Groundwater contamination is one of the most concerning issues from uranium mining activities. Radionuclides cannot be destroyed or degraded, unlike some organic contaminants (and similar to metals). Besides, sites, where radionuclides may be found, are mainly radioactive and mixed waste disposal areas, and therefore many other contaminants may also be present in groundwater.
1. Introduction
Historically, mine sites have been a significant source of contamination to the environment, particularly in sites where the mining activity has ceased without an existing closure plan or a rehabilitation project. Such practices are no longer acceptable. Upon closure, decommissioning and environmental remediation of the site must take place, and in most cases, long-term monitoring to ensure the long-time stability of the site. Closure and decommissioning plans are mandatory at the initial design stage of the project.
Uranium mining can impact groundwater in several ways, mainly from the exploration, mining, processing, and waste management phases of the project. The contamination can occur from the ground surface (by infiltration of contaminated surface water, waste piles, tailings facilities, airborne particles, etc.), from above the water table (leaks in pipelines, leachates, etc.), or from below the water table (drainage wells, groundwater withdrawal, etc.) [1,2,3]. When considering impacts associated with uranium in water, it is essential to note that the risk of chemical toxicity can be much more significant than the radiological risk for a given uranium concentration.
Different in-situ and ex-situ remediation options exist for contaminated groundwater: containment of the contaminants to prevent migration (e.g., using geotechnical measures), removal of the contamination source (e.g., by excavation and subsequent soil washing), groundwater treatment at the point of use, removal of the groundwater for treatment and replacement, or remediation of the groundwater in situ (e.g., the in-situ transformation of the contaminants to reduce their mobility and the toxicity). Many of these options are put in operation through the pump-and-treat systems, in situ permeable treatment wall system, monitored natural attenuation, enhanced attenuation technologies, and biological processes.
All these mechanisms have their advantages and limitations. Moreover, they are contaminant specific and strongly dependent on the subsurface environmental conditions of the site, which may constrain the entire remediation of the site or the application of some technologies which may be too costly to implement on a large scale or inadequate to address the magnitude and combinations of contamination problems. Besides, multiple technologies may be involved simultaneously at a particular site and change over time. Therefore, it is recognized that there is no single technology or a single combination of technologies that would apply to all contaminants under all subsurface environmental conditions.
On the other hand, sites, where radionuclides may be found, are mainly radioactive and mixed waste disposal areas, and therefore many other contaminants may also be present in groundwater. In all situations, designing and implementing effective prevention measures to avoid contamination are preferred over-relying on groundwater remediation after contamination has occurred. The groundwater clean-up systems which are widely used are expensive and need to be applied in practice for a long time, although, in recent years, remediation research has been focused on the development of new clean-up systems and improvement of the efficiency of the existing ones.
Remediation of groundwater impacted with dissolved metals, metalloids, and radionuclides is perhaps one of the biggest challenges for in situ environmental remediation today. Remedial strategies for the treatment of metals in groundwater generally involve direct precipitation or sorption/coprecipitation, with the goal of permanently sequestering and immobilizing the metals in the aquifer soil matrix. The success of this process is dependent upon many factors, such as the kinetics of the reaction, the equilibrium solubility (the solubility of the precipitated solid phases as they form), and durability/permanence (long-term stability of the precipitated solids) [4]. The result is a reduction in the groundwater radionuclide and metals concentrations, but these remain in situ.
2. Remediation Technologies
Remediation technologies can be grouped into three broad categories: physical, chemical, and biological methods (plant and microorganism methods). Each method has limitations and strengths, but the applicable remediation approach should be determined by the site-specific conditions.
Several chemical, physical, biological, and combined methods have been developed to remediate groundwater contaminated with radionuclides originating from uranium mining activities. The available technologies include vertical barriers (U, Ra, Th, Rn), phytoremediation (U), ion exchange (U, Ra), chemical precipitation (U, Ra), permeable reactive barrier (U, Ra), membrane process (U, Ra, Th, Rn), adsorption (U, Ra) and, monitored natural attenuation (U, Ra) [
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32].
The most straightforward approach seems to be the well-established methods of wastewater treatment. With the ‘pump-and-treat’ systems, the contaminated groundwater is removed from the ground by pumping and treated in a treatment plant on the surface. The approach relies on proven treatment techniques, is simple to manage, and the treated groundwater can be re-injected into the subsurface or discharged into surface water sources. However, this process has two main disadvantages: it disturbs the groundwater flow regime and requires steady energy and other inputs.
In the literature, there are references to other technologies that have been bench- or pilot-tested, with successful performance, such as those based on photo-induced technology (e.g., photocatalysis) [
33], electro-remediation (e.g., electro-coagulation) [
34,
35], electrodialysis, and electrodialysis reversal (these systems are generally considered economically viable only for very small installations) [
36]. Still, in what concerns full-scale demonstration, the information is scarce.
2.1. Chemical Separation
Chemical separation technologies for liquid media consist of separating and concentrating the contaminants from groundwater. The process generates residuals such as filters, filter cakes, carbon units, and ion exchange resins requiring further treatment, storage, or disposal. According to the types and concentrations of the contaminants, the extractability rates of the different chemical separation technologies may vary considerably. Still, site-conditions and characteristics will determine the applicability of chemical separation technologies when considering a specific site. Another issue remains from the ex-situ chemical separation technologies (ion exchange and chemical precipitation) in which a groundwater extraction and delivery system are required. These technologies will generate a treated effluent and a contaminated residual that requires further treatment or disposal.
2.2. Physical Separation
Physical separation technologies are ex-situ processes requiring the construction and operation of groundwater extraction and injection system. They are based on contaminants’ physical properties to separate the contaminated media into clean and contaminated fractions. The separation results in a liquid fraction and a contaminated solid residue (sludge, filter cake, or carbon adsorption unit), requiring further treatment or disposal. It can be applied to groundwater, surface water, wastewater, and slurried sludge or sediment.
The applicable processes to radionuclides present in this media, resulting from the uranium mining, are membrane filtration (reverse osmosis and microfiltration) and carbon adsorption.
2.3. Biological Treatment
The treatment of radioactively contaminated groundwater, surface water, and wastewater by biological processes is done through the plant root systems and, for some radionuclides, with the transpiration to the air, through the uptake of groundwater by plants. The process is known as phytoremediation and is implemented at lower costs than conventional treatments; however, the process demands a more extended period of time to reach remediation goals. Phytoremediation uses hyper-accumulator plants and their rhizosphere microorganisms to remove, transfer, stabilize, or destroy contaminants in groundwater, surface water, or wastewater [
13].
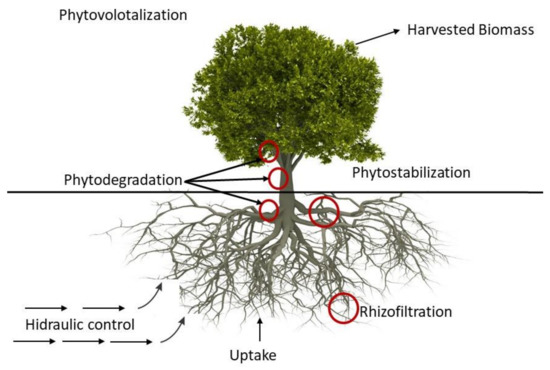
Phytoremediation
The process can be applied in-situ or ex-situ (e.g., hydroponically) to groundwater or surface water. For contaminants, in general, several phytoremediation mechanisms are available for liquid media, however as radionuclides cannot be destroyed, these mechanisms are reduced to rhizofiltration, hydraulic control, and phytovolatilization [
107,
108], although with limited effectiveness for this type of contaminants (Figure 6).
Figure 6. Phytoremediation processes [
13].
In rhizofiltration, contaminants are uptake by the roots of hydroponically grown plants, translocated, and accumulated into plant shoots and leaves. Rhizofiltration has been demonstrated at several scales (bench-scale and field tests), reducing the uranium concentration effectively in water [
108,
109,
110,
111].
Rhizofiltration has been used to clean up waters contaminated with heavy metals (Pb, Cd, Cu, Fe, Ni, Mn, Zn, Cr) and radionuclides (U, Pu, Sr, Cs, I) [
112].
In particular, the process was used to remove uranium from wastewater at DOE’s Ashtabula site (Ohio) with a reduction in uranium concentration by 90% [
13,
111,
113]. It was also used to remove cesium and strontium from pond water at Chernobyl, Ukraine, with a removal efficiency of 95% for cesium and strontium.
The uranium uptake by rhizofiltration has been studied considering several aspects related to site characteristics and contaminant conditions (speciation, retention, mobility, and bioavailability phenomena), plant species, and the effect of microbial activity. Wang et al. [
114] found out that 87.1% of uranium was fixed on root surfaces of common reed in a tailing wetland. However, these authors verified that the uranium complex-forming particles were not tightly adhered to the root surface but surrounding the root loosely. Results from laboratory-scale studies demonstrate that the rhizofiltration technique using beans efficiently removes uranium and cesium from groundwater [
115]. In Portugal, macrophytes are used in the passive treatment system for water resources (surface water, groundwater, mine water) in the already rehabilitated mines. The process is part of the monitoring plan and control mine effluents to meet regulatory values limits for Utotal, Ra-226, Fe, and Mn [
116]. In Germany, a pilot system was constructed to remove U, Ra-226, As, Fe, and Mn from the water of a flooded mine (Wismut) with an average pH of 7.3. Three years of monitoring data showed that Ra-226, As, Fe, and Mn were removed effectively and that the removal was based on the geochemical characteristics of the contaminants. The removal rates for Ra-226, As, Fe, and Mn were 70.6%; 40–70%; 100%, and ~70%, respectively. However, under the pH value of 7.3 and in the presence of high bicarbonate concentration in this mine water, uranium was not removed [
117].
Phytoremediation hydraulic control consists of slowing the movement of contaminants in groundwater through the use of deep-rooted plants. This process is similar to a pump with the roots establishing a dense root mass at the water table, taking up large quantities of groundwater. The root systems should reach and grow directly into the groundwater table. Some of the trees that have been used successfully to “pump” water include poplar, cottonwood, and willow family (reaching as much as 757 L of water per day) [
118].
The process can contain the movement of a groundwater plume toward clean areas off-site, reduce or prevent infiltration and leaching [
119].
The USDOE is responsible for several former uranium mill sites concerning site characterization and groundwater remediation. Groundwater contamination at these sites resulted mostly from the large volumes of processing liquids seeped from tailings impoundments during mills operation. For these sites, evapotranspiration by native plants is evaluated to hydraulically control groundwater flow as an alternative to pump-and-treat remedies at three sites in Arizona and New Mexico [
120].
One special radionuclide is tritium (although not related to uranium mining and milling activities). For tritium-contaminated plumes, the hydraulic control of groundwater plumes by plant uptake has been effectively demonstrated at Argonne National Laboratory [
121,
122], and the remediation of tritium-contaminated groundwater by phytovolatilization has been in operation at the Savannah River Site since 2000 [
123,
124].
Phytovolatilization, or phytoevaporation, is addressed to treat water with volatile or evaporable contaminants (e.g., tritium). In this process, plants uptake the contaminated water and transpire the contaminants into the air through their leaves. The root systems of the selected plants should reach and grow directly into the groundwater table.
A phytovolatilization process has been in operation at Savannah River Site (South Carolina, USA) as an enhanced-passive system since 2000. Although the process is low-energy-consumption and low-carbon-emission, it is not completely passive. Contaminated water with tritium is collected and discharged to a dam/pond system. This water is used to irrigate a pine forest where trees uptake this water through the roots and release very low concentrations of tritium vapor into the atmosphere, where it is diluted.
This semi-passive system combines the natural processes of hydrology and evapotranspiration to reduce the volume of tritium-contaminated water entering site streams and, ultimately, the Savannah River [
31,
123,
124]. The process has resulted in the reduction of tritium by 70% [
31,
124]. Evapotranspiration has been determined to be 80–90% effective [
31].
At Argonne National Laboratory, the phytovolatilization of tritium-contaminated groundwater resulted in a reduction of the average tritium concentration by 73% over a period of three years [
122].
There are a few constraints for the application of phytoremediation mechanisms to liquid media: the process is limited to shallow groundwater requiring a significant land surface area for implementation. There are also aspects that will constrain the applicability and effectiveness of phytoremediation hydraulic control, such as the confinement of shallow groundwater and the vertical flow downward of the plume [
125].
-
2.4. Natural Attenuation
In-situ natural attenuation refers to the natural physical, chemical, and biological processes that reduce the concentration of contaminants in the subsurface [
130].
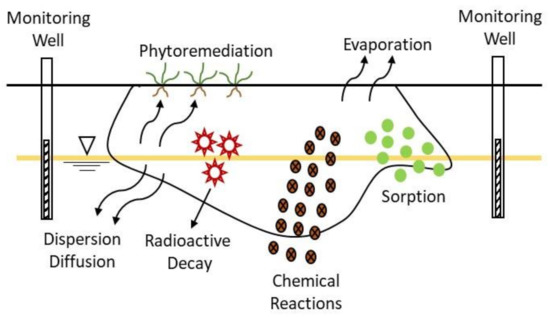
Remediation of groundwater using attenuation-based technologies relies on natural processes to clean up or decrease radionuclides concentration in groundwater. These processes occur in the subsurface at most radioactively contaminated sites and include different mechanisms: abiotic degradation, dispersion, sorption, and evaporation, and radioactive decay (for some radionuclides) (Figure 7). In most cases, the source of radioactive contamination is treated or removed previously of the initiation of the process.
Figure 7. Mechanisms involved in the natural attenuation process [
13].
Natural attenuation is used within the context of a carefully controlled and monitored site cleanup approach to achieve site-specific remediation objectives. Using natural attenuation as a remedial strategy is not equivalent to ‘no action’ and nor is it a ‘walk away’ option; monitoring of these processes is to confirm that natural attenuation is taking place (Monitored Natural Attenuation—MNA), which implies a certain degree of institutional control [
12]. The application of this technology can require multidisciplinary expertise in several technical areas, including radiochemistry, hydrogeology, geochemistry, and phytoremediation.
Monitored Natural Attenuation
Monitored natural attenuation can be selected for the remediation of sites with different radionuclides [
12], where the natural subsurface processes are able to progressively reduce radionuclide concentrations to remediation goal levels. However, it should not be applied to radionuclides with a longer half-life or more toxic and mobile daughter products [
131].
Radionuclides cannot be biodegraded, but the radioactive contaminants’ chemical state may be transformed by microbial action modifying their solubility and mobility, typically through coprecipitation or sorption processes [
53,
132]. Long-term monitoring is necessary to confirm that the contaminant reduction is occurring at rates consistent with meeting cleanup objectives [
133].
Removing or containing metal or radionuclide contaminants in groundwater below specific concentrations are often inefficient and quite costly (that may still be above regulatory criteria). Also, dispersed low-level contamination may be especially challenging at many cleanup sites.
Possible changes due to geochemistry reactions that may result in the remobilization of previously stabilized contaminants must be taken into attention when considering MNA as a remedial alternative. Since with this remediation option, metal and radionuclide contaminants remain in place; MNA is generally acceptable only for sites that intrinsically have a low potential for contaminant migration. This technology is therefore coupled with institutional controls (land-use restrictions and groundwater use restrictions) and with source treatment or removals [
13].
Modeling is required as well as the evaluation of radionuclide reduction rates, pathways, and prediction of the radionuclide concentration at the downgradient exposed receptor. It is necessary to demonstrate that MNA will achieve radionuclides concentrations meeting remedial goals [
133]. Data for the input parameters of models is essential: soil and groundwater quality data (three-dimensional plume definition, historical data, and geochemical data), aquifer characteristics, and locations of potential receptors (wells and surface water discharge points) [
133]. It is also necessary to install monitoring wells for surveillance.
Applicable regulatory policies and available technical guidance should be considered before proceeding with the application of this remediation option at radionuclides contaminate the site. It should be noted that performance monitoring and contingency plans, respectively, are required to evaluate the long-term effectiveness of the process and to provide a fallback option should the solution fail [
12]. In general, monitored natural attenuation can be an appropriate remediation approach when the contaminants degrade or disperse readily, and there are no significant risks to public health and the environment while they attenuate, in particular when the contamination source has been removed or contained. Therefore, in general, monitored natural attenuation is not an appropriate technology when (1) the site contains a significant amount of non-aqueous phase liquids (NAPLs); (2) concentration of contaminants are so high that they represent an unacceptable threat to public health and or an ecosystem, or become toxic to microorganisms; (3) where imminent site risks are present; (4) where radionuclide levels are meaningfully above remediation goals, and (3) the rate of attenuation is unacceptably slow [
18]. The timeframe for reaching the remediation goals should be compatible with anticipated future land use and groundwater use [
134].
Sites with complex, heterogeneous geology, folded and faulted areas, or highly jointed rock, are not suitable for monitored natural attenuation as modeling might not predict groundwater flow, and representative monitoring and sampling might not be possible [
131].
Monitored natural attenuation has been selected as the groundwater remedy option for several sites in the United States: the Teledyne Wah Chang Superfund site in Oregon (radium) [
135,
136]; the Hanford Site 300-Area (uranium, tritium) [
137,
138]; the DOE’s Weldon Spring Site in Missouri (uranium) [
9,
139]; Monticello Mill Tailing Site (uranium) (Utah) [
61,
140]. It was also selected as the groundwater remedy option at the Savannah River Site, but for strontium. However, the behavior of an acidic-U(VI) plume was studied through reactive transport modeling evolution and long-term mobility focus on the pH range where U(VI) is highly mobile [
31,
141].
Although monitored natural attenuation has been applied at several contaminated sites with radionuclides, the information available on process rates and total reductions achieved is scarce. This lack of documented efficiency is caused, in part, by the length of the process in comparison to other remediation technologies.
In the last few years, protocols have been developed to guide evaluations of the potential for natural attenuation to occur (these protocols outline a strategy and methodology to be followed). These documents continue to increase in number over the years [
142].
-
This entry is adapted from the peer-reviewed paper 10.3390/geosciences11060250