanosystems, and small active substance responsive nanosystem) [27]. DNA-inorganic nanoparticles hybrid system including non-stimuli responsive systems has been designed for better cancer treatment. This system included both non-stimuli responsive and stimuli-responsive nanocarrier systems, which are commonly constructed, based on the change DNA configuration [28]. Present literature mentioned that nanoflower inorganic nanoparticles have a spherical shape and increased the concentration of drug at the malignant site [28][29]. They developed AS1411/magnetic nanoparticles for targeted TMPyP4 delivery in this type of non-stimuli inorganic nanoparticle method. They also developed an Sgc8/MNP nanosystem and peptide/Au NPs for targeted DOX delivery [30][31]. Jiang and Zhang et al. engineered DNA nanoflower/polyhedron on nanoparticles for DOX delivery and photosensitizer co-delivery [32]. Ding et al. developed a triangle DNA origami-gold nanorod complex that showed distinguish increase in cellular uptake and enhanced photothermal effect of Au against tumor cells. Light responsive nanosystems used dsDNA to connect with inorganic nanoparticles. AuNPs are representative of light-responsive nanosystem because AuNPs can convert light into heat to assist in the degradation of dsDNA and further release of drugs [33]. Huang’s group developed AS1411 aptamer conjugated dsDNA hybrid nanostructures for co-delivering of Dox and TMPyP4. By applying heat or light effect on Au-Ag nanorod drug can be accumulated in higher concentration in the nuclei to efficiently kill the cancer cells. In a study, mesoporous silica nanoparticles were developed to perform on-demand stimuli response of therapeutics. Single-stranded DNA was ligated to magnetic nanoparticles. Magnetic nanoparticles were then decorated with complementary DNA sequences. The uncapping and subsequent release of mesopore-filled model drug was caused by DNA double stranded melting as a result of temperature increase [34]. DNA lipid hybrid nanosystems are another type of DNA assessed delivery system, where functional DNA can connect to lipid to form hybrid nanosystems for tumor targeting. DNA polymer hybrid nanosystems are called hybrids as they can self-assemble into spherical structures without any complex design structure. Additionally, they are very supportive of other active agents like paclitaxel in the hydrophobic parts. Another type of DNA nanocargo includes polymer hybrid nanosystem that has greater encapsulation efficiency and can protect the drug against premature degradation. This property of the polymer hybrid system further helped to design the stimuli-responsive nanosystem [35]. Willners et al. developed a poly-function core and multilayer shell-based DNA polymer hybrid system for controlled release. Table 1 demonstrated the prerequisite of DNA nanostructures along with their surface characteristics for particular organ targeting. These specific DNA assemblies were designed to identify the specific stimuli like pH, light, ATP to modify their conformation for drug release [36].
Table 1. Prerequisite of DNA nanostructures to fulfill the mean particle diameter for specific organ targeting.
| Targeting Site | Mean Particle Diameter | Surface Characteristics | Ref |
|---|---|---|---|
| Bone | Undefined | Substances like aspartic acid, alendronate can adhere to the bone and can be used for bone targeting. | [37] |
| Liver | Less than 100 nm to cross the liver fenestrae and target the hepatocytes. Greater than 100 nm uptake by Kupffer cells. | No define surface property needed | [37][38] |
| Lung | Particles larger than 200 nm are trapped into lung capillaries | Cationic surface charge | [39] |
| Brain | 5–100 nm: nanoparticles uptake efficiency decreases with size | Hydrophobic moieties and neutral charge enhance the brain uptake | [37][40] |
| Lymph nodes | 1–40 nm: intra-tracheal administration 80 nm: Subcutaneous application |
Non-pegylated, Non-cationic, and sugar-based particles. | [37][38] |
DNA-based nanosystems developed circular DNA nanotechnology for ligand functionalization (neuregulin-1/NRG-1) and its biological application [41]. A group of researchers developed DNA nanospindals (DNA-NS) to efficiently load daunorubicin (DR) and target the HER2/neu receptors on the plasma membrane of drug-resistant MCF-7 (breast cancer) cells. DR loading onto DNA-NS was confirmed by the UV-shift analysis. The MTT results showed reduced viability of the MCF-7 cells after treatment with DNA-NS. Further results of apoptosis/proliferation obtained via flow cytometry showed enhanced apoptosis up to (64%) after treating with DNA-NS. Hence, all the types of DNA nanostructures in cancer therapy showed stiffer, uniform, and more biocompatible-targeted therapy [42]. Figure 2 shows types of DNA nanostructures.
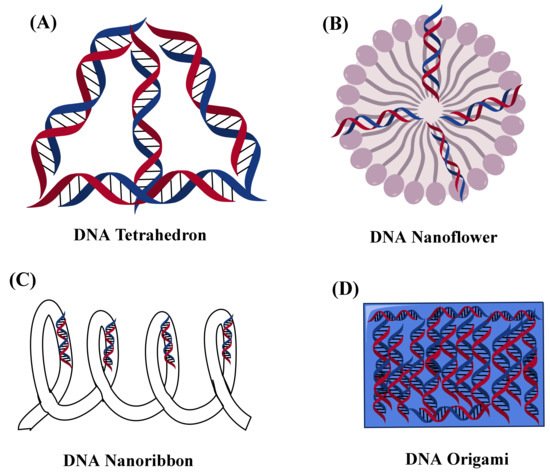
Figure 2. Showed the nanostructure representation of different types of DNA (A) DNA tetrahedron, (B) DNA nanoflower, (C) DNA nanoribbon, and (D) DNA Origami.
2. DNA Assessed Stimuli-Responsive Nanoparticle System for Cancer Targeting
2.1. Exogenous Stimuli-Responsive Nanocarrier System for Diagnosis and Treatment of Cancer
The application of external stimuli has several advantages for targeting delivery to tumors: (I) the location and intensity of applied stimuli could be precisely controlled; (II) the stimuli can be added or removed based on available treatment requirements; (III) several different types of stimuli could be used for multifunction in cancer theranostics; (IV) the possibility to provide continuous or multi-times stimuli for drug therapy and delivery [43]. Table 2 shows different types of stimuli for gene and drug delivery for cancer targeting.
Table 2. Exogenous and endogenous stimuli and nanocarriers system for gene and drug delivery at the tumor site.
| Exogenous and Endogenous Stimuli and Delivery System | Encapsulated Moiety | Application | Advantages | Limitation | Ref |
|---|---|---|---|---|---|
NIR light
|
DOX | Ablation of the tumor via photothermal chemotherapy | Easily tuned, Deep penetration, greater precision, no damaging, minimally invasive | Ionization radiation, Expensive equipment | [43] |
|
DOX and Camptothecin | Photodynamic and Chemotherapy | |||
Ultrasound nanoparticles
|
DOX | Targeted drug delivery to the tumor site | Low cost, greater patient compatibility, no ionizing radiations | Difficult to remove the remote and moving targets | [44][45] |
|
siRNA | Image modulated therapy | |||
Magnetic field
|
Paclitaxel, Curcumin, Camptothecin |
Targeted delivery against tumor imaging and therapy, targeted delivery by magnetic hyperthermia | No ionizing radiation, deep penetration, imaging opportunity, energy modulation with atomic force microscopy (AFM) | Expensive, limited to the surface tumors, increased cytotoxicity, accumulation can lead to emboli formation | [43][46] |
Temperature
|
DOX and curcumin | Targeted drug release | High mobility of matrix, High precision, inexpensive | Limited tissue penetration | [15] |
pH
|
Plasmid DNA | Cytoplasmic delivery | Cationic polymer induces membrane fusion at endosomal pH, Improved anti-cancer property in a murine tumor model, Increased gene transfection to hepatocytes | Heterogeneity and diversity of cancer cells can limit the targeted delivery | [47] |
Redox sensitive
|
Plasmid DNA | Targeted delivery | Thioplexes release DNA in a reductive environment | Heterogenicity of cancer cells and accumulation of nanoparticles may cause toxicity | [48][49] |
|
Lucifer yellow Iodoacetamide | More than 90% release was achieved at 42 °C at the targeting site | Showed several-fold increase in targeting moiety in tumor-bearing mice | Heterogenicity of cancer cells, Toxicity of nanoparticles inside the vital organs | [50][51] |
2.2. Ultrasound Responsive Nanocarriers
A high-intensity sound wave could affect nanocarriers for controlled release at malignant sites. For various applications, the ultrasound intensity may be changed. The ultrasonic intensity can be modified for different uses. At low frequencies, it could be used for imaging, and at higher frequencies, it could be used as a catalyst to release drugs from nanocarriers or increase the permeability of malignant cell membranes. There are several sizes of microbubbles that have been developed for ultrasound imaging and also commercialized as Albunex, Sonazoid, Optison, etc [41][52]. Microbubbles’ large size (1–10 µm), short half-life, and low stability restricted access to the vascular compartment in tumor tissues. As a result, several switchable microbubbles or nanocarriers for ultrasound imaging have been produced. The ultrasound-sensitive nanocarriers include air, perfluorocarbons, N2, etc. or gases releases in the biological environment.
Porphyrin microbubbles (1–10 µm) may be transformed into nanobubbles (5–500 nm) for tumor imaging using an ultrasound-responsive nanocarriers strategy [53]. Due to the collapse of microbubbles in response to low-intensity ultrasound waves, phase-changeable polymeric nanoparticles could be produced for tumor imaging and doxorubicin release. The large size of ultrasound-sensitive nanoparticles may limit the penetration across the malignant site. Furthermore, the drug encapsulated ultrasound-sensitive nanomaterials can be applied for tumor application, theranostics, and image-guided therapy. One study group developed a nanocarrier emulsion made up of perfluoropentane nanodroplet within the aqueous layer of a liposome, along with anticancer drug doxorubicin [54]. The liposomes encapsulated with DOX showed its release on insonation with low-intensity ultrasound at 20-kHz, 1.0 MHz, and 3.0 MHz. More release occurs in vitro at 20 kHz than at greater frequencies. The results showed that this system promises to have more efficient therapy and tumor treatment to decrease the adverse effects of cardiotoxicity caused by Dox. In another research, liposomes were encapsulated with docetaxel and NH4HCO3 to generate CO2 bubbles in tumors for dual ligand-based targeted delivery and ultrasound imaging. One study claimed multimodal ultrasound imaging and molecular biosensors application of nanodroplets bubble vesicles by using genetically encoded nanostructure from microorganisms [55].
Gaspar et al. developed DOX and DNA micelleplexes for co-delivery via stimuli sensitive polymeric nanocarriers. The obtained results showed that minicircle DNA (mcDNA) encapsulated micelleplexes into in vitro tumor spheroid models with specific kinetic and show enhanced gene expression in comparison to other nanocarriers. Moreover, dual-loaded micelleplexes showed a significant uptake and cytotoxic effect in cancer. The findings revealed that triblock micelles are effective for in vivo delivery and have the potential to be used in DNA therapy. Gaspar et al. developed a gas penetrating stimuli sensitive hollow microspheres as a strategy to co-deliver Dox and minicircle DNA. The results demonstrate that microcarriers produced gas-mediated Dox release and dual loaded particles achieved 5.2-fold greater cellular internalization in comparison to non-pegylated microspheres [56]. A stronger cytotoxic effect occurred from the increased cellular concentration. The enhanced transgene expression was obtained after nanoparticle-mcDNA co-delivery in the microspheres. The results showed that nanoparticle-microsphere systems achieve efficient co-delivery of different drug-mcDNA combinations [57]. Figure 3 demonstrate the application of liposomes nanosystem to the cancer site. The stimuli used was ultrasound that releases the payloads with insonation at low intensity to the targeted cell.
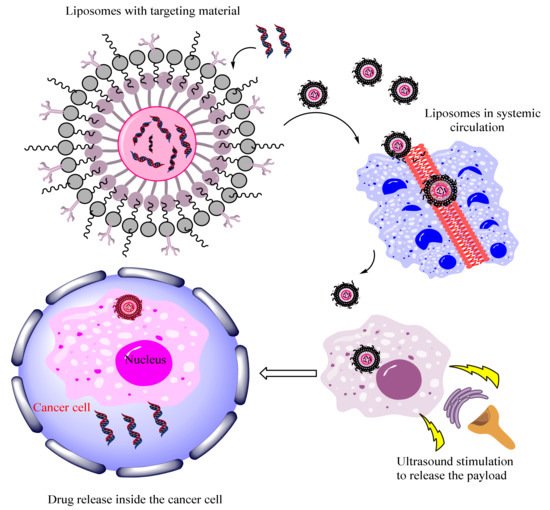
Figure 3. Demonstrated the application of ultrasound responsive liposome-carrier system for cancer targeting.
2.3. Magnetic Field Triggered Therapy
Magnetic stimulation candidates include core shell-dependent nanoparticles coated with silica polymer or magnetoliposome. Magnetically coated nanoparticles may also be used to transport genetic information. When held under an oscillating magnetic field, magnetic nanocarriers can generate heat in close proximity. The structure of nanocarriers can be altered by heat. Attractive Magnetic nanoparticles (MNPs) with the ability to react to a magnetic field can be used in gene and drug delivery using magnetic targeting. Different malignant cells, such as brain, lung, breast, and prostate cancer, have been targeted with magnetic targeting. Similarly, a magnetic field may cause the targeted transmission to a specific location, and MNPs have been used to transfect DNA and RNA [43][58]. The drug delivery system based on MNPs not only delivers the drugs to a particular location but also regulates their release. Drugs can be attached to MNPs by conjugation on a heat-sensitive linker or through p-p interaction and in some situations by co-embedding within thermally sensitive polymers. Under an alternating magnetic field, MNPs can produce heat that can improve the drug release due to the cracking of the polymer or linker [59]. The MNPs heat can generate pressure inside the porous NPs, triggering the drug release. Dobson et al. attributed it to the association of magnetic vectors with membranes and transmission of mechanical forces from the lateral movement of the magnetic field to cellular membranes [60]. The magnetic materials can be applied for tumor imaging via magnetic resonance imaging (MRI). Moreover, besides contrast agents’ plasmids, anti-bodies, photosensitizer can also be incorporated inside the magnetic sensitive nanoparticles to achieve multiple multimodal therapeutic effects. The alternating magnetic field sensitive hyperthermia can induce the release of drug from nanocarriers in diseased regions that is tumor or cancer cells [61]. The PEGylated MoS2/Fe3O4 nanocomposites made via two-step hydrothermal method have shown greater efficiency for tumor targeting. The two-step hydrothermal method demonstrated greater potential for tumor diagnosis by T2-weighted imaging and photoacoustic tomography. Moreover, it further allowed both T1 and T2 weighted MRI of tumors by doping Mn into core of Fe3O4@MoS2 multifunctional nanoflowers [62].
2.4. Thermo-Responsive Nanocarriers Applied for Diagnosis and Treatment of Cancer
Thermo-responsiveness can be defined as the ability of a substance or material to undergo drastic changes in at least one of its physicochemical properties upon variation in temperature [63]. Due to the phase transition behavior, tunable and versatile design, temperature responsive polymers have been extensively studied as smart drug delivery systems [64]. A temperature change can be easily controlled and implemented in vitro/in vivo with convenience. Temperature is also a unique stimulus than others as it can be utilized as an external as well as an internal stimulus.
Temperature acts as an external stimulus when heat is provided from outside of the body or by irradiation, electric field, magnetic field, etc. External heating can also result in the direct killing of cancer cells, as they are naturally susceptible to heat. Temperature is utilized as an internal stimulus when certain pathological conditions elevate the temperature of the specific site in the body. Due to the Warburg effect, tumors show a slight 2–3 degree elevated temperature (40–42 °C) than the normal tissues (37 °C). A change in temperature around the drug-carrying system leads to a sharp non-linear change in the temperature sensitive element of the carrier system resulting in drug release. Ideally, these nanocarriers should be able to maintain the drug load at normal body temperature and should only release the drug in an elevated temperature environment [65][66][67].
To date, many thermo-responsive nanocarriers have been successfully synthesized including liposomes, nanocomposites, nanogels, polymeric micelles, nanocapsules and vesicles. These nanocarriers are either developed with a material that changes their physicochemical properties upon variation in temperature or by incorporating a thermally unstable polymer [68]. For example, liposomes incorporated with NH4HCO3 generated CO2 from local hyperthermia of tumor resulting in swelling and collapsing of the system. This resulted in an efficient drug release [69].
Generally, temperature responsive materials or polymeric nanoparticles can be prepared from techniques like free radical polymerization followed by hydrolysis, phase separation, emulsion, foaming and graft copolymerization mediated by UV irradiation, etc. [70]. Recently advanced polymerization techniques are being used for developing and functionalizing new thermo-responsive polymers. Reversible deactivation radical polymerization (RDRP) techniques, which include atom transfer radical polymerization (ATRP), nitro-oxide mediated polymerization (NMP), and reversible addition-fragmentation chain transfer (RAFT) enables the development of complex macromolecular structures with low variance and high chain-end precision along with other wide range of functionalization options. Ring-opening polymerization (ROP) technique allows the synthesis of well-defined polymers [63].
The fundamental principle of thermo-responsive polymers is based on critical solution temperature (CST). These polymers exhibit a change in their solubility in response to changes in temperature. CST is a temperature at which separation of polymer phase occurs. CST is further divided into lower critical solution temperature (LCST) or upper critical solution temperature (UCST) [71]. Controlled drug delivery systems can be achieved by controlling LCST or UCST which results in phase transition followed by either swelling or shrinking. The majority of polymers are synthesized based on their LCST. The LCST transition is dependent on the nature of the polymer rather than the carrier state like micelles, hydrogels, etc. Below the LCST, the polymer exists in a monophasic and hydrophilic state. Above the LCST it exists in an insoluble, biphasic, and hydrophobic state [1]. At this stage the polymer solution becomes cloudy and the effect is known as the ‘cloud point’. This effect is related to the concentration of the polymer and other constituents [72]. An increase in temperature above LCST disintegrates the network due to coil to globule transition. As a consequence, volume shrinkage occurs that forces the encapsulated contents to squeeze out and subsequently drug release. Such polymers are termed negative thermosensitive polymers [70].
In case of UCST polymers, the increase in temperature above UCST increases the solubility of the polymer and subsequently swelling. However, only a little research has been conducted on these thermo-positive polymers. It should be noted that the changes in the volume are reversible and referred to as ‘swelling-shrinking’ behavior [70][73].
Factors that can affect the LCST and UCST values include pendant functional groups, polymer concentration, and polarity of the medium and molecular weight of the polymer [74]. Since the temperature range from normal physiological sites of the body (37 °C) to diseased sites (40–42 °C) is narrow, thermo-responsive carriers should be able to undergo phase transition precisely. This is important to avoid the advanced release of drugs at normal body temperature [75].
Out of various temperature sensitive polymers, poly (N-isopropyl acrylamide) or PNIPam is the most studied thermo-negative polymer. PNIPam is a non-ionic polymer that is synthesized by radical polymerization of N-isopropyl acrylamide. The LCST value of PNIPam is around 32 °C, closer to the normal body temperature. An adjustment in its phase transition temperature can be achieved by copolymerizing with other polymers. Hydrophilic monomers like acrylic acid cause the temperature to increase while a hydrophobic monomer decreases the temperature [73]. Fu et al. synthesized a semi-interpenetrating network via a free radical polymerization process. Upon increasing the acrylic acid concentration beyond 5.5%, the LCST of PNIPam increased to 41 °C [76].
PNIPam has the disadvantage of not being biodegradable. Polymers like polyethylene glycol (PEG) could be a useful alternative due to better biocompatibility [77]. For example, Hu et al. carried out research work for evaluating the potential of PLA/PEG-based micelles as thermo-sensitive targeted delivery of the anti-cancer drug curcumin. ATRP was implemented for the synthesis of amphiphilic triblock copolymers. The drug was entrapped using the membrane hydration method. Drug release was studied below and above LCST and the release profile was compared with previously reported results of PNIPam based micelles. According to the results, PEG-based micelles showed a broader phase transition than PNIPam based micelles. The drug release profile in both cases was faster above LCST. However, the drug release rate was slower in PEG-based micelles which is a desired characteristic for controlled delivery in treating cancer [78].
Natural polymers, e.g., hyaluronic acid (HA), chitosan, alginate and dextran, etc. can also be used owing to non-toxicity, good biodegradability, and biocompatibility [77]. For example, κ-carrageenan polysaccharide-based thermo-responsive nanogels were synthesized by Danield-Silva et al. using methylene blue (MB) as a model drug. Their results showed that an increase in temperature from (25 °C to 37 °C) and 45 °C resulted in swelling of the nanogel followed by the release of MB [79].
Thermo-responsive nanocarriers have extensive applications in the field of tumor chemotherapy. Thermodox, a thermo-responsive nanocarrier is already in clinical trials for the treatment of breast cancer [75]. Core-shell thermo-responsive drug delivery systems can be utilized for overcoming the insolubility issues of hydrophobic and anti-cancer drugs. These nanocarriers have a temperature-sensitive shell with a hydrophobic core like polystyrene that acts as a reservoir for loaded drugs [77].
Wang and co-workers synthesized a PNIPam based thermo-responsive nanocarrier system for mitochondrial-targeted delivery using Paclitaxel (PTX) as a model drug. They also used a non-thermo-responsive PAM (propylacrylamide) based system as control. Since the temperature of mitochondria is high in cancer cells, their results showed an enhanced release profile of drug from PNIPam-PTX system evidenced by better colocalization of PTX in mitochondria of MB49 cancer cell line, whereas PAM-PTX failed to release drug in mitochondria with poor colocalization of the drug. They also stated that the developed nanoparticles were more cytotoxic to the cancer cells in comparison to free drug and PAM based non-thermo-responsive control [80].
In another investigation carried out by Ghamkhari et al., novel thermo-responsive star like micelles were developed using hyperbranched aliphatic polyesters poly(ε-caprolactone)-b-poly(N-isopropylacrylamide) (HAPs-g-PCL-b-PNIPAM) via ring-opening polymerization and RAFT techniques. They used docetaxel (DTX) as a model drug to overcome the loading and pharmacokinetics issue associated with the drug. The release profile of the developed system showed an increase in release with an increase in temperature. MTT assay, intracellular uptake and DAPI staining confirmed that the prepared micelles with loaded DTX had significant pharmacokinetics and cytotoxicity in breast cancer cell line (MCF7) compared to free DTX [81].
2.5. Light-Responsive Nanocarriers Applied for Diagnosis and Treatment of Cancer
Light as an external stimulus has grabbed considerable attention because of high spatiotemporal precision. Light responsive polymers are non-invasive and release cargo on-demand. Upon exposure to high radiation (ultraviolet, near-infrared, visible) from an external source, these nanocarriers release the encapsulated agents. Generally, these light-responsive carriers can be prepared by introducing a photo-cleavable linker or a chromophore as a light-responsive moiety into the polymer backbone or matrix of the nanocarriers. Under the irradiation of optimum wavelength, intensity, and exposure time, these photo-cleavable molecules undergo photochemical reactions. These light-induced reactions do not require the prerequisite of chemical changes in the environment and can be categorized into (a) photo-isomerization; (b) photo-cleavage; (c) photo-dimerization (d); photo-rearrangement; or (e) photocrosslinking [82][83][84][85][86][87].
Various chromophores have been studied but certain chromophores, e.g., azobenzene [88], spiropyran [89], spiroxazine [90], and nitrobenzyl [91] are considered more efficient than others. In azobenzene, changes in the molecular symmetry occur when the thermally stable trans orientation converts to a less stable cis form. In spiropyrans, irradiation induces a ring opening reaction. UV absorption results in the reversible isomerization of cis to transform the photo-sensitive groups in nanocarriers which are converted back to cis form by the visible light. Hence, results in disruption of the carriers occur resulting in drug release [92]. Various nanocarriers, e.g., micelles, liposomes, polymeric nanoparticles, hollow metal nanoparticles, etc. are being utilized in photochemical reactions for targeted release of therapeutic agents [93][94]. Additionally, the process of photo-isomerization which is reversible and reproducible functionalizes the nanocarriers as an ‘on-off switch’ [95]. The safety profile and efficacy of a light-responsive nanocarrier are affected by the wavelength and power of the irradiation. Hence, the photo-toxicity and penetration depth of light should be taken into account. Generally, the light with a high wavelength results in deeper penetration through the skin. For example, according to research, a light at 360, 700 and 1200 nm penetrates 190, 400, and 800 μm, respectively into the skin [96].
Based on wavelength, non-ionizing light can be categorized into three:
-
(a)Ultraviolet light (UV)—200 nm to 400 nm
-
(b)Visible light (Vis)—400 nm to 700 nm
-
(c)Near-infrared light (NIR)—700 nm to 1000 nm
Among these regions, light-responsive drug delivery systems mostly respond to UV light because of two main reasons: (i) sensitivity of light-responsive materials towards UV; (ii) ability of UV to provide sufficient energy for triggering photochemical reactions. However, UV light suffers from poor penetration and high toxicity rendering the drug release inefficient along with tissue damage [97][98][99]. Light energy depends upon per-photon energy which is inversely related to the wavelength of light. UV light has high energy per photon along with high tissue absorbance, hence a low MPE (maximum permissible exposure) that makes it unsuitable for most clinical applications [98]. On the other hand, NIR and partially visible light have low energy per photon. Their high MPE with high tissue penetration depth due to decreased attenuation with minimum damage to healthy cells making them more suitable for clinical applications [96][100]. NIR responsive nanocarriers are based on three mechanisms; Photo-thermal effect is the most widely studied drug delivery system due to tunable and flexible properties. Metal sulfides/oxides, gold, and carbon nanomaterials are common photo-thermal agents. Two-photon absorption drug delivery systems impart higher excitation while overcoming low penetration issues associated with UV responsive DDS. Up-conversion nanoparticles (UCNP) are nano-scale particles that are inorganic and crystalline in nature that converts NIR excitation to UV emission i-e photon up-conversion. The decreased light scattering results in deeper penetration of biological samples [101].
One drawback with NIR light is only a few compounds respond to this light as NIR is unable to provide sufficient energy for triggering photo-responsive reactions. To overcome this issue, nanomaterials are being formulated that are capable of converting low-energy NIR to high-energy UV photons. This results in efficient drug release encompassing a two-photon absorption process and up-converting using up-conversion nanoparticles [100]. Light responsive nanocarriers have high potential as drug delivery systems. These carriers could be utilized for tumor therapy guided by imaging as well as in theranostics. Exploiting the photo-thermal effect and generation of reactive oxygen species triggered by light can be a useful ablation of cancers. When combined with other anti-cancer therapeutics, they can be implemented in multimodal cancer theranostics. They have also proven to be highly effective in MDR cancers [102].
Tong et al. developed a photosensitive nanoparticle-based drug delivery system using spiropyran as chromophore and UV light as a source of irradiation. This triggered on-demand drug release as well as enhanced tissue penetration because of reversible change in the volume of particles [103]. Yan and coworkers addressed the drawback of light-responsive drug delivery systems that require UV/Vis excitation, by demonstrating an efficient strategy. Making the use of continuous-wave diode NIR laser showed NaYF4: TmYb UCNPs encapsulated in block copolymer micelles emitted photons in UV region upon exposure to 980 nm light. O-nitrobenzyl groups resulting in activation of photo-cleavage reaction absorbed these photons. This led to the disruption of block copolymer micelles and thus release co-loaded agents [104].
In another investigation, Luo et al. reported the development of long-circulating nanoparticles that demonstrated the ability to release drugs upon irradiation. They established a systematic approach for designing stealth liposomes with porphyrin-phospholipid (pop) using doxorubicin as the therapeutic agent. NIR was used for triggering the release of a drug. The developed delivery system exhibited enhanced stability and extended circulation time in blood. They stated that chemo-phototherapy with pop stealth liposomes showed far more efficacy than conventional phototherapy [105]. In a study conducted by Croissant et al., a mesoporous silica nanoparticles-based two-photon triggered drug delivery system was developed using azobenzene and two-photon fluorophore. At the low power of the laser, the fluorescence of fluorophore resulted in efficient two-photon imaging of the cancer cells. At high power and a short duration of exposure, the nanovalves exhibited two-photon triggered release in cancer cells [106].
2.6. Advancement in Endogenous Stimuli Sensitive DNA Based Smart Nanocarriers
Several endogenous stimuli in pathological environments including temperature, low pH, oligonucleotides can be applied for particular triggers. As a result of malignancy, cell proliferation results in imbalances in nutrient, oxygen levels. The relative differences in pH between the extracellular and intracellular cancer cells are the most distinguish pathophysiological feature [107].
2.6.1. pH Responsive Cancer Targeting
Various DNA-assisted and pH-responsive drug delivery systems have been identified by the researchers. Several researchers studied the pH sensitive i-motif structure DNAyzme and structure stabilization. The i-motif is a motif that can be used in a variety of in acidic environments; DNA has structures that form stable links between anti-parallel, cytosine-rich four-strand sequences, forming the tetraplex structure through C base protonation, which favors interactions with other cytosine bases over guanine. The nucleic acids are made up of a duplex structure called nucleic acid bridges, which is made up of the i-motif and its sequences [108]. Rolling circle amplification was created by Tian et al. to produce polymeric DNA composed of tandem units of functional sequences. The i-motif forms a structure and releases the drug to cause apoptosis when exposed to acidic conditions. Wang et al. developed a pH-responsive anti-cancer drug delivery system using a self-catalyzing DNAyzme and a rolling circle amplification method [109].
Coated polymer/DNA nanocomplexes containing a high mobility group box 1 (HMGB1) were developed as a competent non-viral gene delivery system by Mingyue Wang et al. Nanocomplexes with a pH-sensitive core shell system have been formed and characterized. Free folic acid blocked gene transfection and expression in KB cells, according to the findings. The developed nanocomplexes showed enhance fluorescence protein expression at the tumor site [110].
Olcay Boyacioglu et al. created a DNA aptamer to prostate specific antigen with fixed sequences to facilitate Dox binding and dimeric aptamer complexes. The cellular was directly internalized by prostate-specific membrane antigen (PSMA+) cancer cells. Dimeric aptamer complexes (DACs) are complexes that carry Dox to PSMA+ cancer cells. Under physiological conditions, Dox was released from the DAC-D with an 8-h half-life. Dox was delivered to C4-2 cells using DAC-D with nuclear localization and endosomal release. DAC-D has specificity and durability, which could help with Dox delivery to tumor tissues in vivo [111].
Nanoparticles made of polyethyleneimine (PEI) and a pH-sensitive diblock copolymer were formed by Sethuraman et al. Due to the shielding of PEI by poly(methacryloyl sulfadimethoxine) PSD-b-PEG, the nanoparticles containing DNA/PEI/PSD-b-PEG were small and had low cytotoxicity at pH 7.4. PSD-b-PEG attached to the PEI/DNA complex reduced the interaction of PEI positive charges with cells by 60% and reduced cytotoxicity. At pH 6.6, the nanoparticles showed increased cytotoxicity, indicating PSD-b-PEG detachment from nanoparticles, allowing PEI to attach to cells. The following forms of nanoparticles can distinguish minor pH differences between normal and tumor tissues and have a lot of potential for targeting tumor tissues [112].
2.6.2. Oligonucleotide Responsive Nanocarriers
There are a large number of applications of oligonucleotides (microRNA and small interfering RNA) in tumors. Oligonucleotides such as siRNA and microRNA are active agents that have been used for active drug delivery at the malignant site. Nanoparticles are applied to deliver oligonucleotides at malignant sites. The application of iron oxide, gold and quantum dots ligated with contrast agents has facilitated the early diagnosis and analysis of therapeutic efficacy. By strand displacement, the nano-carriers can be reconfigured and released. A single stranded oligonucleotide that is complementary to the region of double stranded DNA is used to rehybridize and dehybridize the double stranded DNA [113]. One group of researchers created an oligonucleotide-responsive DNA nanosuitcase that encapsulates siRNA by connecting two opposite DNA and siRNA end terminals in a complementary manner. Under biological conditions, the targeting moiety within the nanocarrier was covered, but it was released when an oligonucleotide trigger, such as miRNA or mRNA, was recognized. Li et al. created a nanocarrier with DNA and multilocked DNA valves for mRNA-responsive drug delivery. The researchers encapsulated Dox in mesoporous nanoparticles, which were then capped with two gate DNAs via electrostatic interactions. These DNAs were found to be complementary to tumor-associated GT mRNAs or Tk1 [114]. The cargo can be released by nanoparticles in cells that overexpress mRNAs. Shi et al. created DNA nanoflowers with MUC1 apartmers for tumor targeting and anti-miR-21 for miR-21 responsive release. For CRISPR/Cas9 genome editing, the DNA nanoflower was encapsulated with Cas9/sgRNA into nanoflower through hybridization between the stem loop of the sgRNA and the anti-miR-21. When tumor cells were incubated with a miR-21 mimic resulting from miR-21 responsive Cas9/sgRNA release, the genome modulating efficiency was increased [114][115].
2.6.3. Multiples and Molecular Biomarker Responsive Nanocarriers
For more specific targeted drug delivery, the researchers have developed a delivery system with more than two stimuli. In which activation of the responsive moiety is compulsory for the release of the loaded compound. A group of researchers developed mesoporous silica nanoparticles, that are dual responsive to enzymes and biomarkers for controlled release of drugs and also for dye [115]. Another group of researchers developed DNA conjugated gold nanoparticles that disassembled in result to low pH and specific enzyme for tumor associated drug delivery. In this study, the pH and telomerase stimulated thiolated DNA was adsorbed onto gold nanoparticles via Au-S binding that results in the assembly of nanocarriers at physiological pH. Moreover, it can cause the disassembly in the tumor environment via pH-responsive triplex structure formation. Zhou et al. developed mesoporous silica nanoparticles that are triggered via redox reactions, enzymes, and heat [116]. The calcein was encapsulated in the capped and pores via self-complementary duplex DNA [117].
The loaded compound was released after denaturation by DNase and bond cleavage by disulfide reducing agents such as dithiothreitol or glutathione. Biomolecules such as ions, protein, and small molecules are recognized as potential triggers for controlled release in drug delivery applications because of their increased bioavailability at the disease site. ATP was utilized as a trigger mechanism for drug release through conformation reconfiguration [118]. These locks are made up of various aptamer combinations that recognize single or double biomarkers expressed in tumor cells. When the biomarker was bound to both locks, the locks were unfastened, and the origami box unlatched and released the filled compound thermodynamically. Liu et al. designed and introduced a doll-like DNA nanocage with DNA tetrahedra of different sizes but similar structures for ATP-sensitive disassembly. Each layer was hybridized with an anti-ATP aptamer and its complementary sequence, and the small tetrahedra were sequenced with larger tetrahedra. Figure 4A schematic representation of different types of exogenous and endogenous stimuli Figure 4B showed various types of stimuli-responsive nanoparticles for tumor targeting. Aptamer adhesion was engineered to be preferable to duplex formation. As a result, in the presence of ATP, the hybridized tetrahedra dissociate, resulting in the isolation of the tetrahedral structure [5][119][120].
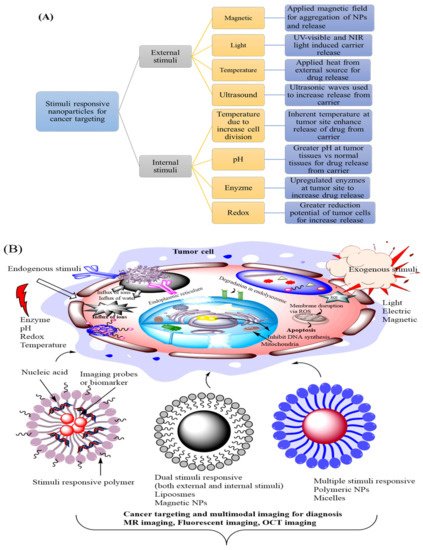
Figure 4. (A) Schematic representation of different types of exogenous and endogenous stimuli (B) Showed different types of stimuli-responsive nanoparticle for cancer targeting.
2.6.4. Redox and Enzyme Responsive Smart Carrier System
Enzymes play key functions in a number of disease states and many of them catalyze the breaking of the particular peptide bonds. The substrates of these enzymes are present at the surface in the cytoplasm or within various cellular organelles. These tumor-associated enzymes are connected to different key events including tumor progression, tumor growth, extravasation, and metastasis. The enhanced levels of particular enzymes including glycosidases, proteases, and phospholipases are signals of various types of tumor cells. Many enzyme responsive delivery systems explore the outside the cell environment [121][122]. Metalloproteinases (MMPs) are the most trigger for controlled drug release. These MMPs are over-expressed in the extracellular environments in many kinds of tumors. Singh et al. synthesize a stimulus-responsive system based on polymer-coated mesoporous silica nanoparticles that encapsulate drugs into both shell and core domains. Another researcher group developed a class of multifunctional type nanoparticles to achieve stimuli-responsive targeting drug delivery. However, anti-cancer drugs could be effectively encapsulated in the nanoparticles and produced the cell death of MMP tumor cells. There are some intracellular enzymes including cathepsin B, elastase, or glycosidases are also exploited for controlled drug release. Cathepsin B is a lysosomal protease that is responsible for cancer cell progression with a particular peptide. Therefore, it gives an attractive option for triggering specific cancer targeting. The differences in reduction efficiency between tumor and normal tissues between extracellular and intracellular environments can be useful for targeted release at the malignant site [123][124]. The GSH concentration is very low in the extracellular environment but is concentrated within the cell inside the cytosol. These differences are more visible in tumor tissues. Wu et al. synthesis a biocompatible and biodegradable 1-cysteine based poly(disulfide amide) for fabricating reduction sensitive nano-carriers with greater hydrophobic drug encapsulated properties. The following GSH sensitive crosslinking agents can also be encapsulated either inside the shell or in the core of micelle-based nanoparticles [125][126].
References
- Aflori, M. Smart Nanomaterials for Biomedical Applications—A Review. Nanomaterials 2021, 11, 396.
- Yan, H.; Zhang, X.; Shen, Z.; Seeman, N.C. A robust DNA mechanical device controlled by hybridization topology. Nat. Cell Biol. 2002, 415, 62–65.
- Kallenbach, N.R.; Ma, R.-I.; Seeman, N.C. An immobile nucleic acid junction constructed from oligonucleotides. Nat. Cell Biol. 1983, 305, 829–831.
- Chao, J.; Liu, H.; Su, S.; Wang, L.; Huang, W.; Fan, C. Structural DNA Nanotechnology for Intelligent Drug Delivery. Small 2014, 10, 4626–4635.
- Kim, T.; Nam, K.; Kim, Y.M.; Yang, K.; Roh, Y.H. DNA-Assisted Smart Nanocarriers: Progress, Challenges, and Opportunities. ACS Nano 2021, 15, 1942–1951.
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113.
- Li, L.; Yang, Z.; Chen, X. Recent Advances in Stimuli-Responsive Platforms for Cancer Immunotherapy. Accounts Chem. Res. 2020, 53, 2044–2054.
- Ruiz-Hernández, E.; Baeza, A.; Vallet-Regí, M. Smart Drug Delivery through DNA/Magnetic Nanoparticle Gates. ACS Nano 2011, 5, 1259–1266.
- Chen, Y.-X.; Huang, K.-J.; He, L.-L.; Wang, Y.-H. Tetrahedral DNA probe coupling with hybridization chain reaction for competitive thrombin aptasensor. Biosens. Bioelectron. 2018, 100, 274–281.
- Li, H.; Fan, J.; Buhl, E.M.; Huo, S.; Loznik, M.; Göstl, R.; Herrmann, A. DNA hybridization as a general method to enhance the cellular uptake of nanostructures. Nanoscale 2020, 12, 21299–21305.
- Juul, S.; Iacovelli, F.; Falconi, M.; Kragh, S.L.; Christensen, B.; Frøhlich, R.; Franch, O.; Kristoffersen, E.L.; Stougaard, M.; Leong, K.W.; et al. Temperature-Controlled Encapsulation and Release of an Active Enzyme in the Cavity of a Self-Assembled DNA Nanocage. ACS Nano 2013, 7, 9724–9734.
- Ouyang, X.; Chang, Y.-N.; Yang, K.-W.; Wang, W.-M.; Bai, J.-J.; Wang, J.-W.; Zhang, Y.-J.; Wang, S.-Y.; Xie, B.-B.; Wang, L.-L. A DNA nanoribbon as a potent inhibitor of metallo-β-lactamases. Chem. Commun. 2017, 53, 8878–8881.
- Ouyang, X.; Wang, M.-F.; Guo, L.-J.; Cui, C.-J.; Liu, T.; Ren, Y.-A.; Zhao, Y.; Ge, Z.-L.; Guo, X.-Q.; Xie, G.; et al. DNA Nanoribbon-Templated Self-Assembly of Ultrasmall Fluorescent Copper Nanoclusters with Enhanced Luminescence. Angew. Chem. 2020, 132, 11934–11942.
- Roh, Y.H.; Lee, J.B.; Kiatwuthinon, P.; Hartman, M.R.; Cha, J.J.; Um, S.H.; Muller, D.; Luo, D. DNAsomes: Multifunctional DNA-Based Nanocarriers. Small 2010, 7, 74–78.
- Liang, H.F.; Hong, M.H.; Ho, R.M.; Chung, C.K.; Lin, Y.H.; Chen, C.H.; Sung, H.W. Novel Method Using a Temperature-Sensitive Polymer (Methylcellulose) to Thermally Gel Aqueous Alginate as a pH-Sensitive Hydrogel. Biomacromolecules 2004, 5, 1917–1925.
- Chu, T.C. Aptamer mediated siRNA delivery. Nucleic Acids Res. 2006, 34, e73.
- Li, W.; Yang, X.; He, L.; Wang, K.; Wang, Q.; Huang, J.; Liu, J.; Wu, B.; Xu, C. Self-assembled DNA nanocentipede as multivalent drug carrier for targeted delivery. ACS Appl. Mater. Interfaces 2016, 8, 25733–25740.
- Wang, T.; Chen, C.; Larcher, L.; Barrero, R.; Veedu, R.N. Three decades of nucleic acid aptamer technologies: Lessons learned, progress and opportunities on aptamer development. Biotechnol. Adv. 2019, 37, 28–50.
- Yuan, Y.; Gu, Z.; Yao, C.; Luo, D.; Yang, D. Nucleic Acid–Based Functional Nanomaterials as Advanced Cancer Therapeutics. Small 2019, 15, e1900172.
- Yang, K.; Chang, Y.; Wen, J.; Lu, Y.; Pei, Y.; Cao, S.; Wang, F.; Pei, Z. Supramolecular Vesicles Based on Complex of Trp-Modified Pillararene and Galactose Derivative for Synergistic and Targeted Drug Delivery. Chem. Mater. 2016, 28, 1990–1993.
- Li, F.; Tang, J.; Geng, J.; Luo, D.; Yang, D. Polymeric DNA hydrogel: Design, synthesis and applications. Prog. Polym. Sci. 2019, 98, 101163.
- Shi, L.; Mu, C.; Gao, T.; Chen, T.; Hei, S.; Yang, J.; Li, G. DNA nanoflower blooms in nanochannels: A new strategy for miRNA detection. Chem. Commun. 2018, 54, 11391–11394.
- Hu, R.; Zhang, X.; Zhao, Z.; Zhu, G.; Chen, T.; Fu, T.; Tan, W. DNA Nanoflowers for Multiplexed Cellular Imaging and Traceable Targeted Drug Delivery. Angew. Chem. 2014, 126, 5931–5936.
- Cao, M.; Sun, Y.; Xiao, M.; Li, L.; Liu, X.; Jin, H.; Pei, H. Multivalent Aptamer-modified DNA Origami as Drug Delivery System for Targeted Cancer Therapy. Chem. Res. Chin. Univ. 2019, 36, 1–7.
- Hu, Q.; Wang, S.; Wang, L.; Gu, H.; Fan, C. DNA Nanostructure-Based Systems for Intelligent Delivery of Therapeutic Oligonucleotides. Adv. Healthc. Mater. 2018, 7, 1701153.
- Pan, Q.; Nie, C.; Hu, Y.; Yi, J.; Liu, C.; Zhang, J.; He, M.; He, M.; Chen, T.-T.; Chu, X. Aptamer-Functionalized DNA Origami for Targeted Codelivery of Antisense Oligonucleotides and Doxorubicin to Enhance Therapy in Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2019, 12, 400–409.
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332.
- Wu, D.; Wang, L.; Li, W.; Xu, X.; Jiang, W. DNA nanostructure-based drug delivery nanosystems in cancer therapy. Int. J. Pharm. 2017, 533, 169–178.
- Shcharbin, D.; Halets-Bui, I.; Abashkin, V.; Dzmitruk, V.; Loznikova, S.; Odabaşı, M.; Acet, Ö.; Önal, B.; Özdemir, N.; Shcharbina, N.; et al. Hybrid metal-organic nanoflowers and their application in biotechnology and medicine. Colloids Surf. B Biointerfaces 2019, 182, 110354.
- Rai, A.; Ferreira, L. Biomedical applications of the peptide decorated gold nanoparticles. Crit. Rev. Biotechnol. 2021, 41, 186–215.
- Bamrungsap, S. DNA-Conjugated Magnetic Nanoparticles for Bio-Analytical and Biomedical Applications; University of Florida: Gainesville, FL, USA, 2011.
- Zhang, F.; Jiang, S.; Wu, S.; Li, Y.; Mao, C.; Liu, Y.; Yan, H. Complex wireframe DNA origami nanostructures with multi-arm junction vertices. Nat. Nanotechnol. 2015, 10, 779–784.
- Hong, F.; Zhang, F.; Liu, Y.; Yan, H. DNA Origami: Scaffolds for Creating Higher Order Structures. Chem. Rev. 2017, 117, 12584–12640.
- Meng, H.-M.; Fu, T.; Zhang, X.-B.; Tan, W. Cell-SELEX-based aptamer-conjugated nanomaterials for cancer diagnosis and therapy. Natl. Sci. Rev. 2015, 2, 71–84.
- Seaberg, J.; Montazerian, H.; Hossen, N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142.
- Wilner, O.I.; Willner, I. Functionalized DNA Nanostructures. Chem. Rev. 2012, 112, 2528–2556.
- Iinuma, R.; Ke, Y.; Jungmann, R.; Schlichthaerle, T.; Woehrstein, J.B.; Yin, P. Polyhedra Self-Assembled from DNA Tripods and Characterized with 3D DNA-PAINT. Science 2014, 344, 65–69.
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2018, 119, 6459–6506.
- Meng, H.-M.; Liu, H.; Kuai, H.; Peng, R.; Mo, L.; Zhang, X.-B. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 2016, 45, 2583–2602.
- Calvo, P.; Gouritin, B.; Brigger, I.; Lasmezas, C.; Deslys, J.-P.; Williams, A.; Andreux, J.P.; Dormont, D.; Couvreur, P. PEGylated polycyanoacrylate nanoparticles as vector for drug delivery in prion diseases. J. Neurosci. Methods 2001, 111, 151–155.
- Baig, M.M.F.A.; Zou, T.; Neelakantan, P.; Zhang, C. Development and functionalization of DNA nanostructures for biomedical applications. J. Chin. Chem. Soc. 2021, 68, 228–238.
- Zhang, C.; Yang, C.; Whitham, S.A.; Hill, J.H. Development and Use of an Efficient DNA-Based Viral Gene Silencing Vector for Soybean. Mol. Plant Microbe Interact. 2009, 22, 123–131.
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117.
- Wang, P.; Yin, T.; Li, J.; Zheng, B.; Wang, X.; Wang, Y.; Zheng, J.; Zheng, R.; Shuai, X. Ultrasound-responsive microbubbles for sonography-guided siRNA delivery. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1139–1149.
- Papa, A.-L.; Korin, N.; Kanapathipillai, M.; Mammoto, A.; Mammoto, T.; Jiang, A.; Mannix, R.; Uzun, O.; Johnson, C.; Bhatta, D.; et al. Ultrasound-sensitive nanoparticle aggregates for targeted drug delivery. Biomaterials 2017, 139, 187–194.
- Muñoz de Escalona, M.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Arias, J.L. Magnetic solid lipid nanoparticles in hyperthermia against colon cancer. Int. J. Pharm. 2016, 504, 11–19.
- Wu, P.; Gao, W.; Su, M.; Nice, E.C.; Zhang, W.; Lin, J.; Xie, N. Adaptive mechanisms of tumor therapy resistance driven by tumor microenvironment. Front. Cell Dev. Biol. 2021, 9, 357–362.
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212.
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204.
- An, H.; Xu, K.; Chang, L.; Wang, Y.; Qin, J.; Li, W. Thermo-responsive self-healable hydrogels with extremely mild base degradability and bio-compatibility. Polymer 2018, 147, 38–47.
- Luckanagul, J.A.; Pitakchatwong, C.; Na Bhuket, P.R.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127.
- Yildirim, T.; Yildirim, I.; Yañez-Macias, R.; Stumpf, S.; Fritzsche, C.; Hoeppener, S.; Guerrero-Sanchez, C.; Schubert, S.; Schubert, U.S. Dual pH and ultrasound responsive nanoparticles with pH triggered surface charge-conversional properties. Polym. Chem. 2017, 8, 1328–1340.
- Di Ianni, T.; Bose, R.J.; Sukumar, U.K.; Bachawal, S.; Wang, H.; Telichko, A.; Herickhoff, C.; Robinson, E.; Baker, S.; Vilches-Moure, J.; et al. Ultrasound/microbubble-mediated targeted delivery of anticancer microRNA-loaded nanoparticles to deep tissues in pigs. J. Control. Release 2019, 309, 1–10.
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Basri, S.M.M.; Mirshekari, H.; Amiri, M.; Pishabad, Z.S.; Aslani, A.; Bozorgomid, M.; et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem. Soc. Rev. 2016, 45, 1457–1501.
- Pitt, W.G.; Husseini, G.A.; Roeder, B.L.; Dickinson, D.J.; Warden, D.R.; Hartley, J.M.; Jones, P.W. Preliminary Results of Combining Low Frequency Low Intensity Ultrasound and Liposomal Drug Delivery to Treat Tumors in Rats. J. Nanosci. Nanotechnol. 2011, 11, 1866–1870.
- Gaspar, V.M.; Moreira, A.F.; de Melo-Diogo, D.; Costa, E.C.; Queiroz, J.A.; Sousa, F.; Pichon, C.; Correia, I.J. Chapter 6—Multifunctional nanocarriers for codelivery of nucleic acids and chemotherapeutics to cancer cells. In Nanobiomaterials in Medical Imaging; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 163–207.
- Eisenbrey, J.; Burstein, O.M.; Kambhampati, R.; Forsberg, F.; Liu, J.-B.; Wheatley, M. Development and optimization of a doxorubicin loaded poly(lactic acid) contrast agent for ultrasound directed drug delivery. J. Control. Release 2010, 143, 38–44.
- Bhattacharya, S.; Eckert, F.; Boyko, V.; Pich, A. Temperature-, pH-, and Magnetic-Field-Sensitive Hybrid Microgels. Small 2007, 3, 650–657.
- Yu, S.; Wu, G.; Gu, X.; Wang, J.; Wang, Y.; Gao, H.; Ma, J. Magnetic and pH-sensitive nanoparticles for antitumor drug delivery. Colloids Surf. B Biointerfaces 2013, 103, 15–22.
- Dobson, J. Magnetic micro- and nano-particle-based targeting for drug and gene delivery. Nanomedicine 2006, 1, 31–37.
- Schenck, J.F. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol. 2005, 87, 185–204.
- Xie, W.; Gao, Q.; Wang, D.; Guo, Z.; Gao, F.; Wang, X.; Cai, Q.; Feng, S.-S.; Fan, H.; Sun, X.; et al. Doxorubicin-loaded Fe3O4@MoS2-PEG-2DG nanocubes as a theranostic platform for magnetic resonance imaging-guided chemo-photothermal therapy of breast cancer. Nano Res. 2018, 11, 2470–2487.
- Bordat, A.; Boissenot, T.; Nicolas, J.; Tsapis, N. Thermoresponsive polymer nanocarriers for biomedical applications. Adv. Drug Deliv. Rev. 2019, 138, 167–192.
- Pippa, N.; Meristoudi, A.; Pispas, S.; Demetzos, C. Temperature-dependent drug release from DPPC:C12H25-PNIPAM-COOH liposomes: Control of the drug loading/release by modulation of the nanocarriers’ components. Int. J. Pharm. 2015, 485, 374–382.
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The Smart Drug Delivery System and Its Clinical Potential. Theranostics 2016, 6, 1306–1323.
- Huda, S.; Alam, A.; Sharma, P.K. Smart nanocarriers-based drug delivery for cancer therapy: An innovative and developing strategy. J. Drug Deliv. Sci. Technol. 2020, 60, 102018.
- Schwerdt, A.; Zintchenko, A.; Concia, M.; Roesen, N.; Fisher, K.; Lindner, L.H.; Issels, R.; Wagner, E.; Ogris, M. Hyperthermia-Induced Targeting of Thermosensitive Gene Carriers to Tumors. Hum. Gene Ther. 2008, 19, 1283–1292.
- Hooshmand, S.; Hayat, S.M.; Ghorbani, A.; Khatami, M.; Pakravanan, K.; Darroudi, M. Preparation and Applications of Superparamagnetic Iron Oxide Nanoparticles in Novel Drug Delivery Systems: An Overview Article. Curr. Med. Chem. 2020, 1, 1–12.
- Chen, K.-J.; Liang, H.-F.; Chen, H.-L.; Wang, Y.; Cheng, P.-Y.; Liu, H.-L.; Xia, Y.; Sung, H.-W. A Thermoresponsive Bubble-Generating Liposomal System for Triggering Localized Extracellular Drug Delivery. ACS Nano 2013, 7, 438–446.
- Karimi, M.; Zangabad, P.S.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Asl, H.G.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-Responsive Smart Nanocarriers for Delivery of Therapeutic Agents: Applications and Recent Advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133.
- Alsehli, M. Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: Recent advances in drug delivery. Saudi Pharm. J. 2020, 28, 255–265.
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242.
- Bergueiro, J.; Calderón, M. Thermoresponsive Nanodevices in Biomedical Applications. Macromol. Biosci. 2014, 15, 183–199.
- Le, P.N.; Huynh, K.; Tran, N.Q. Advances in thermosensitive polymer-grafted platforms for biomedical applications. Mater. Sci. Eng. C 2018, 92, 1016–1030.
- Chen, Q.; Li, C.; Yang, X.; Huang, J.; Liu, S.; Liu, W. Self-assembled DNA nanowires as quantitative dual-drug nanocarriers for antitumor chemophotodynamic combination therapy. J. Mater. Chem. B 2017, 5, 7529–7537.
- Fu, G.; Soboyejo, W. Swelling and diffusion characteristics of modified poly (N-isopropylacrylamide) hydrogels. Mater. Sci. Eng. C 2010, 30, 8–13.
- Hajebi, S.; Rabiee, N.; Bagherzadeh, M.; Ahmadi, S.; Rabiee, M.; Roghani-Mamaqani, H.; Tahriri, M.; Tayebi, L.; Hamblin, M.R. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019, 92, 1–8.
- Hu, Y.; Darcos, V.; Monge, S.; Li, S. Thermo-responsive drug release from self-assembled micelles of brush-like PLA/PEG analogues block copolymers. Int. J. Pharm. 2015, 491, 152–161.
- Daniel-Da-Silva, A.L.; Ferreira, L.; Gil, A.; Trindade, T. Synthesis and swelling behavior of temperature responsive κ-carrageenan nanogels. J. Colloid Interface Sci. 2011, 355, 512–517.
- Wang, D.; Huang, H.; Zhou, M.; Lu, H.; Chen, J.; Chang, Y.-T.; Gao, J.; Chai, Z.; Hu, Y. A thermoresponsive nanocarrier for mitochondria-targeted drug delivery. Chem. Commun. 2019, 55, 4051–4054.
- Ghamkhari, A.; Sarvari, R.; Ghorbani, M.; Hamishehkar, H. Novel thermoresponsive star-liked nanomicelles for targeting of anticancer agent. Eur. Polym. J. 2018, 107, 143–154.
- Muhammad, K.; Zhao, J.; Gao, B.; Feng, Y. Polymeric nano-carriers for on-demand delivery of genes via specific responses to stimuli. J. Mater. Chem. B 2020, 8, 9621–9641.
- Yan, L.; Li, X. Biodegradable Stimuli-Responsive Polymeric Micelles for Treatment of Malignancy. Curr. Pharm. Biotechnol. 2016, 17, 227–236.
- Fomina, N.; Sankaranarayanan, J.; Almutairi, A. Photochemical mechanisms of light-triggered release from nanocarriers. Adv. Drug Deliv. Rev. 2012, 64, 1005–1020.
- Fleige, E.; Quadir, M.A.; Haag, R. Stimuli-responsive polymeric nanocarriers for the controlled transport of active compounds: Concepts and applications. Adv. Drug Deliv. Rev. 2012, 64, 866–884.
- Xiong, Q.; Lim, Y.; Li, D.; Pu, K.; Liang, L.; Duan, H. Photoactive Nanocarriers for Controlled Delivery. Adv. Funct. Mater. 2020, 30, 1903896.
- Ebrahimi, G.; Asadikaram, G.; Akbari, H.; Nematollahi, M.H.; Abolhassani, M.; Shahabinejad, G.; Khodadadnejad, L.; Hashemi, M. Elevated levels of DNA methylation at the OPRM1 promoter region in men with opioid use disorder. Am. J. Drug Alcohol Abus. 2018, 44, 193–199.
- Sun, S.; Liang, S.; Xu, W.-C.; Xu, G.; Wu, S. Photoresponsive polymers with multi-azobenzene groups. Polym. Chem. 2019, 10, 4389–4401.
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184.
- Paramonov, S.V.; Lokshin, V.; Fedorova, O.A. Spiropyran, chromene or spirooxazine ligands: Insights into mutual relations between complexing and photochromic properties. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 209–236.
- Bertrand, O.; Gohy, J.-F. Photo-responsive polymers: Synthesis and applications. Polym. Chem. 2016, 8, 52–73.
- Molla, M.R.; Rangadurai, P.; Antony, L.; Swaminathan, S.; De Pablo, J.J.; Thayumanavan, S. Dynamic actuation of glassy polymersomes through isomerization of a single azobenzene unit at the block copolymer interface. Nat. Chem. 2018, 10, 659–666.
- Khatami, M.; Heli, H.; Jahani, P.M.; Azizi, H.; Nobre, M.A.L. Copper/copper oxide nanoparticles synthesis using Stachys lavandulifolia and its antibacterial activity. IET Nanobiotechnol. 2017, 11, 709–713.
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an alternative for liposomal delivery. PLoS ONE 2018, 13, e0194179.
- Wang, B.; Chen, K.; Yang, R.; Yang, F.; Liu, J. Stimulus-responsive polymeric micelles for the light-triggered release of drugs. Carbohydr. Polym. 2014, 103, 510–519.
- Jia, S.; Fong, W.-K.; Graham, B.; Boyd, B.J. Photoswitchable Molecules in Long-Wavelength Light-Responsive Drug Delivery: From Molecular Design to Applications. Chem. Mater. 2018, 30, 2873–2887.
- Silva, J.M.; Silva, E.; Reis, R.L. Light-triggered release of photocaged therapeutics—Where are we now? J. Control. Release 2019, 298, 154–176.
- Olejniczak, J.; Carling, C.-J.; Almutairi, A. Photocontrolled release using one-photon absorption of visible or NIR light. J. Control. Release 2015, 219, 18–30.
- Khatami, M.; Sharifi, I.; Nobre, M.A.L.; Zafarnia, N.; Aflatoonian, M.R. Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green Chem. Lett. Rev. 2018, 11, 125–134.
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote Light-Responsive Nanocarriers for Controlled Drug Delivery: Advances and Perspectives. Small 2019, 15, e1903060.
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: A review. J. Mater. Res. Technol. 2019, 8, 1497–1509.
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588.
- Tong, R.; Hemmati, H.D.; Langer, R.; Kohane, D.S. Photoswitchable Nanoparticles for Triggered Tissue Penetration and Drug Delivery. J. Am. Chem. Soc. 2012, 134, 8848–8855.
- Yan, B.; Boyer, J.-C.; Branda, N.R.; Zhao, Y. Near-Infrared Light-Triggered Dissociation of Block Copolymer Micelles Using Upconverting Nanoparticles. J. Am. Chem. Soc. 2011, 133, 19714–19717.
- Luo, D.; Carter, K.A.; Razi, A.; Geng, J.; Shao, S.; Giraldo, D.; Sunar, U.; Ortega, J.; Lovell, J.F. Doxorubicin encapsulated in stealth liposomes conferred with light-triggered drug release. Biomaterials 2016, 75, 193–202.
- Croissant, J.; Chaix, A.; Mongin, O.; Wang, M.; Clément, S.; Raehm, L.; Durand, J.-O.; Hugues, V.; Blanchard-Desce, M.; Maynadier, M.; et al. Two-Photon-Triggered Drug Delivery via Fluorescent Nanovalves. Small 2014, 10, 1752–1755.
- Cui, Y.-X.; Sun, Y.-X.; Li, Y.H.; Tang, A.N.; Zhu, L.N.; Kong, D.M. DNA-Based pH-Responsive Core–Shell Drug Nanocarrier for Tumor-Targeted Chemo-Photodynamic Therapy. Adv. Mater. Interfaces 2020, 7, 2000292.
- Yu, D.; Li, W.; Zhang, Y.; Zhang, B. Anti-tumor efficiency of paclitaxel and DNA when co-delivered by pH responsive ligand modified nanocarriers for breast cancer treatment. Biomed. Pharmacother. 2016, 83, 1428–1435.
- Tian, Q.; Wang, Y.; Deng, R.; Lin, L.; Liu, Y.; Li, J. Carbon nanotube enhanced label-free detection of microRNAs based on hairpin probe triggered solid-phase rolling-circle amplification. Nanoscale 2015, 7, 987–993.
- Wang, M.; Hu, H.; Sun, Y.; Qiu, L.; Zhang, J.; Guan, G. A pH-sensitive gene delivery system based on folic acid-PEG-chitosan—PAMAM-plasmid DNA complexes for cancer cell targeting. Biomaterials 2013, 34, 10120–10132.
- Boyacioglu, O.; Stuart, C.H.; Kulik, G.; Gmeiner, W.H. Dimeric DNA Aptamer Complexes for High-capacity–targeted Drug Delivery Using pH-sensitive Covalent Linkages. Mol. Ther. Nucleic Acids 2013, 2, e107.
- Sethuraman, V.A.; Na, K.; Bae, Y.H. pH-Responsive Sulfonamide/PEI System for Tumor Specific Gene Delivery: An in Vitro Study. Biomacromolecules 2006, 7, 64–70.
- Andersen, E.S.; Dong, M.; Nielsen, M.; Jahn, K.; Subramani, R.; Mamdouh, W.; Golas, M.M.; Sander, B.; Stark, H.; Oliveira, C.; et al. Self-assembly of a nanoscale DNA box with a controllable lid. Nat. Cell Biol. 2009, 459, 73–76.
- Li, Y.; Chen, Y.; Pan, W.; Yu, Z.; Yang, L.; Wang, H.; Li, N.; Tang, B. Nanocarriers with multi-locked DNA valves targeting intracellular tumor-related mRNAs for controlled drug release. Nanoscale 2017, 9, 17318–17324.
- Shi, J.; Yang, X.; Li, Y.; Wang, D.; Liu, W.; Zhang, Z.; Liu, J.; Zhang, K. MicroRNA-responsive release of Cas9/sgRNA from DNA nanoflower for cytosolic protein delivery and enhanced genome editing. Biomaterials 2020, 256, 120221.
- Ye, W.; Chen, X.; Li, X.; Liu, Y.; Jia, F.; Jin, Q.; Ji, J. Structure-Switchable DNA Programmed Disassembly of Nanoparticles for Smart Size Tunability and Cancer-Specific Drug Release. ACS Appl. Mater. Interfaces 2020, 12, 22560–22571.
- Zhao, N.; Deng, L.; Luo, D.; Zhang, P. One-step fabrication of biomass-derived hierarchically porous carbon/MnO nanosheets composites for symmetric hybrid supercapacitor. Appl. Surf. Sci. 2020, 526, 146696–146703.
- Kuzuya, A.; Ohya, Y. Nanomechanical Molecular Devices made of DNA Origami. Accounts Chem. Res. 2014, 47, 1742–1749.
- Zangabad, P.S.; Karimi, M.; Mehdizadeh, F.; Malekzad, H.; Ghasemi, A.; Bahrami, S.; Zare, H.; Moghoofei, M.; Hekmatmanesh, A.; Hamblin, M.R. Nanocaged platforms: Modification, drug delivery and nanotoxicity. Opening synthetic cages to release the tiger. Nanoscale 2017, 9, 1356–1392.
- Douglas, S.; Bachelet, I.; Church, G.M. A Logic-Gated Nanorobot for Targeted Transport of Molecular Payloads. Science 2012, 335, 831–834.
- Chong, S.C.; Blake, R. Exogenous attention and endogenous attention influence initial dominance in binocular rivalry. Vis. Res. 2006, 46, 1794–1803.
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003.
- Zhao, H.; Liu, X.; Yu, L.; Lin, S.; Zhang, C.; Xu, H.; Leng, Z.; Huang, W.; Lei, J.; Li, T.; et al. Comprehensive landscape of epigenetic-dysregulated lncRNAs reveals a profound role of enhancers in carcinogenesis in BC subtypes. Mol. Ther. Nucleic Acids 2021, 23, 667–681.
- Pierce, A.P.; De Waal, E.; McManus, L.M.; Shireman, P.; Chaudhuri, A.R. Oxidation and structural perturbation of redox-sensitive enzymes in injured skeletal muscle. Free Radic. Biol. Med. 2007, 43, 1584–1593.
- Chen, W.-H.; Liao, W.-C.; Sohn, Y.S.; Fadeev, M.; Cecconello, A.; Nechushtai, R.; Willner, I. Stimuli-Responsive Nucleic Acid-Based Polyacrylamide Hydrogel-Coated Metal-Organic Framework Nanoparticles for Controlled Drug Release. Adv. Funct. Mater. 2017, 28, 1705137.
- Wu, X.; Gao, Y.; Dong, C.-M. Polymer/gold hybrid nanoparticles: From synthesis to cancer theranostic applications. RSC Adv. 2015, 5, 13787–13796.




