| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Priyani Ashoka Paranagama | + 1851 word(s) | 1851 | 2021-06-28 06:10:02 | | | |
| 2 | Peter Tang | Meta information modification | 1851 | 2021-07-06 03:35:22 | | |
Video Upload Options
A lichen is a symbiotic relationship between a fungus and a photosynthetic organism, which is algae or cyanobacteria. Endolichenic fungi are a group of microfungi that resides asymptomatically within the thalli of lichens. Endolichenic fungi can be recognized as luxuriant metabolic artists that produce propitious bioactive secondary metabolites.
1. Introduction
2. Antimicrobial Compounds Extracted from Endolichenic Fungi
|
Lichen |
Endolichenic Fungi |
No. |
Compound |
Microorganism |
Activity (µg/mL) |
Positive Control |
Activity (µg/mL) |
Reference |
|---|---|---|---|---|---|---|---|---|
|
Diorygma hieroglyphicum |
Talaromyces funiculosus |
1 |
Funiculosone |
Escherichia coli |
IC50 = 25 |
- |
- |
[17] |
|
Staphylococcus aureus |
IC50 = 58 |
- |
- |
|||||
|
2 |
Mangrovamide J |
Escherichia coli |
IC50 = 65 |
- |
- |
|||
|
Staphylococcus aureus |
IC50 = 104 |
- |
- |
|||||
|
3 |
Ravenilin |
Escherichia coli |
IC50 = 23 |
- |
- |
|||
|
Pseudomonas aeruginosa |
IC50 = 96 |
- |
- |
|||||
|
Staphylococcus aureus |
IC50 = 25 |
- |
- |
|||||
|
Everniastrum sp. |
Ulocladium sp. |
4 |
6-hydroxy-8-methoxy-3a-methyl-3a,9b-dihydro-3H-furo[3,2-c]isochromene-2,5-dione |
Bacillus subtilis |
IC50 = 25.0 |
Gentamicin |
IC50 < 0.048 |
[18] |
|
5 |
6-O-methylnorlichexanthone |
Bacillus subtilis |
IC50 = 0.39 |
|||||
|
6 |
Altenusin |
Bacillus subtilis |
IC50 = 11.3 |
|||||
|
7 |
Alterlactone |
Bacillus subtilis |
IC50 = 11.8 |
|||||
|
8 |
Griseoxanthone C |
Bacillus subtilis |
IC50 = 0.35 |
|||||
|
9 |
Isoaltenuene |
Bacillus subtilis |
IC50 = 14.7 |
|||||
|
10 |
Norlichexanthone |
Bacillus subtilis |
IC50 = 0.58 |
|||||
|
Methicillin Resistant Staphylococcus aureus. |
IC50 = 5.4 |
Vancomycin |
IC50 < 1.03 |
|||||
|
11 |
Tricycloalternarene 1b |
Bacille Calmette-Guérin strain |
MIC = 125 |
- |
- |
[19] |
||
|
Ulocladium sp. (CHMCC 5507) |
12 |
Ophiobolin P |
Bacillus subtilis |
MIC = 12.6 |
Gentamicin |
MIC = 0.05 |
[20] |
|
|
Methicillin Resistant Staphylococcus aureus. |
MIC = 25.1 |
Vancomycin |
MIC = 1.0 |
|||||
|
13 |
Ophiobolin T |
Bacille Calmette-Guérin strain |
MIC = 12.7 |
Hygromycin |
MIC = 0.35 |
|||
|
Bacillus subtilis |
MIC = 6.3 |
Gentamicin |
MIC = 0.05 |
|||||
|
Methicillin Resistant Staphylococcus aureus |
MIC = 12.7 |
Vancomycin |
MIC = 1.0 |
|||||
|
Parmelinella wallichiana |
Nigrospora sphaerica |
14 |
Alternariol |
Bacillus subtilis |
MIC = 31.2 |
Amikacin sulfate |
MIC = 0.45 |
[21] |
|
Escherichia coli |
MIC = 62.5 |
MIC = 0.90 |
||||||
|
Staphylococcus aureus |
MIC = 62.5 |
MIC = 0.90 |
||||||
|
15 |
Alternariol-9-methyl ether |
Bacillus subtilis |
MIC = 62.5 |
MIC = 0.45 |
||||
|
Pseudomonas fluorescens |
MIC = 31.2 |
MIC = 0.90 |
||||||
|
Staphylococcus aureus |
MIC = 62.5 |
MIC = 0.90 |
||||||
|
Parmotrema rampoddense |
Fusarium proliferatum |
16 |
Acetyl tributyl citrate |
Klebsiella pneumoniae |
MIC = 125 |
- |
- |
[22] |
|
Pseudomonas aeruginosa |
MIC = 125 |
- |
- |
|||||
|
Staphylococcus aureus |
MIC = 125 |
- |
- |
|||||
|
17 |
Fusarubin |
Escherichia coli |
MIC = 1.56 |
- |
- |
|||
|
Pseudomonas aeruginosa |
MIC = 1.56 |
- |
- |
|||||
|
Staphylococcus aureus |
MIC = 1.56 |
- |
- |
|||||
|
Parmotrema ravum |
Aspergillus niger |
18 |
Asperpyrone A |
Staphylococcus aureus MTCC 737 |
IC50 = 112 |
- |
- |
[23] |
|
19 |
Aurasperone A |
Dickeya solani GBBC 1502 |
IC50 = 63 |
- |
- |
|||
|
Listeria innocua LMG11387 |
IC50 = 141 |
- |
- |
|||||
|
Pectobacterium sp. |
IC50 = 76 |
- |
- |
|||||
|
Pseudomonas aeruginosa MTCC 424 |
IC50 = 160 |
- |
- |
|||||
|
Pseudomonas syringae pv. Maculicola I11004 |
IC50 = 80 |
- |
- |
|||||
|
Staphylococcus aureus MTCC 737 |
IC50 = 135 |
- |
- |
|||||
|
20 |
Carbonarone A |
Dickeya solani GBBC 1502 |
IC50 = 88 |
- |
- |
|||
|
21 |
Fonsecinone A |
Escherichia coli MTCC 443 |
IC50 = 47 |
- |
- |
|||
|
Pseudomonas syringae pv. Maculicola I11004 |
IC50 = 154 |
- |
- |
|||||
|
Staphylococcus aureus MTCC 738 |
IC50 = 120 |
- |
- |
|||||
|
22 |
Pyrophen |
Aeromonas hydrophila ATCC 7966 |
IC50 = 78 |
- |
- |
|||
|
Listeria innocua LMG11387 |
IC50 = 86 |
- |
- |
|||||
|
Micrococcus luteus DPMB3 |
IC50 = 63 |
- |
- |
|||||
|
Sticta fuliginosa |
Xylariaceae sp. (CR1546C) |
23 |
(R)-4,6,8-trihydroxy-3,4-dihydro-1(2H)-naphthalenone |
Bacillus subtilis |
IC50 = 104.2 |
Streptomycin sulphate |
IC50 = 5.2 |
[24] |
|
24 |
18-O-acetylambuic acid |
Staphylococcus aureus ATCC 6538 |
IC50 = 10.9 |
Antimicrobial peptide (AMP) |
[25] |
|||
|
25 |
6,8-dihydroxy-(3R)-(2-oxopropyl)-3,4-dihydroisocoumarin |
Bacillus subtilis |
IC50 = 106.4 |
Streptomycin sulphate |
IC50 = 5.2 |
[24] |
||
|
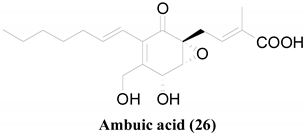
26 |
Ambuic acid |
Staphylococcus aureus ATCC 6538 |
IC50 = 15.4 |
Antimicrobial peptide (AMP) |
[25] |
|||
|
Usnea sp. |
Hypoxylon fuscum |
27 |
16-α-D-mannopyranosyloxyisopimar-7-en-19-oic acid |
Staphylococcus aureus CGMCC 1.2465 |
MIC = 46.4 |
Vancomycin Hydrochloride |
MIC = 3.12 |
[26] |
|
28 |
8-methoxy-1-naphthyl-β-glucopyranoside |
Staphylococcus aureus CGMCC 1.2465 |
MIC = 30.1 |
|||||
|
29 |
Phomol |
Staphylococcus aureus CGMCC 1.2465 |
MIC = 21.1 |
|||||
|
- |
Coniochaeta sp. |
30 |
Coniothienol A |
Enterococcus faecalis (CGMCC 1.2535) |
IC50 = 4.89 |
Ampicillin |
IC50 = 2.61 |
[27] |
|
Enterococcus faecium (CGMCC 1.2025) |
IC50 = 2.00 |
IC50 = 0.51 |
||||||
|
31 |
Coniothiepinols A |
Enterococcus faecalis (CGMCC 1.2535) |
IC50 = 11.51 |
IC50 = 2.61 |
||||
|
Enterococcus faecium (CGMCC 1.2025) |
IC50 = 3.93 |
IC50 = 0.51 |
||||||
|
Cetraria islandica |
Myxotrichum sp. |
32 |
Myxodiol A |
Candida albicans SC 5314 |
MIC = 128 |
Fluconazole |
MIC = 2 |
[28] |
|
Pestalotiopsis sp. |
33 |
Ambuic acid derivative 1 |
Fusarium oxysporum |
MIC = 8 |
Ketoconazole |
MIC = 8 |
[29] |
|
|
34 |
Ambuic acid derivative 2 |
Fusarium oxysporum |
MIC = 32 |
MIC = 8 |
||||
|
35 |
Ambuic acid derivative 4 |
Verticillium dahlia |
MIC = 32 |
MIC = 1 |
||||
|
36 |
Ambuic acid derivative 5 |
Fusarium gramineum |
MIC = 8 |
MIC = 8 |
||||
|
Fusarium oxysporum |
MIC = 8 |
MIC = 8 |
||||||
|
Verticillium dahlia |
MIC = 16 |
MIC = 1 |
||||||
|
37 |
Ambuic acid derivative 6 |
Fusarium gramineum |
MIC = 8 |
MIC = 8 |
||||
|
38 |
Ambuic acid derivative 7 |
Rhizoctonia solani |
MIC = 32 |
MIC = 8 |
||||
|
39 |
Ambuic acid derivative 8 |
Rhizoctonia solani |
MIC = 32 |
MIC = 8 |
||||
|
40 |
Ambuic acid derivative 9 |
Fusarium gramineum |
MIC = 32 |
MIC = 8 |
||||
|
Fusarium oxysporum |
MIC = 16 |
MIC = 8 |
||||||
|
41 |
Ambuic acid derivative 11 |
Fusarium gramineum |
MIC = 32 |
MIC = 8 |
||||
|
Cetrelia sp. |
Aspergillus sp. CPCC 400810 |
42 |
Isocoumarindole A |
Candida albicans |
MIC = 32.0 |
Caspofungin |
MIC = 0.03 |
[30] |
|
Diorygma hieroglyphicum |
Talaromyces funiculosus |
1 |
Funiculosone |
Candida albicans |
IC50 = 35 |
- |
- |
[17] |
|
Everniastrum sp. |
Ulocladium sp. |
43 |
7-hydroxy-3-(2-hydroxy-propyl)-5-methyl-isochromen-1-one |
Candida albicans SC 5314 |
IC50 = 45.4 |
Amphotericin B |
IC50 = 1.03 |
[18] |
|
44 |
7-hydroxy-3,5-dimethyl-isochromen-1-one |
Candida albicans SC 5314 |
IC50 = 18.7 |
|||||
|
6 |
Altenusin |
Aspergillus fumigatus |
IC50 = 57.5 |
IC50 = 0.74 |
||||
|
8 |
Griseoxanthone C |
Candida albicans SC 5314 |
IC50 = 40.6 |
IC50 = 1.03 |
||||
|
10 |
Norlichexanthone |
Aspergillus fumigatus |
IC50 = 43.6 |
IC50 = 0.74 |
||||
|
45 |
Rubralactone |
Aspergillus fumigatus |
IC50 = 63.3 |
IC50 = 0.74 |
||||
|
Candida albicans SC 5314 |
IC50 = 54.7 |
IC50 = 1.03 |
||||||
|
Lethariella zahlbruckner |
Tolypocladium cylindrosporum |
46 |
Pyridoxatin |
Candida albicans (Multiple strains) |
MIC = 0.5 − 8.0 |
Fluconazole |
MIC = 1.0 − 2.0 |
[31] |
|
Candida glabrata (Multiple strains) |
MIC = 1.0 − 8.0 |
MIC = 1.0 − 2.0 |
||||||
|
Candida krusei (Multiple strains) |
MIC = 1.0 − 4.0 |
MIC = 1.0 − 2.0 |
||||||
|
Candida tropicalis CT2 |
MIC = 32 |
MIC = 2.0 |
||||||
|
Lobaria quercizans |
Aspergillus versicolor |
47 |
3,7-dihydroxy-1,9-dimethyldibenzofuran |
Candida albicans |
MIC = 64 |
Fluconazole |
MIC = 2 |
[32] |
|
48 |
Cordyol C |
Candida albicans |
MIC = 8 |
|||||
|
49 |
Diorcinol D |
Candida albicans |
MIC = 8 |
|||||
|
50 |
Diorcinol I |
Candida albicans |
MIC = 32 |
|||||
|
51 |
Violaceol I |
Candida albicans |
MIC = 8 |
|||||
|
52 |
Violaceol II |
Candida albicans |
MIC = 8 |
|||||
|
Parmelia sp. |
Periconia sp. |
53 |
3-(2-oxo-2H-pyran-6-yl)propanoic acid |
Aspergillus niger |
MIC = 31 |
Cycloheximide |
MIC < 16 |
[33] |
|
54 |
Pericocin A |
Aspergillus niger |
MIC = 31 |
Cycloheximide |
MIC < 16 |
|||
|
55 |
Pericocin B |
Aspergillus niger |
MIC = 31 |
|||||
|
56 |
Pericocin C |
Aspergillus niger |
MIC = 31 |
|||||
|
57 |
Pericocin D |
Aspergillus niger |
MIC = 31 |
|||||
|
58 |
Pericoterpenoid A |
Aspergillus niger |
MIC = 31 |
[34] |
||||
|
Tolypocladium sp. (4259a) |
46 |
Pyridoxatin |
Candida albicans |
MIC = 0.5 |
- |
- |
[35] |
|
|
Parmelinella wallichiana |
Nigrospora sphaerica |
14 |
Alternariol |
Candida albicans |
MIC = 80.0 |
Ketoconazole |
MIC = 1.90 |
[21] |
|
Parmotrema ravum |
Aspergillus niger |
59 |
Aspergyllone |
Candida parapsilosis |
IC50 = 52 |
- |
- |
[23] |
|
19 |
Aurasperone A |
Candida krusei MTCC 9215 |
IC50 = 373 |
- |
- |
|||
|
20 |
Carbonarone A |
Candida albicans MTCC 227 |
IC50 = 103 |
- |
- |
|||
|
Candida krusei MTCC 9215 |
IC50 = 31 |
- |
- |
|||||
|
22 |
Pyrophen |
Candida albicans MTCC 227 |
IC50 = 74 |
- |
- |
|||
|
Candida glabrata |
IC50 = 97 |
- |
- |
|||||
|
Candida utilis IHEM 400 |
IC50 = 62 |
- |
- |
|||||
|
Pseudosyphellaria sp. |
Biatriospora sp. |
60 |
2-acetonyl-3-methyl-5-hydroxy-7-methoxynaphthazarin |
Candida albicans |
MIC = 64 |
Fluconazole |
MIC = 2 |
[36] |
|
61 |
6-deoxy-7-O-demethyl-3,4-anhydrofusarubin |
Candida albicans |
MIC = 32 |
|||||
|
62 |
Biatriosporin D |
Candida albicans |
MIC = 16 |
|||||
|
63 |
Biatriosporin K |
Candida albicans |
MIC = 64 |
|||||
|
Sticta fuliginosa |
Xylariaceae sp. (CR1546C) |
64 |
(3R,4S)-3,4,8-trihydroxy-3,4-dihydro-1(2H)-naphthalenone |
Candida albicans |
IC50 = 63.2 |
Amphotericin B |
IC50 = 1.3 |
[24] |
|
65 |
(3S,4S)-3,4,6,8-tetrahydroxy-3,4-dihydro-1(2H)-naphthalenone |
Candida albicans |
IC50 = 67.8 |
|||||
|
23 |
(R)-4,6,8-trihydroxy-3,4-dihydro-1(2H)-naphthalenone |
Candida albicans |
IC50 = 78.2 |
|||||
|
66 |
2,4-dihydroxy-6-(2-oxopropyl)-benzoic acid |
Candida albicans |
IC50 = 101.3 |
|||||
|
67 |
5,6,8-trihydroxy-3(R)-methyl-3,4-dihydroisocoumarin |
Candida albicans |
IC50 = 71.4 |
|||||
|
68 |
6,8-dihydroxy-(3)-(2-oxopropyl)-isocoumarin |
Candida albicans |
IC50 = 98.1 |
|||||
|
25 |
6,8-dihydroxy-(3R)-(2-oxopropyl)-3,4-dihydroisocoumarin |
Candida albicans |
IC50 = 71.2 |
|||||
|
69 |
6,8-dihydroxy-3(R)-methyl-3,4-dihydroisocoumarin |
Candida albicans |
IC50 = 65.1 |
|||||
|
70 |
6,8-dihydroxy-3-[(2S)-2-hydroxypropyl]-isocoumarin |
Candida albicans |
IC50 = 99.1 |
|||||
|
71 |
6,8-dihydroxy-3-methylisocoumarin |
Candida albicans |
IC50 = 67.2 |
|||||
|
Umbilicaria sp. |
Floricola striata |
72 |
Floricolin A |
Candida albicans |
MIC = 16 |
- |
- |
[37] |
|
73 |
Floricolin B |
Candida albicans |
MIC = 8 |
- |
- |
|||
|
74 |
Floricolin C |
Candida albicans |
MIC = 8 |
- |
- |
|||
|
75 |
Floricolin D |
Candida albicans |
MIC = 64 |
- |
- |
|||
|
76 |
Terphenyl 2 |
Candida albicans |
MIC = 64 |
- |
- |
|||
|
Usnea baileyi |
Xylaria venustula |
77 |
N-dodecyldiethanolamine (DDE) |
Candida albicans NCTC713 |
MIC = 5.5 |
- |
- |
|
|
78 |
Piliformic acid |
Colletotrichum gloeosporioides |
MIC = 625.2 |
Captan |
MIC = 5000 |
|||
|
Difenoconazole |
MIC = 8.1 |
|||||||
|
- |
Coniochaeta sp. |
31 |
Coniothiepinols A |
Fusarium oxysporum (CGMCC 3.2830) |
IC50 = 13.12 |
Carbendazim |
IC50 = 0.44 |
[27] |
|
Parmelinella wallichiana |
Nigrospora sphaerica |
14 |
Alternariol |
Herpes Simplex Virus |
IC50 = 34.9 |
- |
- |
[41] |
|
15 |
Alternariol-9-methyl ether |
Herpes Simplex Virus |
IC50 = 64.0 |
- |
- |
|||
|
Usnea baileyi |
Xylaria venustula |
79 |
Isoplysin A |
Plasmodium falciparum |
MIC = 0.97 |
- |
- |
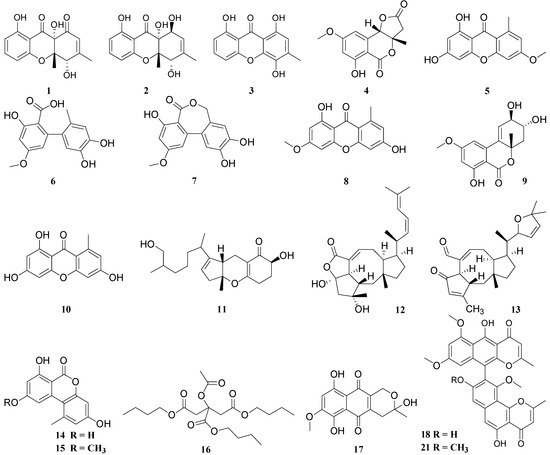
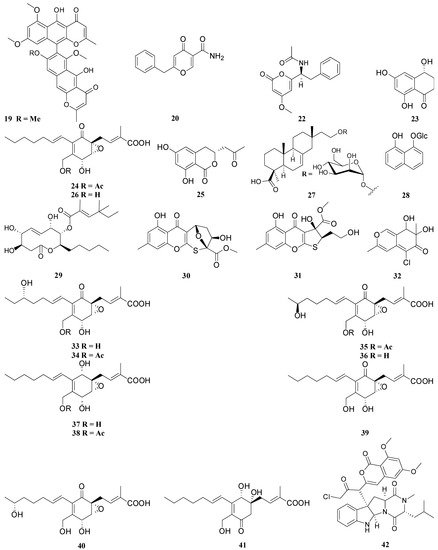
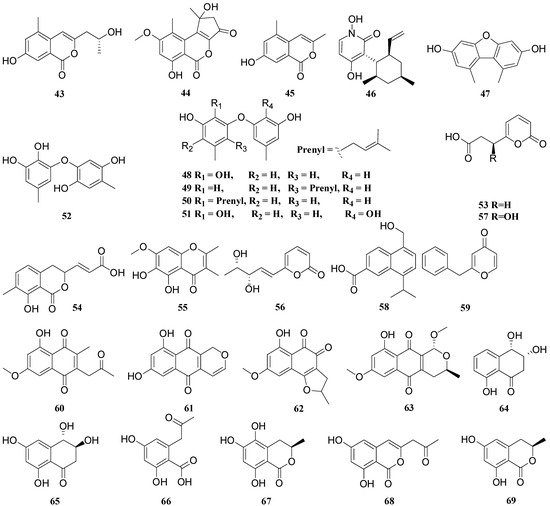
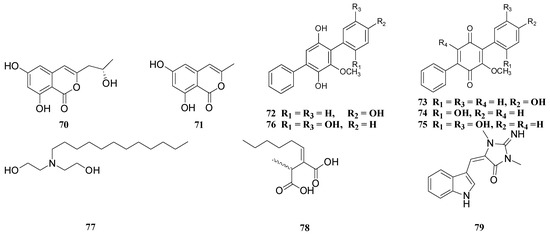
3. Structural Features Which Affect the Antimicrobial Activity of the Compounds
|
Scaffold |
Compounds |
|---|---|
|
|
4, 9, 14, 15, 25, 42, 43, 44, 45, 53, 54, 56, 57, 66, 67, 68, 69, 70, 71 |
|
|
1, 2, 3, 5, 8, 10, 18, 19, 20, 21, 30, 31, 55, 59 |
|
|
24, 26, 33, 34, 35, 36, 37, 38, 39, 40, 41 |
|
|
48, 49, 50, 51, 52 |
|
|
17, 61, 63 |
|
|
23, 64, 65 |
References
- Gunatilaka, A.A.L. Natural products from plant-associated microorganisms: Distribution, structural diversity, bioactivity, and implications of their occurrence. J. Nat. Prod. 2006, 69, 509–526.
- Petrini, O. Fungal endophytes of tree leaves. In Microbial Ecology of Leaves; Springer: New York, NY, USA, 1991; pp. 179–197.
- Strobel, G.; Daisy, B. Bioprospecting for microbial endophytes and their natural products. Microbiol. Mol. Biol. Rev. 2003, 67, 491–502.
- Henskens, F.L.; Green, T.G.A.; Wilkins, A. Cyanolichens can have both cyanobacteria and green algae in a common layer as major contributors to photosynthesis. Ann. Bot. 2012, 110, 555–563.
- Basnet, B.B.; Liu, H.; Liu, L.; Suleimen, Y.M. Diversity of anticancer and antimicrobial compounds from lichens and lichen-derived fungi: A systematic review (1985–2017). Curr. Org. Chem. 2018, 22, 2487–2500.
- Gao, H.; Zou, J.; Li, J.; Zhao, H. Endolichenic fungi: A potential treasure trove for discovery of special structures and bioactive compounds. In Studies in Natural Products Chemistry; Atta-Ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 48, pp. 347–397.
- Agrawal, S.; Deshmukh, S.K.; Reddy, M.S.; Prasad, R.; Goel, M. Endolichenic fungi: A hidden source of bioactive metabolites. S. Afr. J. Bot. 2020, 134, 163–186.
- Boustie, J.; Tomasi, S.; Grube, M. Bioactive lichen metabolites: Alpine habitats as an untapped source. Phytochem. Rev. 2011, 10, 287–307.
- Lawrey, J.D. Biological role of lichen substances. Bryologist 1986, 89, 111–122.
- Shukla, V.; Joshi, G.P.; Rawat, M.S.M. Lichens as a potential natural source of bioactive compounds: A review. Phytochem. Rev. 2010, 9, 303–314.
- Galloway, D.J. Biodiversity: A lichenological perspective. Biodivers. Conserv. 1992, 1, 312–323.
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota-approaching one thousand genera. Bryologist 2016, 119, 361–416.
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268.
- Sarasan, M.; Puthumana, J.; Job, N.; Han, J.; Lee, J.S.; Philip, R. Marine algicolous endophytic fungi-a promising drug resource of the era. J. Microbiol. Biotechnol. 2017, 27, 1039–1052.
- Alwis, Y.V.; Wethalawe, A.N.; Udukala, D.N.; Paranagama, P.A. Endophytic microflora of sri lankan plants: An overview of the therapeutic and agricultural applications of the secondary metabolites. In Endophytes; Patil, R.H., Maheshwari, V.L., Eds.; Springer: Singapore, 2021; pp. 153–175.
- Nguyen, K.H.; Chollet-Krugler, M.; Gouault, N.; Tomasi, S. UV-protectant metabolites from lichens and their symbiotic partners. Nat. Prod. Rep. 2013, 30, 1490–1508.
- Padhi, S.; Masi, M.; Cimmino, A.; Tuzi, A.; Jena, S.; Tayung, K.; Evidente, A. Funiculosone, a substituted dihydroxanthene-1,9-dione with two of its analogues produced by an endolichenic fungus Talaromyces funiculosus and their antimicrobial activity. Phytochemistry 2019, 157, 175–183.
- Wang, Q.X.; Bao, L.; Yang, X.L.; Guo, H.; Yang, R.N.; Ren, B.; Zhang, L.X.; Dai, H.Q.; Guo, L.D.; Liu, H.W. Polyketides with antimicrobial activity from the solid culture of an endolichenic fungus Ulocladium sp. Fitoterapia 2012, 83, 209–214.
- Wang, Q.X.; Bao, L.; Yang, X.L.; Guo, H.; Ren, B.; Guo, L.D.; Song, F.H.; Wang, W.Z.; Liu, H.W.; Zhang, L.X. Tricycloalternarenes F-H: Three new mixed terpenoids produced by an endolichenic fungus Ulocladium sp. using OSMAC method. Fitoterapia 2013, 85, 8–13.
- Wang, Q.X.; Bao, L.; Yang, X.L.; Liu, D.L.; Guo, H.; Dai, H.Q.; Song, F.H.; Zhang, L.X.; Guo, L.D.; Li, S.J.; et al. Ophiobolins P-T, five new cytotoxic and antibacterial sesterterpenes from the endolichenic fungus Ulocladium sp. Fitoterapia 2013, 90, 220–227.
- Gu, W. Bioactive metabolites from Alternaria brassicicola ML-P08, an endophytic fungus residing in Malus halliana. World J. Microbiol. Biotechnol. 2009, 25, 1677–1683.
- Tan, M.A.; Castro, S.G.; Oliva, P.M.P.; Yap, P.R.J.; Nakayama, A.; Magpantay, H.D.; dela Cruz, T.E.E. Biodiscovery of antibacterial constituents from the endolichenic fungi isolated from Parmotrema rampoddense. 3 Biotech 2020, 10, 1–7.
- Padhi, S.; Masi, M.; Panda, S.K.; Luyten, W.; Cimmino, A.; Tayung, K.; Evidente, A. Antimicrobial secondary metabolites of an endolichenic Aspergillus niger isolated from lichen thallus of Parmotrema ravum. Nat. Prod. Res. 2020, 34, 2573–2580.
- Kim, K.H.; Beemelmanns, C.; Murillo, C.; Guillén, A.; Umaña, L.; Tamayo-Castillo, G.; Kim, S.N.; Clardy, J.; Cao, S. Naphthalenones and isocoumarins from a Costa Rican fungus Xylariaceae sp. CR1546C. J. Chem. Res. 2014, 38, 722–725.
- Ding, G.; Li, Y.; Fu, S.; Liu, S.; Wei, J.; Che, Y. Ambuic acid and torreyanic acid derivatives from the endolichenic fungus Pestalotiopsis sp. J. Nat. Prod. 2009, 72, 182–186.
- Basnet, B.B.; Chen, B.; Suleimen, Y.M.; Ma, K.; Guo, S.; Bao, L.; Huang, Y.; Liu, H. Cytotoxic secondary metabolites from the endolichenic fungus Hypoxylon fuscum. Planta Med. 2019, 85, 1088–1097.
- Wang, Y.; Niu, S.; Liu, S.; Guo, L.; Che, Y. The first naturally occurring thiepinols and thienol from an endolichenic fungus Coniochaeta sp. Org. Lett. 2010, 12, 5081–5083.
- Yuan, C.; Wang, H.Y.; Wu, C.S.; Jiao, Y.; Li, M.; Wang, Y.Y.; Wang, S.Q.; Zhao, Z.T.; Lou, H.X. Austdiol, fulvic acid and citromycetin derivatives from an endolichenic fungus, Myxotrichum sp. Phytochem. Lett. 2013, 6, 662–666.
- Yuan, C.; Ding, G.; Wang, H.Y.; Guo, Y.H.; Shang, H.; Ma, X.J.; Zou, Z.M. Polyketide-terpene hybrid metabolites from an endolichenic fungus Pestalotiopsis sp. Biomed Res. Int. 2017, 2017, 1–10.
- Chen, M.; Wang, R.; Zhao, W.; Yu, L.; Zhang, C.; Chang, S.; Li, Y.; Zhang, T.; Xing, J.; Gan, M.; et al. Isocoumarindole A, a chlorinated isocoumarin and indole alkaloid hybrid metabolite from an endolichenic fungus Aspergillus sp. Org. Lett. 2019, 21, 1530–1533.
- Chang, W.; Zhang, M.; Li, Y.; Li, X.; Gao, Y.; Xie, Z.; Lou, H. Lichen endophyte derived pyridoxatin inactivates Candida growth by interfering with ergosterol biosynthesis. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1762–1771.
- Li, X.; Zhou, Y.; Zhu, R.; Chang, W.; Yuan, H. Identification and biological evaluation of secondary metabolites from the endolichenic fungus Aspergillus versicolor. Chem. Biodivers. 2015, 12, 575–592.
- Wu, Y.H.; Xiao, G.K.; Chen, G.D.; Wang, C.X.; Hu, D.; Lian, Y.Y.; Lin, F.; Guo, L.D.; Yao, X.S.; Gao, H. Pericocins A-D, new bioactive compounds from Periconia sp. Nat. Prod. Commun. 2015, 10, 2127–2130.
- Wu, Y.H.; Chen, G.D.; Wang, C.X.; Hu, D.; Li, X.X.; Lian, Y.Y.; Lin, F.; Guo, L.D.; Gao, H. Pericoterpenoid A, a new bioactive cadinane-type sesquiterpene from Periconia sp. J. Asian Nat. Prod. Res. 2015, 17, 671–675.
- Hu, C.H.; Zhou, Y.H.; Xie, F.; Li, Y.L.; Zhao, Z.T.; Lou, H.X. Two new α-pyrone derivatives from an endolichenic fungus Tolypocladium sp. J. Asian Nat. Prod. Res. 2017, 19, 786–792.
- Zhou, Y.H.; Zhang, M.; Zhu, R.X.; Zhang, J.Z.; Xie, F.; Li, X.B.; Chang, W.Q.; Wang, X.N.; Zhao, Z.T.; Lou, H.X. Heptaketides from an endolichenic fungus Biatriospora sp. and their antifungal activity. J. Nat. Prod. 2016, 79, 2149–2157.
- Li, W.; Gao, W.; Zhang, M.; Li, Y.L.; Li, L.; Li, X.B.; Chang, W.Q.; Zhao, Z.T.; Lou, H.X. P-Terphenyl derivatives from the endolichenic fungus Floricola striata. J. Nat. Prod. 2016, 79, 2188–2194.
- Santiago, K.A.A.; Edrada-Ebel, R.; Dela Cruz, T.E.E.; Cheow, Y.L.; Ting, A.S.Y. Biodiscovery of potential antibacterial diagnostic metabolites from the endolichenic fungus Xylaria venustula using LC–MS-based metabolomics. Biology 2021, 10, 191.
- Lambert, P.A.; Smith, A.R.W. The mode of action of N-(n-dodecyl)diethanolamine with particular reference to the effect of protonation on uptake by Escherichia coli. J. Gen. Microbiol. 1977, 103, 367–374.
- Elias, L.M.; Fortkamp, D.; Sartori, S.B.; Ferreira, M.C.; Gomes, L.H.; Azevedo, J.L.; Montoya, Q.V.; Rodrigues, A.; Ferreira, A.G.; Lira, S.P. The potential of compounds isolated from Xylaria spp. as antifungal agents against anthracnose. Brazilian J. Microbiol. 2018, 49, 840–847.
- He, J.W.; Chen, G.D.; Gao, H.; Yang, F.; Li, X.X.; Peng, T.; Guo, L.D.; Yao, X.S. Heptaketides with antiviral activity from three endolichenic fungal strains Nigrospora sp., Alternaria sp. and Phialophora sp. Fitoterapia 2012, 83, 1087–1091.
- Bialonska, D.; Zjawiony, J.K. Aplysinopsins—marine indole alkaloids: Chemistry, bioactivity and ecological significance. Mar. Drugs 2009, 7, 166–183.