
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Maria De Lurdes Dinis | + 2940 word(s) | 2940 | 2021-06-24 05:52:28 | | | |
| 2 | Catherine Yang | Meta information modification | 2940 | 2021-07-05 06:12:09 | | |
Video Upload Options
Groundwater contamination is one of the most concerning issues from uranium mining activities. Radionuclides cannot be destroyed or degraded, unlike some organic contaminants (and similar to metals). Besides, sites, where radionuclides may be found, are mainly radioactive and mixed waste disposal areas, and therefore many other contaminants may also be present in groundwater.
1. Introduction
Historically, mine sites have been a significant source of contamination to the environment, particularly in sites where the mining activity has ceased without an existing closure plan or a rehabilitation project. Such practices are no longer acceptable. Upon closure, decommissioning and environmental remediation of the site must take place, and in most cases, long-term monitoring to ensure the long-time stability of the site. Closure and decommissioning plans are mandatory at the initial design stage of the project.
Uranium mining can impact groundwater in several ways, mainly from the exploration, mining, processing, and waste management phases of the project. The contamination can occur from the ground surface (by infiltration of contaminated surface water, waste piles, tailings facilities, airborne particles, etc.), from above the water table (leaks in pipelines, leachates, etc.), or from below the water table (drainage wells, groundwater withdrawal, etc.) [1][2][3]. When considering impacts associated with uranium in water, it is essential to note that the risk of chemical toxicity can be much more significant than the radiological risk for a given uranium concentration.
Different in-situ and ex-situ remediation options exist for contaminated groundwater: containment of the contaminants to prevent migration (e.g., using geotechnical measures), removal of the contamination source (e.g., by excavation and subsequent soil washing), groundwater treatment at the point of use, removal of the groundwater for treatment and replacement, or remediation of the groundwater in situ (e.g., the in-situ transformation of the contaminants to reduce their mobility and the toxicity). Many of these options are put in operation through the pump-and-treat systems, in situ permeable treatment wall system, monitored natural attenuation, enhanced attenuation technologies, and biological processes.
All these mechanisms have their advantages and limitations. Moreover, they are contaminant specific and strongly dependent on the subsurface environmental conditions of the site, which may constrain the entire remediation of the site or the application of some technologies which may be too costly to implement on a large scale or inadequate to address the magnitude and combinations of contamination problems. Besides, multiple technologies may be involved simultaneously at a particular site and change over time. Therefore, it is recognized that there is no single technology or a single combination of technologies that would apply to all contaminants under all subsurface environmental conditions.
On the other hand, sites, where radionuclides may be found, are mainly radioactive and mixed waste disposal areas, and therefore many other contaminants may also be present in groundwater. In all situations, designing and implementing effective prevention measures to avoid contamination are preferred over-relying on groundwater remediation after contamination has occurred. The groundwater clean-up systems which are widely used are expensive and need to be applied in practice for a long time, although, in recent years, remediation research has been focused on the development of new clean-up systems and improvement of the efficiency of the existing ones.
Remediation of groundwater impacted with dissolved metals, metalloids, and radionuclides is perhaps one of the biggest challenges for in situ environmental remediation today. Remedial strategies for the treatment of metals in groundwater generally involve direct precipitation or sorption/coprecipitation, with the goal of permanently sequestering and immobilizing the metals in the aquifer soil matrix. The success of this process is dependent upon many factors, such as the kinetics of the reaction, the equilibrium solubility (the solubility of the precipitated solid phases as they form), and durability/permanence (long-term stability of the precipitated solids) [4]. The result is a reduction in the groundwater radionuclide and metals concentrations, but these remain in situ.
2. Remediation Technologies
2.1. Chemical Separation
2.2. Physical Separation
2.3. Biological Treatment
Phytoremediation
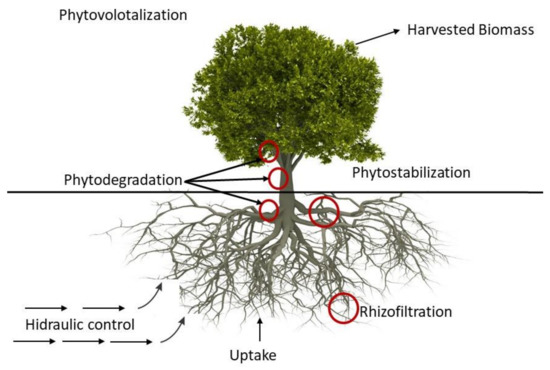
2.4. Natural Attenuation
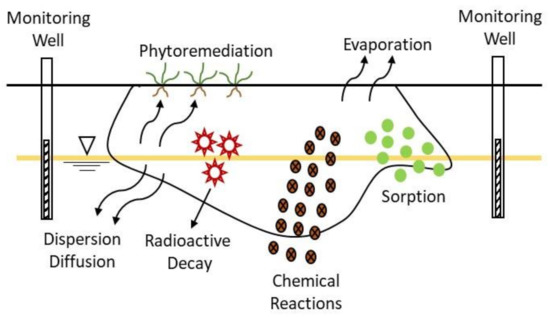
Monitored Natural Attenuation
References
- Beneš, P. The Environmental Impacts of Uranium Mining and Milling and the Methods of Their Reduction. In Chemical Separation Technologies and Related Methods of Nuclear Waste Management; Choppin, G.R., Khankhasayev, M.K., Eds.; NATO Science Series (Series 2: Environmental Security); Springer: Dordrecht, The Netherlands, 1999; Volume 53.
- Nuclear Energy Agency. Managing Environmental and Health Impacts from Uranium Mining; No. 7062; OECD: Paris, France, 2014.
- Heard, B. Environmental Impacts of Uranium Mining in Australia, History, Progress and Current Practice. Minerals Council of Australia. 2007. Available online: (accessed on 3 June 2021).
- Interstate Technology & Regulatory Council. PRB-5 Permeable Reactive Barrier: Technology Update. PRB-5. Washington, DC: Interstate Technology & Regulatory Council, PRB: Technology Update. 2011. Available online: (accessed on 19 April 2021).
- Patel, R.; Clifford, C. Radium Removal from Water by Manganese Dioxide Adsorption and Diatomaceous Erath Filtration; Project Summary, EPA/600/S2-91/063; EPA: Washington, DC, USA, 1992.
- U.S. Department of Energy. Innovative Technology Summary Report: Permeable Reactive Treatment (PeRT) Wall for Rads and Metals. Subsurface Contaminants Focus Area. September 2000. DOE/EM-0557. Available online: (accessed on 3 June 2021).
- U.S. Environmental Protection Agency. Atomic Energy of Canada, Limited (Chemical Treatment and Ultrafiltration). Superfund Innovative Technology Evaluation Program, Technology Profiles, Tenth Edition. August 2000. EPA/540/C-99/500. Available online: (accessed on 3 June 2021).
- Simon, F.G.; Meggyes, T.; McDonald, C. Advanced Groundwater Remediation. Active and Passive Technologies, 1st ed.; Thomas Telford Publishing, Thomas Telford Ltd.: London, UK, 2002; Available online: (accessed on 19 April 2021).
- U.S. Environmental Protection Agency. EPA Superfund Record of Decision: Weldon Spring, Quarry/Plant/Pits (USDOE/Army), EPA ID: MO3210090004, OU 6, St. Charles County, Mo., 02/20/2004. EPA/ROD/R07-04/036. Available online: (accessed on 3 June 2021).
- U.S. Environmental Protection Agency. EPA Superfund Record of Decision: Savannah River Site, (USDOE), EPA ID: SC1890008989, OU 12, Aiken, S.C., 03/10/2004. EPA/ROD/R04-04/007. Available online: (accessed on 3 June 2021).
- International Atomic Energy Agency. Treatment of Liquid Effluent from Uranium Mines and Mills. Report of a Co-Ordinated Research Project, 1996–2000; IAEA-TECDOC-1419; IAEA: Vienna, Austria, 2004.
- International Atomic Energy Agency. Applicability of Monitored Natural Attenuation at Radioactively Contaminated Sites; Technical Reports Series No. 445, STI/DOC/010/445; IAEA: Vienna, Austria, 2006.
- U.S. Environmental Protection Agency. Technology Reference Guide for Radioactively Contaminated Media, EPA 402-R-07-004. 2007. Available online: (accessed on 3 June 2021).
- Csővári, M.; Földing, G.; Csicsák, J.; Frucht, É. Experience gained from the experimental permeable reactive barrier installed on the former uranium mining site. In Uranium, Mining and Hydrogeology; Merkel, B.J., Hasche-Berger, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2008.
- Litter, M. Treatment of Chromium, Mercury, Lead, Uranium, and Arsenic in Water by Heterogeneous Photocatalysis. In Advances in Chemical Engineering; de Lasa, H.I., Rosales, B.S., Eds.; Academic Press: Cambridge, MA, USA, 2009; Volume 36, pp. 37–67.
- U.S. Environmental Protection Agency. Monitored Natural Attenuation of Inorganic Contaminants in Ground Water Technology, EPA/600/R-10/093, 2010; Volume 3, Assessment for Radionuclides Including Tritium, Radon, Strontium, Technetium, Uranium, Iodine, Radium, Thorium, Cesium, and Plutonium-Americium. Available online: (accessed on 3 June 2021).
- Interstate Technology & Regulatory Council. A Decision Framework for Applying Monitored Natural Attenuation Processes to Metals and Radionuclides in Groundwater. APMR-1. Washington, DC: Interstate Technology & Regulatory Council, Attenuation Processes for Metals and Radionuclides. 2010. Available online: (accessed on 19 April 2021).
- Yeung, A.T. Remediation Technologies for Contaminated Sites. In Advances in Environmental Geotechnics; Chen, Y., Zhan, L., Tang, X., Eds.; Springer: Berlin/Heidelberg, Germany, 2010.
- Wan, J.; Dong, W.; Tokunaga, T.K. Method to Attenuate U(VI) Mobility in Acidic Waste Plumes Using Humic Acids. Environ. Sci. Technol. 2011, 45, 2331–2337.
- Dresel, P.E.; Wellman, D.M.; Cantrell, K.J.; Truex, M.J. Review: Technical and Policy Challenges in Deep Vadose Zone Remediation of Metals and Radionuclides. Environ. Sci. Technol. 2011, 45, 4207–4216.
- Daniel, M.; Ollivier, D.; Merten, D.; Bochel, G.; Bergman, H.; Willscher, S.; Jablonski, L.; Wittig, J.; Werner, P. The New Uranium Mining Boom: Challenges and Lessons Learned; Springer: Berlin/Heidelberg, Germany, 2011; Part 3; pp. 433–442. ISBN 978-3-642-22121-7.
- Patel, R.; Clifford, D. Radium Removal from Water by Manganese Dioxide Adsorption and Diatomaceous-Earth Filtration Final Report (PB--92-115260/XAB). United States. 1991. Available online: (accessed on 3 June 2021).
- Borch, T.; Roche, N.; Johnson, T.E. Determination of contaminant levels and remediation efficacy in groundwater at a former in situ recovery uranium mine. J. Environ. Monit. 2012, 14, 1814–1823.
- Vokál, V.; Mužák, J.; Ekert, V. Remediation of Uranium In-Situ Leaching Area at Stráž pod Ralskem, Czech Republic. In Proceedings of the ASME 2013 15th International Conference on Environmental Remediation and Radioactive Waste Management, Brussels, Belgium, 8–12 September 2013.
- Willscher, S.; Wittig, J.; Bergmann, H.; Büchel, G.; Merten, D.; Werner, P. Phytoremediation as an Alternative Way for the Treatment of Large, Low Heavy Metal Contaminated Sites: Application at a Former Uranium Mining Area. Adv. Mater. Res. 2009, 71–73, 705–708.
- Phillips, E.; Yarmak, E. Frozen Soil Barrier Technology—Facts about the Oak Ridge National Laboratory Barrier—14554. In Proceedings of the WM 20104 Conference, Phoenix, AZ, USA, 2–6 March 2014.
- Truex, M.J.; Jonhnson, C.D.; Becker, D.J.; Lee, M.H.; Nimmons, M.J. Performance Assessment for Pump-and-Treat Closure or Transition. Pacific Northwest National Laboratory PNNL-24696, RPT-DVZ-AFRI-029. 2015. Available online: (accessed on 3 June 2021).
- Rosenberg, E.; Pinson, G.; Tsosie, R.; Tutu, H.; Cukrowska, E.; Edward, R.; Glenn, P.; Ranalda, T.; Hlanganani, T.; Ewa, C. Uranium Remediation by Ion Exchange and Sorption Methods: A Critical Review. Johns. Matthey Technol. Rev. 2016, 60, 59–77.
- Zhang, X.; Gu, P.; Liu, Y. Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 2019, 215, 543–553.
- Denham, M.E.; Amidon, M.B.; Wainwright, H.M.; Dafflon, B.; Ajo-Franklin, J.; Eddy-Dilek, C.A. Improving Long-term Monitoring of Contaminated Groundwater at Sites where Attenuation-based Remedies are Deployed. Environ. Manag. 2020, 66, 1142–1161.
- Savannah River Nuclear Solutions, LLC. Savannah River Site Groundwater Management Strategy and Implementation Plan (U). WSRC-RP-2006-4074. 2020. Available online: (accessed on 3 June 2021).
- Nedjimi, B. Phytoremediation: A sustainable environmental technology for heavy metals decontamination. SN Appl. Sci. 2021, 3, 286.
- Li, C.; Ji, X.; Luo, X. Phytoremediation of Heavy Metal Pollution: A Bibliometric and Scientometric Analysis from 1989 to 2018. Int. J. Environ. Res. Public Health 2019, 16, 4755.
- Nariyan, E.; Sillanpää, M.; Wolkersdorfer, C. Uranium removal from Pyhäsalmi/Finland mine water by batch electrocoagulation and optimization with the response surface methodology. Sep. Purif. Technol. 2018, 193, 386–397.
- Hossain, F. Natural and anthropogenic radionuclides in water and wastewater: Sources, treatments and recoveries. J. Environ. Radioact. 2020, 225, 106423.
- Zaheri, A.; Mohed, A.; Keshtkar, R.A.; Shirani, A.S. Uranium Separation from Wastewater by Electrodialysis. Iran. J. Environ. Health. Sci. Eng. 2010, 7, 429–436.
- U.S. Environmental Protection Agency. Treatment Technologies for Site Cleanup: Annual Status Report (Eleventh Edition), February 2004. EPA/542/R-03/009. Available online: (accessed on 3 June 2021).
- Federal Remediation Technologies Roundtable. Remediation Technologies Screening Matrix and Reference Guide, Version 4.0: Phytoremediation. 2002. Available online: (accessed on 3 June 2021).
- Dushenkov, S.; Vasudev, D.; Kapulnik, Y.; Gleba, D.; Fleisher, D.; Ting, K.C.; Ensley, B. Removal of Uranium from Water Using Terrestrial Plants. Environ. Sci. Technol. 1997, 31, 3468–3474.
- Han, Y.; Lee, J.; Kim, C.; Park, J.; Lee, M.; Yang, M. Uranium Rhizofiltration by Lactuca sativa, Brassica campestris L., Raphanus sativus L., Oenanthe javanica under Different Hydroponic Conditions. Minerals 2020, 11, 41.
- U.S. Environmental Protection Agency. Phytotech—Phytoextraction of Lead from Soil. 2006. Available online: (accessed on 3 June 2021).
- Tomé, F.V.; Rodríguez, P.B.; Lozano, J. Elimination of natural uranium and 226Ra from contaminated waters by rhizofiltration using Helianthus annuus L. Sci. Total. Environ. 2008, 393, 351–357.
- U.S. Department of Energy. Subsurface Contaminants Focus Area: Technology Summary, DOE/EM-0296. August 1996. Available online: (accessed on 3 June 2021).
- Wang, W.Q.; Brackhage, C.; Bäuker, E.; Dudel, E.G. Rhizofiltration of U by plant root surfaces in a tailing wetland. In Uranium—Past and Future Challenges; Merkel, B., Arab, A., Eds.; Springer: Cham, Switzerland, 2015.
- Yang, M.; Jawitz, J.W.; Lee, M. Uranium and cesium accumulation in bean (Phaseolus vulgaris L. var. vulgaris) and its potential for uranium rhizofiltration. J. Environ. Radioact. 2015, 140, 42–49.
- Diamantino, C.; Carvalho, E.; Pinto, R. Water resources monitoring and mine water control in Portuguese old uranium mines. In Proceedings of the IMWA Symposium 2016, Leipzig, Germany, 11–15 July 2016.
- Kalin, M.; Kießig, G.; Küchler, A. Ecological water treatment processes for underground uranium mine water: Progress after three years of operating a constructed wetland. In Proceedings of the Uranium Mining and Hydrogeology III Conference, Freiberg, Germany, 15–21 September 2002; pp. 591–600.
- Rock, S. Introduction to Phytoremediation. In The Standard Handbook of Hazardous Waste Treatment and Disposal, 2nd ed.; McGraw-Hill Inc.: New York, NY, USA, 1997.
- Pivetz, B. Ground Water Issue: Phytoremediation of Contaminated Soil and Ground Water at Hazardous Waste Sites. Prepared for U.S. EPA, Office of Solid Waste and Emergency Response, February 2001. EPA/540/S-01/500. Available online: (accessed on 3 June 2021).
- Waugh, W.J.; Glenn, E.P.; Benson, C.H.; Albright, W.H.; Brusseau, M.L.; Bush, R.P.; Dayvault, J. Applications of Ecological Engineering Remedies for Uranium Processing Sites, USA. 2016. Available online: (accessed on 21 April 2021).
- Negri, M.; Hinchman, R.; Wozniak, J. Capturing a ‘Mixed’ Contaminant Plume: Tritium Phytoevaporation at Argonne National Laboratory East. In Proceedings of the Phytoremediation State of the Science Conference, Boston, MA, USA, 1–2 May 2000.
- U.S. Environmental Protection Agency. Deployment of Phytotechnology in the 317/319 Area at Argonne National Laboratory-East: Innovative Technology Evaluation Report, December 2003. EPA/540/R-05/011. Available online: (accessed on 3 June 2021).
- Hitchcock, D.; Rebel, K.; Barton, C.; Seaman, J.; Riha, S.; Blake, J. Estimating Efficiencies of Tritium Phytoremediation at the Savannah River Site. In Proceedings of the Annual International Conference on Soils, Sediments, and Water, Amherst, MA, USA, 22–24 October 2002; Available online: (accessed on 3 June 2021).
- Lewis, C.; Van Pelt, R. Natural Remediation at Savannah River Site; WSRC-MS-2002-00075; Bechtel Savannah River Inc.: North Augusta, SC, USA, 2002.
- Schnoor, J. Phytoremediation of Soil and Groundwater; Technology Evaluation Report TE-02-01; Ground-Water Remediation Technologies Analysis Center: Pittsburgh, PA, USA, 2002.
- Sellers, K. Fundamentals of Hazardous Waste Site Remediation; Lewis Publishers: Boca Raton, FL, USA, 1999.
- International Atomic Energy Agency. Technologies for Remediation of Radioactively Contaminated Sites; IAEA-TECDOC-1086; International Atomic Energy Agency: Vienna, Austria, 1999.
- International Atomic Energy Agency. Review of the Factors Affecting the Selection and Implementation of Waste Management Technologies; IAEA-TECDOC-1096; IAEA: Vienna, Austria, 1999.
- Adamson, D.T.; Newell, C.J. Frequently Asked Questions About Monitored Natural Attenuation in Groundwater; ESTCP Project ER-201211; Environmental Security and Technology Certification Program: Arlington, Virginia, 2014.
- Federal Remediation Technologies Roundtable. Remediation Technologies Screening Matrix and Reference Guide, Version 4.0: Monitored Natural Attenuation. 2002. Available online: (accessed on 3 June 2021).
- Krupka, K.; Martin, W. Subsurface Contaminant Focus Area: Monitored Natural Attenuation (MNA)—Programmatic, Technical, and Regulatory Issues. Prepared for U.S. DOE by Pacific Northwest National Laboratory, July 2001. PNNL-13569. Available online: (accessed on 3 June 2021).
- U.S. Environmental Protection Agency. EPA Superfund Explanation of Significant Differences: Teledyne Wah Chang, EPA ID: ORD050955848, OU 01, Albany, Ore., 10/08/1996, 1997. EPA/ESD/R10-97/082. Available online: (accessed on 3 June 2021).
- U.S. Environmental Protection Agency. Fourth Five-Year Review Report for Teledyne Wah Chang Superfund Site City of Millersburg Linn County, Oregon, 28 December 2012. Contract No. 68-57-03-04 Task Order No. (011). Available online: (accessed on 3 June 2021).
- U.S. Environmental Protection Agency. EPA Superfund Record of Decision: Hanford 300-Area (USDOE), EPA ID: WA2890090077, OU 01, 02, Benton County, Wash., 07/17/1996, 1996. EPA/ROD/R10-96/143. Available online: (accessed on 3 June 2021).
- U.S. Department of Energy. Hanford Site Groundwater Monitoring Report for 2019. Revision 0. DOE/RL-2019-66. August 2020. Available online: (accessed on 19 April 2021).
- U.S. Environmental Protection Agency. Fifth Five-Year Review Report for Weldon Spring Site, September 2016, LMS/WEL/S13516. Available online: (accessed on 3 June 2021).
- Cujic, M.; Petrovic, J.; Dragovic, S. Review of Remediation Approaches Implemented in Radioactively Contaminated Areas. In Remediation Measures for Radioactively Contaminated Areas; Gupta, D.K., Voronina, A., Eds.; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 1–9.
- U.S. Department of Energy. Monticello Mill Tailings Site Operable Unit III, Annual Groundwater Report May 2015 through April 2016. October 2016. LMS/MNT/S14233. Available online: (accessed on 3 June 2021).
- Bea, S.A.; Wainwright, H.; Spycher, N.; Faybishenko, B.; Hubbard, S.S.; Denham, M.E. Identifying key controls on the behavior of an acidic-U(VI) plume in the Savannah River Site using reactive transport modeling. J. Contam. Hydrol. 2013, 151, 34–54.
- U.S. Environmental Protection Agency. Office of Solid Waste and Emergency Response. Use of Monitored Natural Attenuation for Inorganic Contaminants in Groundwater at Superfund Sites, Directive 9283.1-36. August 2015. Available online: (accessed on 3 June 2021).




