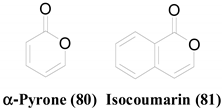
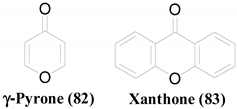
A lichen is a symbiotic relationship between a fungus and a photosynthetic organism, which is algae or cyanobacteria. Endolichenic fungi are a group of microfungi that resides asymptomatically within the thalli of lichens. Endolichenic fungi can be recognized as luxuriant metabolic artists that produce propitious bioactive secondary metabolites.
- endolichenic fungi
- antibacterial
- antifungal
- antiviral
- antiplasmodial
- secondary metabolites
1. Introduction
2. Antimicrobial Compounds Extracted from Endolichenic Fungi
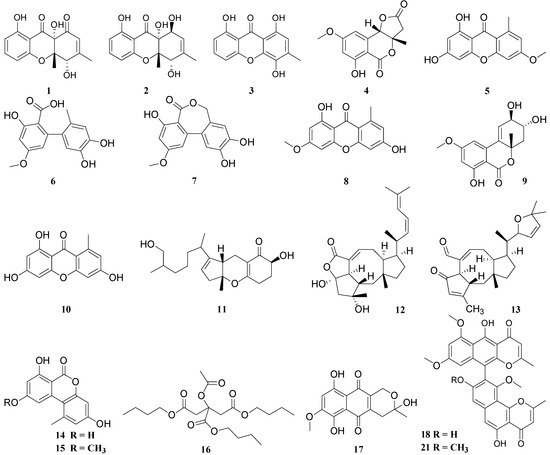
Lichen | Scaffold | Endolichenic Fungi | Compounds | No. | Compound | Microorganism | Activity (µg/mL) | Positive Control | Activity (µg/mL) | Reference | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Diorygma hieroglyphicum | Talaromyces funiculosus | |||||||||||||||||||||||||||||
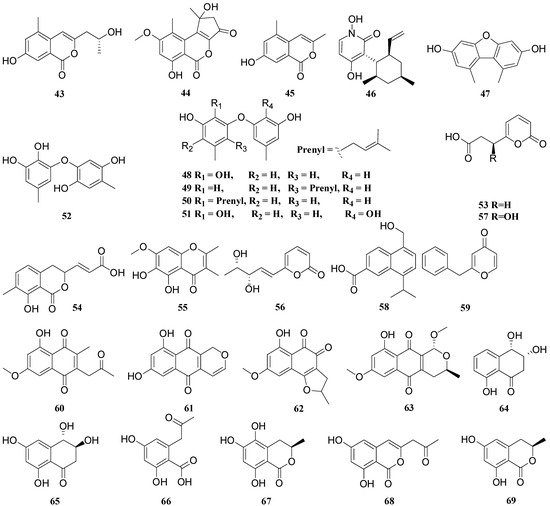
| 4, 9, 14, 15, 25 | 1 | , 42, | Funiculosone | 43, 44, | Escherichia coli | 45, | IC50 = 25 | - | - | 53, 54, 56, 57, 66, 67, 68, 69, 70, 71 |
[17] |
[39] |
|||||||||||||||||
Staphylococcus aureus | ||||||||||||||||||||||||||||||
| IC50 = 58 | 1, 2, 3, 5, 8, 10, 18, 19, 20, 21, 30, | - | 31, 55 | - | |||||||||||||||||||||||||
, | 59 | 2 | Mangrovamide J | Escherichia coli | ||||||||||||||||||||||||||
| IC50 = 65 | - | - | |||||||||||||||||||||||||||
24, 26, 33, 34, 35, 36, 37, 38, 39, 40, 41 | Staphylococcus aureus | |||||||||||||||||||||||||||||
| IC50 = 104 |
| 48, 49, 50, 51, 52 | - | - | |||||||||||||||||||||||||
3 | Ravenilin | Escherichia coli | IC50 = 23 | - | - | |||||||||||||||||||||||||
Pseudomonas aeruginosa | ||||||||||||||||||||||||||||||
| 17, |
IC50 = 96 | - | - | ||||||||||||||||||||||||||
Staphylococcus aureus | IC50 = 25 | - | - | |||||||||||||||||||||||||||
Everniastrum sp. | Ulocladium sp. | 4 | 6-hydroxy-8-methoxy-3a-methyl-3a,9b-dihydro-3H-furo[3,2-c]isochromene-2,5-dione | Bacillus subtilis | IC50 = 25.0 | Gentamicin | IC50 < 0.048 |
[18] |
[40] |
|||||||||||||||||||||
5 | 6-O-methylnorlichexanthone | Bacillus subtilis | IC50 = 0.39 | |||||||||||||||||||||||||||
6 | Altenusin | Bacillus subtilis | IC50 = 11.3 | |||||||||||||||||||||||||||
7 | Alterlactone | Bacillus subtilis | IC50 = 11.8 | |||||||||||||||||||||||||||
8 | Griseoxanthone C | Bacillus subtilis | IC50 = 0.35 | |||||||||||||||||||||||||||
9 | Isoaltenuene | Bacillus subtilis | IC50 = 14.7 | |||||||||||||||||||||||||||
10 | Norlichexanthone | Bacillus subtilis | IC50 = 0.58 | |||||||||||||||||||||||||||
Methicillin Resistant Staphylococcus aureus. | IC50 = 5.4 | Vancomycin | IC50 < 1.03 | |||||||||||||||||||||||||||
11 | Tricycloalternarene 1b | Bacille Calmette-Guérin strain | MIC = 125 | - | - |
[19] |
[41] |
|||||||||||||||||||||||
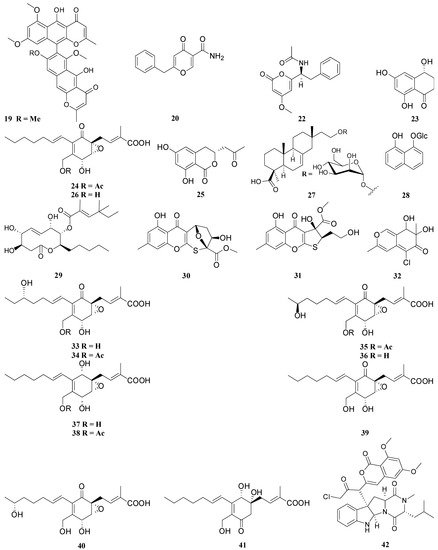
Ulocladium sp. (CHMCC 5507) | 12 | Ophiobolin P | Bacillus subtilis | MIC = 12.6 | Gentamicin | MIC = 0.05 |
[20] |
[27] |
||||||||||||||||||||||
Methicillin Resistant Staphylococcus aureus. | MIC = 25.1 | Vancomycin | MIC = 1.0 | |||||||||||||||||||||||||||
13 | Ophiobolin T | Bacille Calmette-Guérin strain | MIC = 12.7 | Hygromycin | MIC = 0.35 | |||||||||||||||||||||||||
Bacillus subtilis | MIC = 6.3 | Gentamicin | MIC = 0.05 | |||||||||||||||||||||||||||
Methicillin Resistant Staphylococcus aureus | MIC = 12.7 | Vancomycin | MIC = 1.0 | |||||||||||||||||||||||||||
Parmelinella wallichiana | Nigrospora sphaerica | 14 | Alternariol | Bacillus subtilis | MIC = 31.2 | Amikacin sulfate | MIC = 0.45 |
[21] |
[42] |
|||||||||||||||||||||
Escherichia coli | MIC = 62.5 | MIC = 0.90 | ||||||||||||||||||||||||||||
Staphylococcus aureus | MIC = 62.5 | MIC = 0.90 | ||||||||||||||||||||||||||||
15 | Alternariol-9-methyl ether | Bacillus subtilis | MIC = 62.5 | MIC = 0.45 | ||||||||||||||||||||||||||
61 | , 63 | Pseudomonas | fluorescens | MIC = 31.2 | MIC = 0.90 | |||||||||||||||||||||||||
Staphylococcus aureus | MIC = 62.5 | MIC = 0.90 | ||||||||||||||||||||||||||||
Parmotrema rampoddense | Fusarium | proliferatum | 16 | Acetyl tributyl citrate | Klebsiella pneumoniae | MIC = 125 | - | - |
[22] |
[43] |
||||||||||||||||||||
Pseudomonas aeruginosa | MIC = 125 | - | - | |||||||||||||||||||||||||||
Staphylococcus aureus | MIC = 125 | - | - | |||||||||||||||||||||||||||
17 | Fusarubin | Escherichia coli | MIC = 1.56 | - | - | |||||||||||||||||||||||||
Pseudomonas aeruginosa | MIC = 1.56 | - | - | |||||||||||||||||||||||||||
Staphylococcus aureus | MIC = 1.56 | - | - | |||||||||||||||||||||||||||
Parmotrema ravum | Aspergillus niger | 18 | Asperpyrone A | Staphylococcus aureus MTCC 737 | IC50 = 112 | - | - |
[23] |
[44] |
|||||||||||||||||||||
19 | Aurasperone A | Dickeya solani GBBC 1502 | IC50 = 63 | - | - | |||||||||||||||||||||||||
Listeria innocua LMG11387 | IC50 = 141 | - | - | |||||||||||||||||||||||||||
Pectobacterium sp. | IC50 = 76 | - | - | |||||||||||||||||||||||||||
Pseudomonas aeruginosa MTCC 424 | IC50 = 160 | - | - | |||||||||||||||||||||||||||
Pseudomonas syringae pv. Maculicola I11004 | IC50 = 80 | - | - | |||||||||||||||||||||||||||
Staphylococcus aureus MTCC 737 | IC50 = 135 | - | - | |||||||||||||||||||||||||||
20 | Carbonarone A | Dickeya solani GBBC 1502 | IC50 = 88 | - | - | |||||||||||||||||||||||||
21 | Fonsecinone A | Escherichia coli MTCC 443 | IC50 = 47 | - | - | |||||||||||||||||||||||||
Pseudomonas syringae pv. Maculicola I11004 | IC50 = 154 | - | - | |||||||||||||||||||||||||||
Staphylococcus aureus MTCC 738 | IC50 = 120 | - | - | |||||||||||||||||||||||||||
22 | Pyrophen | Aeromonas hydrophila ATCC 7966 | IC50 = 78 | - | - | |||||||||||||||||||||||||
Listeria innocua LMG11387 | IC50 = 86 | - | - | |||||||||||||||||||||||||||
Micrococcus luteus DPMB3 | IC50 = 63 | - | - | |||||||||||||||||||||||||||
Sticta fuliginosa | Xylariaceae sp. (CR1546C) | 23 | (R)-4,6,8-trihydroxy-3,4-dihydro-1(2H)-naphthalenone | Bacillus subtilis | IC50 = 104.2 | Streptomycin sulphate | IC50 = 5.2 |
[24] |
[45] |
|||||||||||||||||||||
24 | 18-O-acetylambuic acid | Staphylococcus aureus ATCC 6538 | IC50 = 10.9 | Antimicrobial peptide (AMP) |
[25] |
[46] |
||||||||||||||||||||||||
25 | 6,8-dihydroxy-(3R)-(2-oxopropyl)-3,4-dihydroisocoumarin | Bacillus subtilis | IC50 = 106.4 | Streptomycin sulphate | IC50 = 5.2 |
[24] |
[45] |
|||||||||||||||||||||||
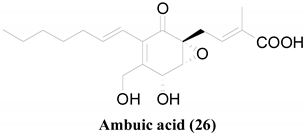
26 | Ambuic acid | Staphylococcus aureus ATCC 6538 | IC50 = 15.4 | Antimicrobial peptide (AMP) |
[25] |
[46] |
||||||||||||||||||||||||
Usnea sp. | Hypoxylon fuscum | 27 | 16-α-D-mannopyranosyloxyisopimar-7-en-19-oic acid | Staphylococcus aureus CGMCC 1.2465 | MIC = 46.4 | Vancomycin Hydrochloride | MIC = 3.12 |
[26] |
[47] |
|||||||||||||||||||||
28 | 8-methoxy-1-naphthyl-β-glucopyranoside | Staphylococcus aureus CGMCC 1.2465 | MIC = 30.1 | |||||||||||||||||||||||||||
29 | Phomol | Staphylococcus aureus CGMCC 1.2465 | MIC = 21.1 | |||||||||||||||||||||||||||
- | Coniochaeta sp. | 30 | Coniothienol A | Enterococcus faecalis (CGMCC 1.2535) | IC50 = 4.89 | Ampicillin | IC50 = 2.61 |
[27] |
[48] |
|||||||||||||||||||||
Enterococcus faecium (CGMCC 1.2025) | IC50 = 2.00 | IC50 = 0.51 | ||||||||||||||||||||||||||||
31 | Coniothiepinols A | Enterococcus faecalis (CGMCC 1.2535) | IC50 = 11.51 | IC50 = 2.61 | ||||||||||||||||||||||||||
Enterococcus faecium (CGMCC 1.2025) | IC50 = 3.93 | IC50 = 0.51 | ||||||||||||||||||||||||||||
Cetraria islandica | Myxotrichum sp. | 32 | Myxodiol A | Candida albicans SC 5314 | MIC = 128 | Fluconazole | MIC = 2 |
[28] |
[49] |
|||||||||||||||||||||
Pestalotiopsis sp. | 33 | Ambuic acid derivative 1 | Fusarium oxysporum | MIC = 8 | Ketoconazole | MIC = 8 |
[29] |
[50] |
||||||||||||||||||||||
34 | Ambuic acid derivative 2 | |||||||||||||||||||||||||||||
| 23, 64 | Fusarium oxysporum | MIC = 32 | MIC = 8 | ||||||||||||||||||||||||||
35 | Ambuic acid derivative 4 | Verticillium dahlia | MIC = 32 | MIC = 1 | ||||||||||||||||||||||||||
36 | Ambuic acid derivative 5 | Fusarium gramineum | MIC = 8 | MIC = 8 | ||||||||||||||||||||||||||
Fusarium oxysporum | MIC = 8 | MIC = 8 | ||||||||||||||||||||||||||||
Verticillium dahlia | MIC = 16 | MIC = 1 | ||||||||||||||||||||||||||||
37 | Ambuic acid derivative 6 | Fusarium gramineum | MIC = 8 | MIC = 8 | ||||||||||||||||||||||||||
38 | Ambuic acid derivative 7 | Rhizoctonia solani | MIC = 32 | MIC = 8 | ||||||||||||||||||||||||||
39 | Ambuic acid derivative 8 | Rhizoctonia solani | MIC = 32 | MIC = 8 | ||||||||||||||||||||||||||
40 | Ambuic acid derivative 9 | Fusarium gramineum | MIC = 32 | MIC = 8 | ||||||||||||||||||||||||||
Fusarium oxysporum | MIC = 16 | MIC = 8 | ||||||||||||||||||||||||||||
41 | Ambuic acid derivative 11 | Fusarium gramineum | MIC = 32 | MIC = 8 | ||||||||||||||||||||||||||
Cetrelia sp. | Aspergillus sp. CPCC 400810 | 42 | Isocoumarindole A | Candida albicans | MIC = 32.0 | Caspofungin | MIC = 0.03 |
[30] |
[51] |
|||||||||||||||||||||
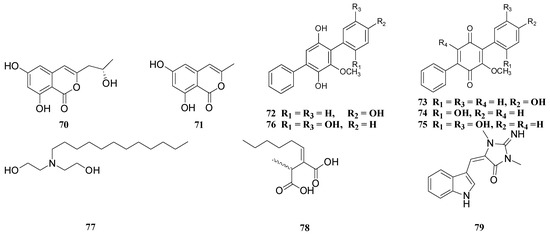
Diorygma hieroglyphicum | Talaromyces funiculosus | 1 | Funiculosone | Candida albicans | IC50 = 35 | - | - |
[17] |
[39] |
|||||||||||||||||||||
Everniastrum sp. | Ulocladium sp. | 43 | 7-hydroxy-3-(2-hydroxy-propyl)-5-methyl-isochromen-1-one | Candida albicans SC 5314 | IC50 = 45.4 | Amphotericin B | IC50 = 1.03 |
[18] |
[40] |
|||||||||||||||||||||
44 | 7-hydroxy-3,5-dimethyl-isochromen-1-one | Candida albicans SC 5314 | IC50 = 18.7 | |||||||||||||||||||||||||||
6 | Altenusin | Aspergillus fumigatus | IC50 = 57.5 | IC50 = 0.74 | ||||||||||||||||||||||||||
8 | Griseoxanthone C | Candida albicans SC 5314 | IC50 = 40.6 | IC50 = 1.03 | ||||||||||||||||||||||||||
10 | Norlichexanthone | Aspergillus fumigatus | IC50 = 43.6 | IC50 = 0.74 | ||||||||||||||||||||||||||
45 | Rubralactone | Aspergillus fumigatus | IC50 = 63.3 | IC50 = 0.74 | ||||||||||||||||||||||||||
Candida albicans SC 5314 | IC50 = 54.7 | IC50 = 1.03 | ||||||||||||||||||||||||||||
Lethariella zahlbruckner | Tolypocladium cylindrosporum | 46 | Pyridoxatin | Candida albicans (Multiple strains) | MIC = | 0.5 − 8.0 | Fluconazole | MIC = | 1.0 − 2.0 |
[31] |
[52] |
|||||||||||||||||||
Candida glabrata (Multiple strains) | MIC = | 1.0 − 8.0 | MIC = | 1.0 − 2.0 | ||||||||||||||||||||||||||
Candida krusei (Multiple strains) | MIC = | 1.0 − 4.0 | MIC = | 1.0 − 2.0 | ||||||||||||||||||||||||||
Candida tropicalis CT2 | MIC = 32 | MIC = 2.0 | ||||||||||||||||||||||||||||
Lobaria quercizans | Aspergillus versicolor | 47 | 3,7-dihydroxy-1,9-dimethyldibenzofuran | Candida albicans | MIC = 64 | Fluconazole | MIC = 2 |
[32] |
[53] |
|||||||||||||||||||||
48 | Cordyol C | Candida albicans | MIC = 8 | |||||||||||||||||||||||||||
49 | Diorcinol D | Candida albicans | MIC = 8 | |||||||||||||||||||||||||||
50 | Diorcinol I | Candida albicans | MIC = 32 | |||||||||||||||||||||||||||
51 | Violaceol I | Candida albicans | MIC = 8 | |||||||||||||||||||||||||||
, | 65 | 52 | Violaceol II | Candida albicans | MIC = 8 | |||||||||||||||||||||||||
Parmelia sp. | Periconia sp. | 53 | 3-(2-oxo-2H-pyran-6-yl)propanoic acid | Aspergillus niger | MIC = 31 | Cycloheximide | MIC < 16 |
[33] |
[54] |
|||||||||||||||||||||
54 | Pericocin A | Aspergillus niger | MIC = 31 | Cycloheximide | MIC < 16 | |||||||||||||||||||||||||
55 | Pericocin B | Aspergillus niger | MIC = 31 | |||||||||||||||||||||||||||
56 | Pericocin C | Aspergillus niger | MIC = 31 | |||||||||||||||||||||||||||
57 | Pericocin D | Aspergillus niger | MIC = 31 | |||||||||||||||||||||||||||
58 | Pericoterpenoid A | Aspergillus niger | MIC = 31 |
[34] |
[55] |
|||||||||||||||||||||||||
Tolypocladium sp. (4259a) | 46 | Pyridoxatin | Candida albicans | MIC = 0.5 | - | - |
[35] |
[56] |
||||||||||||||||||||||
Parmelinella wallichiana | Nigrospora sphaerica | 14 | Alternariol | Candida albicans | MIC = 80.0 | Ketoconazole | MIC = 1.90 |
[21] |
[42] |
|||||||||||||||||||||
Parmotrema ravum | Aspergillus niger | 59 | Aspergyllone | Candida parapsilosis | IC50 = 52 | - | - |
[23] |
[44] |
|||||||||||||||||||||
19 | Aurasperone A | Candida krusei MTCC 9215 | IC50 = 373 | - | - | |||||||||||||||||||||||||
20 | Carbonarone A | Candida albicans MTCC 227 | IC50 = 103 | - | - | |||||||||||||||||||||||||
Candida krusei MTCC 9215 | IC50 = 31 | - | - | |||||||||||||||||||||||||||
22 | Pyrophen | Candida albicans MTCC 227 | IC50 = 74 | - | - | |||||||||||||||||||||||||
Candida glabrata | IC50 = 97 | - | - | |||||||||||||||||||||||||||
Candida utilis IHEM 400 | IC50 = 62 | - | - | |||||||||||||||||||||||||||
Pseudosyphellaria sp. | Biatriospora sp. | 60 | 2-acetonyl-3-methyl-5-hydroxy-7-methoxynaphthazarin | Candida albicans | MIC = 64 | Fluconazole | MIC = 2 |
[36] |
[29] |
|||||||||||||||||||||
61 | 6-deoxy-7-O-demethyl-3,4-anhydrofusarubin | Candida albicans | MIC = 32 | |||||||||||||||||||||||||||
62 | Biatriosporin D | Candida albicans | MIC = 16 | |||||||||||||||||||||||||||
63 | Biatriosporin K | Candida albicans | MIC = 64 | |||||||||||||||||||||||||||
Sticta fuliginosa | Xylariaceae sp. (CR1546C) | 64 | (3R,4S)-3,4,8-trihydroxy-3,4-dihydro-1(2H)-naphthalenone | Candida albicans | IC50 = 63.2 | Amphotericin B | IC50 = 1.3 |
[24] |
[45] |
|||||||||||||||||||||
65 | (3S,4S)-3,4,6,8-tetrahydroxy-3,4-dihydro-1(2H)-naphthalenone | Candida albicans | IC50 = 67.8 | |||||||||||||||||||||||||||
23 | (R)-4,6,8-trihydroxy-3,4-dihydro-1(2H)-naphthalenone | Candida albicans | IC50 = 78.2 | |||||||||||||||||||||||||||
66 | 2,4-dihydroxy-6-(2-oxopropyl)-benzoic acid | Candida albicans | IC50 = 101.3 | |||||||||||||||||||||||||||
67 | 5,6,8-trihydroxy-3(R)-methyl-3,4-dihydroisocoumarin | Candida albicans | IC50 = 71.4 | |||||||||||||||||||||||||||
68 | 6,8-dihydroxy-(3)-(2-oxopropyl)-isocoumarin | Candida albicans | IC50 = 98.1 | |||||||||||||||||||||||||||
25 | 6,8-dihydroxy-(3R)-(2-oxopropyl)-3,4-dihydroisocoumarin | Candida albicans | IC50 = 71.2 | |||||||||||||||||||||||||||
69 | 6,8-dihydroxy-3(R)-methyl-3,4-dihydroisocoumarin | Candida albicans | IC50 = 65.1 | |||||||||||||||||||||||||||
70 | 6,8-dihydroxy-3-[(2S)-2-hydroxypropyl]-isocoumarin | Candida albicans | IC50 = 99.1 | |||||||||||||||||||||||||||
71 | 6,8-dihydroxy-3-methylisocoumarin | Candida albicans | IC50 = 67.2 | |||||||||||||||||||||||||||
Umbilicaria sp. | Floricola striata | 72 | Floricolin A | Candida albicans | MIC = 16 | - | - |
[37] |
[57] |
|||||||||||||||||||||
73 | Floricolin B | Candida albicans | MIC = 8 | - | - | |||||||||||||||||||||||||
74 | Floricolin C | Candida albicans | MIC = 8 | - | - | |||||||||||||||||||||||||
75 | Floricolin D | Candida albicans | MIC = 64 | - | - | |||||||||||||||||||||||||
76 | Terphenyl 2 | Candida albicans | MIC = 64 | - | - | |||||||||||||||||||||||||
Usnea baileyi | Xylaria venustula | 77 | N-dodecyldiethanolamine (DDE) | Candida albicans NCTC713 | MIC = 5.5 | - | - | |||||||||||||||||||||||
78 | Piliformic acid | Colletotrichum gloeosporioides | MIC = 625.2 | Captan | MIC = 5000 | |||||||||||||||||||||||||
Difenoconazole | MIC = 8.1 | |||||||||||||||||||||||||||||
- | Coniochaeta sp. | 31 | Coniothiepinols A | Fusarium oxysporum (CGMCC 3.2830) | IC50 = 13.12 | Carbendazim | IC50 = 0.44 |
[27] |
[48] |
|||||||||||||||||||||
Parmelinella wallichiana | Nigrospora sphaerica | 14 | Alternariol | Herpes Simplex Virus | IC50 = 34.9 | - | - |
[41] |
[26] |
|||||||||||||||||||||
15 | Alternariol-9-methyl ether | Herpes Simplex Virus | IC50 = 64.0 | - | - | |||||||||||||||||||||||||
Usnea baileyi | Xylaria venustula | 79 | Isoplysin A | Plasmodium falciparum | MIC = 0.97 | - | - |