1. Classification and Applications of Smart Nanocarrier System in Cancer Targeting
DNA is a novel and smart biomaterials that can be implied to synthesize the various types of nanocarriers system based on its key GC/AT complementary base pairing. The data from numerous studies revealed that the DNA nanostructure is an effective tool for addressing major issues in cancer care, such as toxicity and drug efficacy. Therefore, some significant improvements have been made in recent years [
24]. One of the advancements to these intelligent nanocarrier systems is multidrug co-delivery, which increases the targetability with the help of various ligands and adaptations of active target strategies. Different methods are adopted for the preparation of DNA based nanocarrier systems. Basically, these nanocarrier systems consist of functional DNA sequences, biomloecules, that are bound using physical, chemical, or biological engineering tools. Back into the history regarding the evolution of DNA based nanocarrier system, first static four-arm structure of DNA was designed by Nadrain Seeman in 1983, consisting of four strands of DNA. Each strand has a different base sequence to make a junction point at specific loci [
25]. These static DNA joints are basic blocks to design a stable and rigid DNA nanostructures. With further advancement in this field more arms including three, five, six, eight, twelve were generated for the production of various DNA nanostructures. Structural DNA nanotechnology has become significantly important in the field of nanoscience since the 1980s [
26]. The various dynamic and static data DNA devices with various dimensions and structures have been introduced and developed. The pure DNA consisted of nanostructure have been divided into various types as shown in schematic representation (
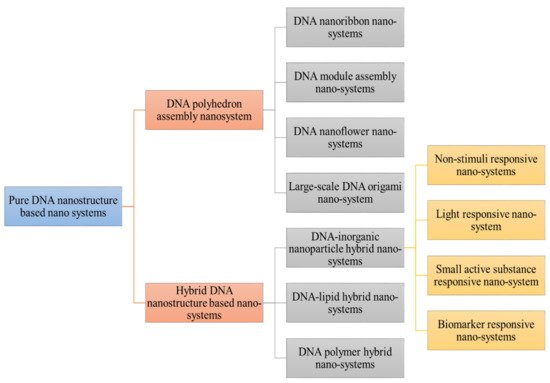
Figure 1) of different classification of DNA based nanosystem [
27].
Figure 1. Demonstrated different classification of DNA nanostructure. Pure DNA nanostructure is divided into DNA polyhedron assembly nanosystem (DNA nanoribbon, DNA module assembly nanosystem, DNA nanoflower system) and hybrid DNA nanosystem (DNA-inorganic nanoparticle hybrid nanosystems (non-stimuli responsive, small active responsive, biomarker responsive) DNA lipid hybrid and DNA polymer hybrid nanocargo.
The DNA polyhedron nanosystems have been designed from tetrahedron and DNA octahedron to DNA icosahedron that served as simple carriers in anti-cancer drug delivery. Tuberfield and his colleagues developed classic DNA tetrahedron for the first time. Thereafter, it has been used as an efficient cargo for anti-cancer drugs including photosensitizer, DOX, siRNA, and other drugs concurrently. The anti-neoplastic drugs (Dox, doxorubicin) encapsulated into DNA tetrahedron can kill the circulating tumor cells (CTC) [
23,
28]. Furthermore, the light will cause the photosensitizer marked on the DNA tetrahedron, resulting in enhanced cytotoxic effects. There are several abilities of DNA nanodevices to increase the endocytotic uptake of anti-neoplastic agents and also increased the drug loading capacity with greater efficacy. Most of the present literature study data emphasize the progress in modification to increase the drug ability and to decrease its adverse effects [
29]. One research group created a DNA tetrahedron to encapsulate DOX with available conjugation sites for attaching cetuximab antibodies that target the epidermal growth factor receptor specifically. The findings of the following study showed that this nanosystem have greater targeting ability and better killing efficacy of malignant cells. Chen et al. developed biotins conjugated to DNA tetrahedron (ruthenium polypyridyl complexes). The DNA cage also increases its specific cellular uptake along with drug cytotoxicity and retention against HepG2 cells [
30,
31].
Lo’s group has produced a DNA nanocage for the first time for mitochondrial delivery of DOX by conjugating lipids. In contrast to DOX localization in lysosomes, DOX retention in mitochondria causes major cytotoxicity and cellular apoptosis in MCF-7 cells, according to the findings. However, with the introduction of stimuli responsive DNA tetrahedrons and switchable DNA nanosuitcases, more stimuli responsive DNA polyhedron drug delivery strategies will be established and used in advanced nanotechnology cancer treatment [
32,
33].
Aside from hybridization, catalytic hairpin conjugation may generate DNA nanoribbons. Rigid and programmable DNA tiles have previously been used to cause significant one-dimensional (1D) nanoribbons, nanotubes, two-dimensional (2D) arrays, and even three-dimensional (3D) crystals [
34]. By use of different technologies number of researchers design different nanoribbons to deliver the siRNA, DOX, photosensitizer, and so on [
35]. Weizmann et al. developed DNA nanoribbon by a modified DNA origami strategy. Furthermore, various studies proved that the DNA nanoribbons was an efficient siRNA delivery cargo in human cells cancer. The functionalized DNA nanoribbon structures and devices show extraordinary performance in cancer diagnosis and treatment because of their small sizes, morphology, and greater biocompatibility. Several research groups collaborated to develop various types of DNA nanoribbons, for example, Liang et al. developed DNA nanoribbon with two compartments, one was loaded with -GC- base pairs for DOX delivery. Another component was the AS1411 aptamer, which is a DNA aptamer. The following system helped to increase the tolerability of human breast cancer cells to the DOX with inhibition of tumor cell proliferation. Self-assembled DNA nanocentipede was developed by Roh et al. to deliver multivalent aptamers to functionalize in cancer targeting [
36,
37]. Chu’s and his colleagues developed an aptamer probe to target the cancer cells via structure switching. Hybridization chain reaction (HCR) accumulated higher encapsulated prodrugs from a drug labeled probe and induced their conversion and uptake into cisplatin in cells for selective tumor targeting using this strategy [
38]. Another type of DNA assembly nanosystems was designed by a group of researchers. They classified these materials into two groups: DNA nanohydrogels and DNA dendrimers [
39]. DNA dendrimers are basically hybridized layer by layer self-assembled functional branched DNA [
40]. DNA nanohydrogels, on the other hand, are made from functional building blocks by base-pairing hybridization or liquid crystallization and dense packaging. Since they can be configured into and provide further docking sites to encapsulate drugs or other functional elements, these DNA nanosystems are denser. Yang et al. developed DNA dendrimers and encapsulate DOX. Other researchers groups developed nanohydrogels from hybridization of different building blocks to synergistic cancer therapy with Dox [
41,
42]. Different researchers have applied different methods for the development of DNA nano-hydrogels for targeting DOX delivery by using building blocks and liquid crystallization without base-pairing hybridization. The DNA nanohydrogel is comprised of three building blocks unit including functional moiety, DNAzyme, and aptamer. Each of these parts have different functionalization. These three parts are self-assembled into nanohydrogels by hybridization between sticky ends [
43].
DNA nanoflower system in comparison to self-assembled, form long DNA strands via rolling circle replication along with liquid crystallization and dense packaging. Despite the drawbacks of large nanostructures, the above type of nanostructure seems to have its own set of characteristics. To deliver anti-cancer drugs, this form of structure is very light in sequence design and its size can be tuned by varying the assembly time and template sequence. A group of researchers had developed series of nanoflowers to encapsulate the anti-cancer agents (CpG, DOX). Furthermore, the researchers modify the nanoflowers to encapsulate different types of agents for multigene therapy [
44,
45].
Since DNA origami is large and dense, it has a high ability to target tumors without the need for targeted modification. The first DNA origami design was complicated because it relied on the hybridization of a long ssDNA from the M13 phage genome with hundreds of short-staple strands. However, further improvements in this design were implied by many researchers to simplify the method of its development by using an RCP-amplified scaffold in replacement to ssDNA from the M13 phage [
46]. Likewise, with other DNA-based nanostructures, it is efficient for DOX, CpG, photosensitizer, etc. More advanced DNA origami structures include DNA rod/tube and DNA triangle to encapsulate a high load of drugs. Another study used DOX encapsulated DNA origami delivery systems that can induce remarkable cytotoxicity in cancer targeting. Bachelet et al. designed a hollow hexagonal barrel-shaped DNA origami as a wonderful logic gated nanorobot to handle the release of encapsulated molecules while identifying specific receptor proteins [
47]. Following that, they build more complex nanorobots that can interact with one another and generate logical outputs to turn molecular payloads on or off [
48].
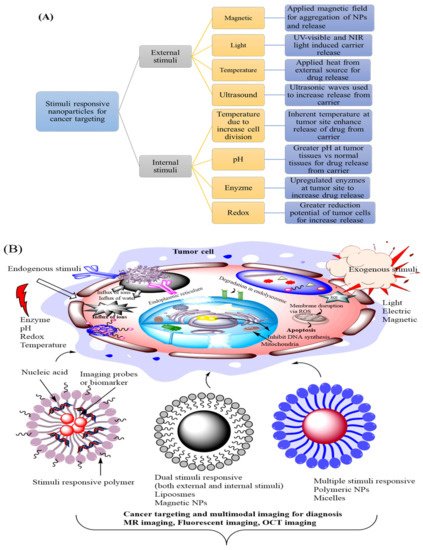
DNA structure is further classified into hybrid DNA nanostructured system that is subdivided into DNA-inorganic nanoparticle (non-stimuli responsive, light-responsive, small molecule, DNA lipid hybrid, DNA polymer hybrid nanosystems, and small active substance responsive nanosystem) [
49]. DNA-inorganic nanoparticles hybrid system including non-stimuli responsive systems has been designed for better cancer treatment. This system included both non-stimuli responsive and stimuli-responsive nanocarrier systems, which are commonly constructed, based on the change DNA configuration [
50]. Present literature mentioned that nanoflower inorganic nanoparticles have a spherical shape and increased the concentration of drug at the malignant site [
50,
51]. They developed AS1411/magnetic nanoparticles for targeted TMPyP4 delivery in this type of non-stimuli inorganic nanoparticle method. They also developed an Sgc8/MNP nanosystem and peptide/Au NPs for targeted DOX delivery [
52,
53]. Jiang and Zhang et al. engineered DNA nanoflower/polyhedron on nanoparticles for DOX delivery and photosensitizer co-delivery [
54]. Ding et al. developed a triangle DNA origami-gold nanorod complex that showed distinguish increase in cellular uptake and enhanced photothermal effect of Au against tumor cells. Light responsive nanosystems used dsDNA to connect with inorganic nanoparticles. AuNPs are representative of light-responsive nanosystem because AuNPs can convert light into heat to assist in the degradation of dsDNA and further release of drugs [
55]. Huang’s group developed AS1411 aptamer conjugated dsDNA hybrid nanostructures for co-delivering of Dox and TMPyP4. By applying heat or light effect on Au-Ag nanorod drug can be accumulated in higher concentration in the nuclei to efficiently kill the cancer cells. In a study, mesoporous silica nanoparticles were developed to perform on-demand stimuli response of therapeutics. Single-stranded DNA was ligated to magnetic nanoparticles. Magnetic nanoparticles were then decorated with complementary DNA sequences. The uncapping and subsequent release of mesopore-filled model drug was caused by DNA double stranded melting as a result of temperature increase [
56]. DNA lipid hybrid nanosystems are another type of DNA assessed delivery system, where functional DNA can connect to lipid to form hybrid nanosystems for tumor targeting. DNA polymer hybrid nanosystems are called hybrids as they can self-assemble into spherical structures without any complex design structure. Additionally, they are very supportive of other active agents like paclitaxel in the hydrophobic parts. Another type of DNA nanocargo includes polymer hybrid nanosystem that has greater encapsulation efficiency and can protect the drug against premature degradation. This property of the polymer hybrid system further helped to design the stimuli-responsive nanosystem [
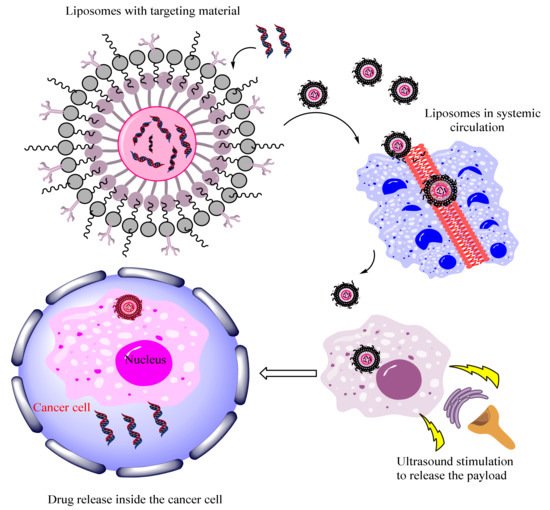
57]. Willners et al. developed a poly-function core and multilayer shell-based DNA polymer hybrid system for controlled release.
Table 1 demonstrated the prerequisite of DNA nanostructures along with their surface characteristics for particular organ targeting. These specific DNA assemblies were designed to identify the specific stimuli like pH, light, ATP to modify their conformation for drug release [
58].
Table 1. Prerequisite of DNA nanostructures to fulfill the mean particle diameter for specific organ targeting.
| Targeting Site |
Mean Particle Diameter |
Surface Characteristics |
Ref |
| Bone |
Undefined |
Substances like aspartic acid, alendronate can adhere to the bone and can be used for bone targeting. |
[59] |
| Liver |
Less than 100 nm to cross the liver fenestrae and target the hepatocytes. Greater than 100 nm uptake by Kupffer cells. |
No define surface property needed |
[59,60] |
| Lung |
Particles larger than 200 nm are trapped into lung capillaries |
Cationic surface charge |
[61] |
| Brain |
5–100 nm: nanoparticles uptake efficiency decreases with size |
Hydrophobic moieties and neutral charge enhance the brain uptake |
[59,62] |
| Lymph nodes |
1–40 nm: intra-tracheal administration
80 nm: Subcutaneous application |
Non-pegylated, Non-cationic, and sugar-based particles. |
[59,60] |
DNA-based nanosystems developed circular DNA nanotechnology for ligand functionalization (neuregulin-1/NRG-1) and its biological application [
63]. A group of researchers developed DNA nanospindals (DNA-NS) to efficiently load daunorubicin (DR) and target the HER2/neu receptors on the plasma membrane of drug-resistant MCF-7 (breast cancer) cells. DR loading onto DNA-NS was confirmed by the UV-shift analysis. The MTT results showed reduced viability of the MCF-7 cells after treatment with DNA-NS. Further results of apoptosis/proliferation obtained via flow cytometry showed enhanced apoptosis up to (64%) after treating with DNA-NS. Hence, all the types of DNA nanostructures in cancer therapy showed stiffer, uniform, and more biocompatible-targeted therapy [
64].
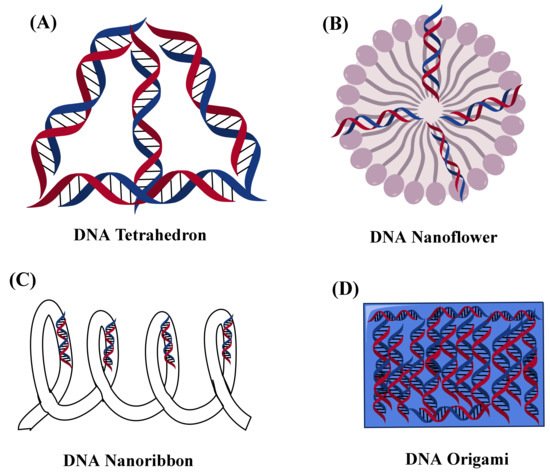
Figure 2 shows types of DNA nanostructures.
Figure 2. Showed the nanostructure representation of different types of DNA (A) DNA tetrahedron, (B) DNA nanoflower, (C) DNA nanoribbon, and (D) DNA Origami.