Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Václav Zumr | + 4055 word(s) | 4055 | 2021-06-30 10:10:21 |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zumr, V. Saproxylic Beetles. Encyclopedia. Available online: https://encyclopedia.pub/entry/11627 (accessed on 07 February 2026).
Zumr V. Saproxylic Beetles. Encyclopedia. Available at: https://encyclopedia.pub/entry/11627. Accessed February 07, 2026.
Zumr, Václav. "Saproxylic Beetles" Encyclopedia, https://encyclopedia.pub/entry/11627 (accessed February 07, 2026).
Zumr, V. (2021, July 03). Saproxylic Beetles. In Encyclopedia. https://encyclopedia.pub/entry/11627
Zumr, Václav. "Saproxylic Beetles." Encyclopedia. Web. 03 July, 2021.
Copy Citation
Saproxylic beetles are dependent on dead wood at any stage of their own development and at any stage of wood decomposition e.g. mycetophages on wood-decay fungi. This group of saproxylic beetles has become a frequently used as a bioindicator of forest biodiversity.
saproxylic beetles
deadwood
integrated forest management
deadwood enrichment
species richness
managed stands
1. Introduction
The importance of deadwood for the biodiversity of saproxylic species of insects and fungi, as well as for the natural functioning of forest ecosystems, has long been the subject of research. Over the last 20 years, this topic has become the focus of attention for commercial forests too, as deadwood is no longer seen as a product of poor forest management. However, this issue has not been comprehensively settled in order to be a tangible and acceptable forestry practice. To date, there have been isolated research studies [1][2][3] or various original experiments, e.g., [4][5][6][7][8]. Saproxylic organisms are dependent on deadwood at all stages of their development, and throughout any stage of wood decomposition [1][9][10][11]. The largest groups bound to deadwood are fungi and insects [12][13]. Fungi are the most important factor in the decomposition process [14][15], especially the division Basidiomycetes [16], and insects are the most important vector with active wood-seeking movement, while their way of life helps to spread fungi to more distant places [17][18][19]. Saproxylic beetles are very popular because they provide reliable data on the preservation of the environment and are often used as indicators of forest biodiversity [2][9][13][20][21][22]. Nature reserves are one of the options to preserve and create conditions for many specific species of animals. A forest area excluded from management, however, may not always be the most advantageous environment for saproxylic beetles. In this respect, suitable habitats for a non-intervention regime are found especially at higher and middle altitudes in stands predominantly consisting of three main tree species—Norway spruce, European beech, and silver fir [23]. The non-intervention regime is also suitable for extreme positions, steep slopes, and drying sites where the canopy is not fully closed, and the stands remain strongly differentiated [24]. Nevertheless, a conservation (non-intervention) strategy is inappropriate for lowland forests where local species depend on sunny habitats, e.g., oak forests [25][26]. The absence of management would lead to the homogenization of species composition, the closure of the canopy, and a strong reduction of species richness of saproxylic beetles [24][27][28][29][30][31][32][33]. In addition, there are a number of typical attributes of the natural forest in the reserves, towards which the development spontaneously leads. In particular, we are talking about large volumes of coarse woody debris (CWD), spatial heterogeneity, and the limited use of tree species, among other factors [1]. Societally, these facets are mostly considered as beneficial, but the owners to some extent view them as negative [1]. It is the wood production function that the owners perceive as positive, while it is excluded in nature reserves. Therefore, a compromise is sought between wood biomass production, and the expansion of the typical characteristics of natural forests, as high volumes of deadwood are problematic for the economy of forest enterprises [34]. One way to combine the production functions of forests and high biodiversity is functionally integrated forest management, with an emphasis on active enrichment of stands with deadwood [6], or retention management, which is preferred in Scandinavia, e.g., [35]. The implementation of different methods of management depends on several factors: socio-cultural, economic, and political [36]. In contrast to nature reserves, the goal of silvicultural interventions in functionally integrated forest management is the gradual increase in stand volumes and the improvement of production quality, accompanied by active enrichment with wood necromass [37]. Active enrichment with wood necromass may be in fact faster than the natural increase of deadwood volumes in newly established reserves [4][5][6][7]. While conventional forest management reduces the amount of deadwood, the number of microhabitats and the diameter differentiation of trees [37], functionally integrated management seeks to take into account all of these attributes [4]. However, it must be remembered that in commercial forests, it is still necessary to observe the basic principles of forest protection and the struggle against pests with special regard to climate change, reflected in rising temperatures and the uneven distribution of precipitation—including periods of intense drought, which has manifested itself over the last years in Central Europe. These factors induce long-term stress on forest stands, reducing the natural resistance of forest tree species, and conversely, increasing the risk of an outbreak of insect pests, e.g., [38][39][40][41].
The application of scientific findings on the importance of deadwood in the management of production forests, which form the main share of woodlands in Central Europe, is essential for the biological diversity in the wider region of forests. Based on a thorough analysis of scientific findings, this work aims to define the attributes of functionally integrated forest management supporting the biological diversity of the saproxylic beetle species. The goal is to propose appropriate management measures in the context of common forestry practice in the Central Europe region.
2. What Is the Optimal Constant Volume of Deadwood?
Discussions are often held on the amount of deadwood left to decompose, which is needed to comprehensively fulfill all its functions, while at the same time balance the reproductive offer for the widest possible range of saproxylic beetles. This question is important for the planning of wood-necromass management, as the number of saproxylic beetles are known to increase with the amount of deadwood [6][42][43][44][45][46][47][48] and wood-inhabiting fungi [23][49][50]. The number of large logs in the late stage of decay, and the constant volume per hectare are the variables that best explain the species richness of this group of beetles [51][52][53] and fungi [50]. With each m3 of deadwood per hectare, the number of saproxylic beetle and fungal species increases on average by an additional 1.2 species [5]. To some extent, even the exact optimal volume is relative, as it encounters acceptable economic loss and other risks associated with deadwood [1]. In some published studies, we can find a specified universal volume of deadwood for increasing and maintaining the biological diversity of saproxylic beetles in commercial forests. This volume most often fluctuates between 20 and 60 m3/ha [34][54][43][55][56].
These values correlate to the diversity of wood-decaying fungi, for which the optimal volume of deadwood might exceed 100 m3/ha, and only from 20 m3/ha do the first endangered species begin to appear [57]. Wood-decaying fungi generally require high volumes of necromass, sometimes exceeding 300 m3/ha [23]. Many saproxylic beetles are linked to wood-decaying fungi [7][17][58][59]. The solar influence is essential for the abundance and diversity of saproxylic beetles [28][60][61], and at the same time, sun exposure can compensate for the amount of deadwood [23]. A greater amount of insolation in sunny stands reduces the required volume of deadwood for saproxylic beetles, and vice versa in shady cold stands [43][62][63]. The strength of the effect of sun exposure on species richness of saproxylic beetles is more likely related to the local climate—the importance of sun exposure diminishes and may not become a limiting factor in warmer climatic conditions. By contrast, wood-decaying fungi react negatively to changed or opened canopy [23]. Humid and warmer environments are very important factors for many fungal decomposers [49][64][65]. Thus, in the case of high volumes of deadwood, sun exposure may be a limiting factor for biodiversity [44]. At the same time, several large logs cannot be replaced by a larger number of thin logs or even smallwood [66]. Logs are more valuable than branches [45], as numerous species cannot live on small dimensions of dead biomass and have a set minimum threshold diameter [45][67]. Similarly, the number of saproxylic beetle species increases with biomass diameter [68][69].
If we compare these recommended volumes of deadwood in managed stands to the current state of forests in 19 European countries, where there is an average of 15.6 m3/ha of deadwood, we find significant differences [70]. More precise volumes of deadwood identified in commercial forests are given in Table 1. The small volume of deadwood is the main reason why commercial stands are very poor in saproxylic beetles [54][71][72]. The same applies to the most endangered species in forest ecosystems, where these volumes are well below the limits at which they begin to appear [54], e.g., typically over 60 m3/ha [34][44][62].
Table 1. Volume of deadwood in managed stands (m3/ha).
| Volume (m3/ha) | Tree Species | Country | |
|---|---|---|---|
| Fridman and Walheim (2000) [73] | 6.1 | Coniferous | Sweden |
| Siitonen (2001) [74] | 14 | Coniferous | Finland |
| Christensen et al. (2005) [75] | 10 | Beech | Europe |
| Vašíček (2007) [76] | 5.5–9 | Mix | Czech Republic |
| Vítková et al. (2018) [3] | 9.1 | Mix | Czech Republic |
| Puletti et al. (2019) [70] | 9.8 | Mix | Czech Republic |
| Roth et al. (2019) [7] | 18.9 | Beech | Germany |
| Kučera and Adolt (2019) [77] | 6.7–13.8 | Mix | Czech Republic |
| Leidinger et al. (2020) [8] | 19.3 | Beech-oak | Germany |
| Bujoczek et al. (2021) [78] | 4.1–15 | Mix | Poland |
Tree species—the main tree species occurring in studied forest stands. Mix: forest stands with coniferous and deciduous tree species combined.
Up to three times higher volumes of deadwood were detected in commercial stands in mountain areas [78]. However, even this condition strongly limits the biodiversity of saproxylic beetles because in such cold locations, a higher volume of deadwood is required compared to warm ecosystems [43][62]. The diversity of deadwood is also important [63]. Increased volumes of deadwood—and consequently, the saproxylic beetles’ diversity—are highly correlated with the diversity of other taxonomic saproxylic groups, which leads to an increase in the overall multidiversity of saproxylics [71]. This is due to the inhabitation of the same or similar types of deadwood microhabitats, e.g. by fungi, lichens, and mosses [34][50][79], also the group of Diptera [80]. This is also confirmed by the proven causality in the number of nesting birds in tree snags only when robustly colonized by saproxylic beetles [81].
3. How to Effectively Enrich the Stands
It is simple to enrich the stands just by leaving the felled logs, felling residues, and fallen wood in place [1]. The enrichment of forest stands with only small fractions of wood biomass is insufficient in terms of increasing biodiversity [3]. It is necessary to focus on bulky specimens of deadwood, as this type of wood is certainly missing in the commercial stands [82]. Therefore, thick wood fractions need to be applied [2][83]. The minimum diameter is 15 cm [84][85], but at the same time, the increasing diameter of deadwood increases the possibility to host bigger species of beetles [45] and a larger number of saproxylic beetles [69] and fungi [50]. Also, Grossner et al. [34] recommend preserving deadwood of larger dimensions in the stands, with a diameter of 50 cm and more, as much as possible. Primeval forest relics, which are species that depend upon forest habitats without interrupting the continuity of the forest with large amounts of bulky (>40 cm) deadwood [11][86][87][88][89][90].
Diameters over 70 cm of veteran trees have a demonstrably positive effect on all saproxylic beetles [44]. Sizeable deadwood deposits can host more saproxylic species, both endangered and common, simultaneously [91]. However, such large fractions are very scarce in forests [92], while they are essential for highly endangered and rare saproxylic beetle species [11][34][56][83][89][90]. Although smaller wood necromass also hosts many saproxylic beetles [69][93], these are mainly groups of common species [56][93]. However, some studies still favor smaller deadwood mass over large fractions [94]. The difference is probably due to the greater abundance of early species of saproxylic beetles rather than the later species, which are dependent upon the later stages of wood decomposition.
Active enrichment brings about a more complicated issue—standing deadwood, the so-called snags, or microhabitat trees [4][5]. Good results in terms of biodiversity are provided by so-called ring-barked trees [95] or stumps of up to 4 m high (Figure 1), often used and studied in Scandinavian countries [35][96][97][98]. High stumps are parts of the trees that remain in the stand after they have been felled at a greater height. Normally, trees are felled at a height just above the ground utilizing the lower part of the trunk to produce wood products. The importance of standing deadwood and its greater impact on biodiversity than that of lying logs, especially in endangered species, is illustrated in many cases [52][66][84][99][100]. They carry the highest number of microhabitats per unit area [101]. For this reason, it is necessary to focus on standing deadwood. The simplest, safest, and at the same time the most economically viable way seems to be the formation of high stumps, when at this height, we can then talk about the equivalent of snags. Nevertheless, leaving live trees in the stands is the most frequently recommended method [1], even though there is a far greater safety risk due to the unpredictable fall of a dying tree [3]. Leaving live coniferous species in commercial stands does not develop high-quality microhabitats for saproxylic organisms even after an extended period of time. It is much more convenient to leave deciduous tree species to die naturally in the stands [102] due to their high potential for microhabitat formation [3][103].
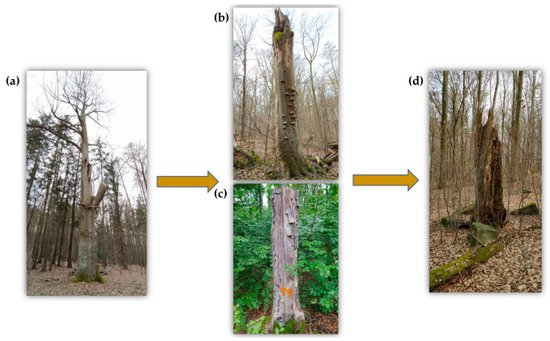
Figure 1. Development of a veteran tree. (a) Solitary tree, which is slowly approaching the limit of physical age and creates a variety of microhabitats. (b) The snags are very valuable for saproxylic beetles. (c) The man-made snags (high stumps) are a suitable substitute in places where trees cannot be left to develop spontaneously. (d) Very advanced stage of decomposition process of the snags.
In high stumps, it will take longer for the wood to become attractive to saproxylic species of the later stages of the decomposition process, because the decomposition of higher stumps takes longer [65]. In some cases, decomposition took up to 3 times longer, and was due to substantially lower moisture of the snag’s wood [73][104][105][106]. The point of the attractiveness to beetles was confirmed by Jonsell and Weslien [96]. It was found that even man-made stumps that are several years old match the species richness of natural stumps [97]. For species of the early stages of deadwood decomposition, insolation is more important than the diameter of the stump [98]. As the decomposition phase of deadwood progresses, the species dependent on these stages of deadwood decomposition will also occur [17]. With larger dimensions, however, deadwood in the form of trunk torsos and high stumps can host more microhabitats such as cavities, and thus be a hotspot for saproxylic beetles as well as nesting birds and bats. The larger the diameter of the tree, the more species of common and endangered beetles [107][108][109], as well as Picidae birds are found [81]. By contrast, fungi prefer lying deadwood [50].
It has been confirmed that the volume of lying wood has effectively increased since the introduction of integrated management, but special support for standing deadwood is still a necessity. Even after the introduction of integrated management, the number of torsos has not increased, while habitat trees have even decreased [4]. The share of standing deadwood is 20–30% (mode) of the total volume of deadwood, exceeding 100 m3/ha. This share was found in natural forests and old reserves [75][101][105][110][111][112]. Too many snags on the local level (e.g., in one stand) can reduce the occupancy of individual snags, as resource availability would be greater than the ability of beetle communities to colonize these habitats [95]. At the same time, keeping in mind that isolation is negative, it is important to maintain the connection between these habitats [108], preferably in groups [2]. Functionally, the trunk torsos and veteran trees are also suitable for biological forest protection due to their great host potential. They often host large numbers of predatory, parasitoid insects, birds, and bats, and the synergistic effect of these groups can inhibit the growing number of pests to some extent. Deadwood itself is the host of many pest antagonists [84]. The enrichment strategy and the subsequent change over several years were evaluated by Doerfler et al. [5], who found that deadwood mass in production forests increased from 8 m3 (set 1) and 18.9 m3 (set 2) to 13.6 m3 and 67.9 m3 (set 1 ≥ 20 cm, set 2 ≥ 12 cm of deadwood for timber inventory). In standard production management, the annual increase in the average level of deadwood is only 0.18 m3/h [73]. As a result of active enrichment, saproxylic species respond positively to the increased amounts of deadwood in stands [5][6][7][113] and simultaneously increase multidiversity, including non-saproxylic insect species [5]. It has even been found that the biodiversity of the saproxylics in production stands has exceeded recent reserves [8], or at least equalized them [7]. This can be proof of how suitable integrated forest management is for supporting an abundance of insects without the need for permanent and strict conservation activities that exclude the production function of forests. A positive effect of close-to-nature integrated management on saproxylic beetles is also confirmed by Jacobsen et al. [114].
4. Maintaining Constant Volume Continuity
The continuity of deadwood over time is essential for the maintenance of biodiversity, so that the supply of microhabitats for invertebrates is constantly evolving and emerging [46][115][116]. If a species does not find their particular type of microhabitat to establish in the landscape, they will persist in their current location, and upon losing it, they will completely disappear and become regionally extinct [117]. Therefore, it is necessary to supply deadwood more often than only once during the entire production period (rotation), as the decomposition process of wood is relatively short in some cases. The metadata assessment shows that wood of European beech (Fagus sylvatica, L.) decomposes fastest, in 20–60 years. The wood of Norway spruce (Picea abies, L. H. Karst) decomposes within 50–100 years on average, and wood of silver fir (Abies alba, Mill.) within 70–110 years, depending on the conditions and dimensions [64][65][105][106][118][119][120][121][122][123][124][125]. By contrast, oak (Quercus sp.) is rather stable, and its wood standardly decomposes for at least 90 years in all circumstances [126].
For this reason, it is necessary to carry out the enrichment at least three times within the European beech rotation period, two times in the case of Norway spruce, and once is sufficient for the average oak rotation period, which is 115 years in the Czech Republic (Information on Forest and Forestry 2019). Similarly, enrichment in the range of 25–40 years is recommended by Přívětivý et al. [64]. Alternatively, it is recommended to keep standing trees in the stand to decay naturally, to bridge the period when deadwood is not left purposely in place after regeneration felling [1][3]. In order to create a practical methodology for forest owners—in connection with financial compensation—it will be necessary to calculate in more detail the necessary enrichment phases according to the site conditions and their original rotation periods.
5. Discussion and Conclusions
The proposed management model suggests a relatively rapid enrichment of stands with deadwood, which is a key factor in increasing the diversity of saproxylic organisms.At the same time, the production function of forest stands—and thus the use of wood is not suppressed. Management is designed to be applicable to current standard forest management models. However, it is obvious that alternative, close-to-nature silvicultural methods are also important for increasing biodiversity in commercial forests. Changing silviculture systems are creating different conditions for many invertebrate communities continuously present in forest stands. In our study was calculated volume of dead wood at 40–60 m3/ha. This is a volume attempting to balance a wide range of requirements for saproxylic beetles, other ecosystem functions and economic considerations. This volume will ensure the survival of many endangered species, with the exception of critically endangered primeval forest relics. The actual enrichment process is assessed in relation to the rate of decomposition of the examined tree species deadwood, and on the models of tending and regeneration silviculture measures. The most intensive management model is designed for beech stands (150 m3/ha per rotation period), while the least intensive enrichment is designed for oak stands (50 m3/ha per rotation period). Enrichment during the stand development is shifted to more advanced stages, when it is possible to leave wood of larger dimensions in the stand, which is especially important for increasing the diversity and abundance of rare species of saproxylic beetles. Deadwood over 15 cm of diameter has the best properties. These larger fractions maintain suitable conditions for extended periods of time. Standing deadwood should be kept in groups, with at least a few individuals representing a share of 20–30% of the total volume of deadwood. High stumps are available and safe standing deadwood. Due to economic aspects, labor intensity, and the effect on biodiversity, it is clear that creating such high stumps should be performed on trees with diameter at breast height (DBH) >35 cm. When created by harvester technology, even occupational safety precautions are met. In terms of the diversity of dead biomass, it is necessary to naturally preserve dead trees and their parts, as they are often damaged and contain important microhabitats—provided that there is no threat to forest stands by pests. This type of deadwood can be expected in the longer term from the trees that will be left standing to die naturally in the stand. However, the forest manager will find it difficult to select convenient places where these unique trees can be left to die naturally. Suitable sites include places where a substantial loss of the stand-growing area does not pose a problem, and where no significant crown projection is an obstacle to the growth of the new forest. From a safety perspective, it is necessary to avoid places frequently visited by people. A continuity of nutrient/residence stability is undoubtedly a key issue. To maintain stability, it is necessary to enrich several times during the rotation period, depending on the type of species. It is three times for F. sylvatica, two times for P. abies, and once for Quercus sp. during their rotation period. In terms of standing wood, the production of high stumps in regeneration felling will be combined with leaving several live trees in place to die naturally. The starting point of functionally integrated management is not tied to specific sites or conditions. This management concept can be used almost wherever the diversity of the landscape is observed. However, from the point of view of saproxylic beetles with rather limited mobility, it is appropriate to start enrichment in the immediate vicinity of forest reserves or other important refugia of endangered beetles. This will create a backbone network in the sense of a green corridor to more distant localities for easier dispersal of these species into the landscape. As a matter of fact, reserves are not a complex solution in the landscape where an area several times larger is occupied by commercial stands. The proposed management for increasing the biodiversity of saproxylic species reduces timber production. Nevertheless, our concept of active forest management can be classified as Medium-Combined Objective Forestry in the sense of the Duncker et al. [[127]] classification, as it attempts to combine the fulfilment of multiple functions and needs in a single stand. The absolute amount of loss is influenced by the proposed management parameters, which reflect the tree species. The lowest production loss is in oak because the proposed volume of wood to be left in the stand is the smallest (50 m3/ha for 130 years). In contrast, the highest loss is recorded for beech (150 m3/ha for 100 years). The relative amount of loss in relation to the total volume production is significantly affected by the quality of the site. It varies between 6.3% (oak, SI 34) and 47.3% (beech, SI 18). If we assume that the level of monetization will be the same, then the potential economic loss would be in the range of ca 20 EUR/ha.year (oak) to 45 EUR/ha.year (spruce). However, when applying the recommended methods in the management proposal, a reduction of this loss can be expected due to the preferential leaving of low-value trees to decay. While the economic loss in the application of the proposed model is comparable between sites, the share of this loss in total profit varies considerably, from ca. 10% (SI 30, oak) to ca. 100% (ASI 28, beech). In our case, however, we calculated the actual potential loss by subtracting the estimated costs of harvesting and transporting the wood to the customer from the value of the deadwood left. If we considered only the value of the left wood, we get an amount of 45–70 EUR/ha.year for spruce and beech, and about 30 EUR/ha.year for oak. Moreover, in the poorest beech and oak habitats (SI 14, 18), standard management is unprofitable, so the application of the proposed management in payments for ecosystem services could mean a positive economic outcome and therefore a high incentive for forest owners. These values correspond to the potential ecosystem function of these stands and could be used to derive payments for this service to the forest owner. By comparison, these amounts are equivalent to 20–30% of the flat rate payment in 2020 for agricultural land in the Czech Republic, or 15–25% of the subsidy for organic farming. When applied generally to all commercial forests in the Czech Republic, this would mean an annual payment of approximately 1.9 billion CZK, which corresponds to about 76 million EUR. Still, these financial values are only indicative ones; it is necessary to provide a more precise analysis in a separate economic study.
References
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537.
- Bače, R.; Svoboda, M. Management Mrtvého Dřeva v Hospodářských Lesích; Certifikovaná metodika; VÚLHM: Strnady, Czechia, 2016; 44p.
- Vítková, L.; Bače, R.; Kjučukov, P.; Svoboda, M. Deadwood management in Central European forests: Key considerations for practical implementation. For. Ecol. Manag. 2018, 429, 394–405.
- Doerfler, I.; Müller, J.; Gossner, M.M.; Hofner, B.; Weisser, W. Success of a deadwood enrichment strategy in production forests depends on stand type and management intensity. For. Ecol. Manag. 2017, 400, 607–620.
- Doerfler, I.; Gossner, M.M.; Müller, J.; Seibold, S.; Weisser, W. Deadwood enrichment combining integrative and segregative conservation elements enhances biodiversity of multiple taxa in managed forests. Biol. Conserv. 2018, 228, 70–78.
- Doerfler, I.; Cadotte, M.W.; Weisser, W.W.; Müller, J.; Gossner, M.M.; Heibl, C.; Bässler, C.; Thorn, S.; Seibold, S. Restoration-oriented forest management affects community assembly patterns of deadwood-dependent organisms. J. Appl. Ecol. 2020, 57, 2429–2440.
- Roth, N.; Doerfler, I.; Bässler, C.; Blaschke, M.; Bussler, H.; Gossner, M.M.; Heideroth, A.; Thorn, S.; Weisser, W.W.; Müller, J. Decadal effects of landscape-wide enrichment of dead wood on saproxylic organisms in beech forests of different historic management intensity. Divers. Distribut. 2019, 25, 430–441.
- Leidinger, J.; Weisser, W.W.; Kienlein, S.; Blaschke, M.; Jung, K.; Kozak, J.; Fischer, A.; Mosandl, R.; Michler, B.; Ehrhardt, M.; et al. Formerly managed forest reserves complement integrative management for biodiversity conservation in temperate European forests. Biol. Conserv. 2020, 242, 108437.
- Speight, M. Saproxylic invertebrates and their conservation. Council of Europe. Nat. Environ. Ser. 1989, 42, 79p.
- Alexander, K.N.A. Tree biology and saproxylic coleoptera: Issues of definitions, and conservation language. Revue d´Écologie la Terre et la vie 2008, 63 (Suppl. 10), 9–13.
- Jaworski, T.; Plewa, R.; Tarwacki, G.; Sućko, K.; Hilszczański, J.; Horák, J. Ecologically similar saproxylic beetles depend on diversified deadwood resources: From habitat requirements to management implications. For. Ecol. Manag. 2019, 449, 117462.
- Stokland, J.N.; Tomter, S.M.; Söderberg, U. Development of dead wood indicators for biodiversity monitoring: Experiences from Scandinavia. Monit. Indic. For. Biodivers. Eur. Ideas Oper. 2004, 51, 207–226.
- Davies, Z.G.; Tyler, C.; Stewart, G.; Pullin, A. Are current management recommendations for saproxylic invertebrates effective? A systematic review. Biodivers. Conserv. 2008, 17, 209–234.
- Boddy, L.; Watkinson, S.C. Wood decomposition, higher fungi, and their role in nutrient redistribution. Can. J. Botan. 1995, 73, 1377–1383.
- Janovský, L.; Vágner, A.; Apltauer, J. The decomposition of wood mass under conditions of climax spruce stands and related mycoflora in the Krkonoše Mountains. J. For. Sci. 2002, 48, 70–79.
- Baldrian, P.; Valášková, V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008, 32, 501–521.
- Weslien, J.; Djupström, L.; Schroeder, M.; Widenfalk, O. Long-term priority effects among insects and fungi colonizing decaying wood. J. Anim. Ecol. 2011, 80, 1155–1162.
- Hofstetter, R.; Dinkins-Bookwalter, J.; Davis, T.S.; Klepzig, K.D. Symbiotic Associations of Bark Beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 209–245.
- Vogel, S.; Alvarez, B.; Bässler, C.; Müller, J.; Thorn, S. The Red-belted Bracket (Fomitopsis pinicola) colonizes spruce trees early after bark beetle attack and persists. Fungal Ecol. 2017, 27, 182–188.
- Horák, J. Ochrana saproxylického hmyzu: Chceme řešit příčiny nebo pouze následky? In Brouci Vázaní na Dřeviny—Beetles Associated with Trees; Sborník referátů; Horák, J., Ed.; Česká Lesnická Společnost: Pardubice, Czech Republic, 2008; pp. 14–17.
- Nieto, A.; Alexander, K.N.A. European Red List of Saproxylic Beetles; Publications Office of the European Union: Luxembourg, 2010; 45p.
- Parisi, F.; Frate, L.; Lombardi, F.; Tognetti, R.; Campanaro, A.; Biscaccianti, A.B.; Marchetti, M. Diversity Patterns Of Coleoptera and Saproxylic Communities in Unmanaged Forests of Mediterranean Mountains. Ecol. Indic. 2020, 110.
- Horák, J.; Kout, J.; Vodka, Š.; Donato, D. Dead wood dependent organisms in one of the oldest protected forests of Europe: Investigating the contrasting effects of within-stand variation in a highly diversified environment. For. Ecol. Manag. 2016, 363, 229–236.
- Krása, A. Ochrana Saproxylického Hmyzu a Opatření na Jeho Podporu; Metodika AOPK ČR; vyd. –Agentura ochrany přírody a krajiny České republiky: Praha, Czechia, 2015; 156p, ISBN 978-80-88076-15-5.
- Vodka, S.; Konvicka, M.; Cizek, L. Habitat preferences of oak-feeding xylophagous beetles in a temperate woodland: Implications for forest history and management. J. Insect Conserv. 2009, 13, 553–562.
- Vogel, S.; Bussler, H.; Finnberg, S.; Müller, J.; Stengel, E.; Thorn, S. Diversity and conservation of saproxylic beetles in 42 European tree species: An experimental approach using early successional stages of branches. Insect Conserv. Divers. 2021, 14, 132–143.
- Ranius, T.; Jansson, N. The influence of forest regrowth, original canopy cover and tree size on saproxylic beetles associated with old oaks. Biol. Conser. 2000, 95, 85–94.
- Widerberg, M.K.; Ranius, T.; Drobyshev, I.; Nilsson, U.; Lindbladh, M. Increased openness around retained oaks increases species richness of saproxylic beetles. Biodivers. Conserv. 2012, 21, 3035–3059.
- Horák, J.; Rébl, K. The species richness of click beetles in ancient pasture woodland benefits from a high level of sun exposure. J. Insect Conserv. 2013, 17, 307–318.
- Sebek, P.; Vodka, S.; Bogusch, P.; Pech, P.; Tropek, R.; Weiss, M.; Zimova, K.; Cizek, L. Open-grown trees as key habitats for arthropods in temperate woodlands: The diversity, composition, and conservation value of associated communities. For. Ecol. Manag. 2016, 380, 172–181.
- Mertlik, J. Review of the saproxylic click-beetles (Coleoptera: Elateridae) in Eastern Bohemia (Czech Republic), with special emphasis on species of the oak forests. Elateridarium 2017, 11, 17–110.
- Horak, J.; Vodka, S.; Kout, J.; Halda, J.P.; Bogusch, P.; Pech, P. Biodiversity of most dead wood-dependent organisms in thermophilic temperate oak woodlands thrives on diversity of open landscape structures. For. Ecol. Manag. 2014, 315, 80–85.
- Horák, J.; Pavliček, J.; Kout, J.; Halda, J. Winners and losers in the wilderness: Response of biodiversity to the abandonment of ancient forest pastures. Biodivers. Conserv. 2018, 27, 3019–3029.
- Gossner, M.M.; Lachat, T.; Brunet, J.; Isacsson, G.; Bouget, C.; Brustel, H.; Brandl, R.; Weisser, W.W.; Müller, J. Current Near-to-Nature Forest Management Effects on Functional Trait Composition of Saproxylic Beetles in Beech Forests. Conserv. Biol. 2013, 27, 605–614.
- Gustafsson, L.; Hannerz, M.; Koivula, M.; Shorohova, E.; Vanha-Majamaa, I.; Weslien, J. Research on retention forestry in Northern Europe. Ecol. Proces. 2020, 9, 3.
- Aggestam, F.; Konczal, A.; Sotirov, M.; Wallin, I.; Paillet, Y.; Spinelli, R.; Lindner, M.; Derks, J.; Hanewinkel, M.; Winkel, G. Can nature conservation and wood production be reconciled in managed forests? A review of driving factors for integrated forest management in Europe. J. Environ. Manag. 2020, 268, 9.
- Dieler, J.; Uhl, E.; Biber, P.; Müller, J.; Rötzer, T.; Pretzsch, H. Effect of forest stand management on species composition, structural diversity, and productivity in the temperate zoneof Europe. Eur. J. For. Res. 2017, 136, 739–766.
- Stadelmann, G.; Bugmann, H.; Wermelinger, B.; Meier, F.; Bigler, C. A predictive framework to assess spatio-temporal variability of infestations by the European spruce bark beetle. Ecography 2013, 36, 1208–1217.
- Netherer, S.; Matthews, B.; Katzensteiner, K.; Blackwell, E.; Henschke, P.; Hietz, P.; Pennerstorfer, J.; Rosner, S.; Kikuta, S.; Schume, H.; et al. Do water-limiting conditions predispose Norway spruce to bark beetle attack? New Phytol. 2015, 205, 1128–1141.
- Marini, L.; Økland, B.; Jönsson, A.M.; Bentz, B.J.; Carroll, A.; Forster, B.; Grégoire, J.-C.; Hurling, R.; Nageleisen, L.M.; Netherer, S.; et al. Climate drivers of bark beetle outbreak dynamics in Norway spruce Forests. Ecography 2017, 40, 1426–1435.
- Matthews, B.; Netherer, S.; Katzensteiner, K.; Pennerstorfer, J.; Blackwell, E.; Henschke, P.; Hietz, P.; Rosner, S.; Jansson, P.-E.; Schume, H.; et al. Transpiration deficits increase host susceptibility to bark beetle attack: Experimental observations and practical outcomes for Ips typographus hazard assessment. Agric. For. Meteorol. 2018, 263, 69–89.
- Ranius, T.; Fahrig, L. Targets for maintenance of dead wood for biodiversity conservation based on extinction thresholds. Scand. J. For. Res. 2006, 21, 201–208.
- Müller, J.; Brustel, H.; Brin, A.; Bussler, H.; Bouget, C.; Obermaier, E.; Heidinger, I.M.M.; Lachat, T.; Förster, B.; Horak, J.; et al. Increasing temperature may compensate for lower amounts of dead wood in driving richness of saproxylic beetles. Ecography 2015, 38, 499–509.
- Lachat, T.; Chumak, M.; Chumak, V.; Jakoby, O.; Müller, J.; Tanadini, M.; Wermelinger, B.; Didham, R.; Jonsell, M. Influence of canopy gaps on saproxylic beetles in primeval beech forests: A case study from the Uholka-Shyrokyi Luh forest, Ukraine. Insect Conserv. Divers. 2016, 9, 559–573.
- Brin, A.; Bouget, C.; Brustel, H.; Jactel, H. Diameter of downed woody debris does matter for saproxylic beetle assemblages in temperate oak and pine forests. J. Insect Conserv. 2011, 15, 653–669.
- Brin, A.; Valladares, L.; Ladet, S.; Bouget, C. Effects of forest continuity on flying saproxylic beetle assemblages in small woodlots embedded in agricultural landscapes. Biodivers. Conserv. 2016, 25, 587–602.
- Sandström, J.; Bernes, C.; Junninen, K.; Lõhmus, A.; Macdonald, E.; Müller, J.; Jonsson, B.G.; Mukul, S. Impacts of dead wood manipulation on the biodiversity of temperate and boreal forests. A systematic review. J. Appl. Ecol. 2019, 56, 1770–1781.
- Cours, J.; Larrieu, L.; Lopez-Vaamonde, C.; Müller, J.; Parmain, G.; Thorn, S.; Bouget, C. Contrasting responses of habitat conditions and insect biodiversity to pest—Or climate-induced dieback in coniferous mountain forests. For. Ecol. Manag. 2021, 482.
- Müller, J.; Engel, H.; Blaschke, M. Assemblages of woodinhabiting fungi related to silvicultural management intensity in beech forests in southern Germany. Eur. J. For. Res. 2007, 126, 513–527.
- Atrena, A.; Banelytė, G.G.; Læssøe, T.; Riis-Hansen, R.; Bruun, H.H.; Rahbek, C.; Heilmann-Clausen, J. Quality of substrate and forest structure determine macrofungal richness along a gradient of management intensity in beech forests. For. Ecol. Manag. 2020, 478, 118512.
- Økland, B.; Bakke, A.; Hagvar, S.; Kvamme, T. What factors influence the diversity of saproxylic beetles? A multi scaled study from a spruce forest in southern Norway. Biodivers. Conserv. 1996, 5, 75–100.
- Sverdrup-Thygeson, A. Can ‘continuity indicator species’ predict species richness or red-listed species of saproxylic beetles? Biodivers. Conserv. 2001, 10, 815–832.
- Janssen, P.; Fuhr, M.; Cateau, E.; Nusillard, B.; Bouget, C. Forest continuity acts congruently with stand maturity in structuring the functional composition of saproxylic beetles. Biol. Conserv. 2017, 205, 1–10.
- Müller, J.; Bütler, R. A review of habitat thresholds for dead wood: A baseline for management recommendations in European forests. Eur. J. For. Res. 2010, 129, 981–992.
- Haase, V.; Topp, W.; Zach, P. Eichen-Totholz im Wirtschaftswald als Lebensraum fur xylobionte Insekten. Z. Okologie Nat. 1998, 7, 137–153.
- Procházka, J.; Schlaghamerský, J. Does dead wood volume affect saproxylic beetles in montane beechfir forests of Central Europe? J. Insect Conserv. 2019, 23, 157–173.
- Penttilä, R.; Siitonen, J.; Kuusinen, M. Polypore diversity in managed and old-growth boreal Picea abies forests in southern Finland. Biol. Conserv. 2004, 117, 271283.
- Müller, J.; Bußler, H.; Kneib, T. Saproxylic beetle assemblages related to silvicultural management intensity and stand structures in a beech forest in Southern Germany. J. Insect Conserv. 2008, 12, 107–124.
- Friess, N.; Müller, J.C.; Aramendi, P.; Bässler, C.; Brändle, M.; Bouget, C.; Brin, A.; Bussler, H.; Georgiev, K.B.; Gil, R.; et al. Arthropod communities in fungal fruitbodies are weakly structured by climate and biogeography across European beech forests. For. Ecol. Manag. 2019, 25, 783–796.
- Müller, J.; Ulyshen, M.; Seibold, S.; Cadotte, M.; Chao, A.; Bässler, C.; Vogel, S.; Hagge, J.; Weiß, I.; Baldrian, P.; et al. Primary Determinants Of Communities In Deadwood Vary Among Taxa But Are Regionally Consistent. Oikos 2020, 129, 1579–1588.
- Vogel, S.; Gossner, M.M.; Mergner, U.; Müller, J.; Thorn, S.; Cheng, L. Optimizing Enrichment Of Deadwood For Biodiversity By Varying Sun Exposure And Tree Species: An Experimental Approach. J. Appl. Ecol. 2020, 57, 2075–2085.
- Lachat, T.; Wermelinger, B.; Gossner, M.M.; Bussler, H.; Isacsson, G.; Müller, J. Saproxylic Beetles As Indicator Species For Dead-Wood Amount And Temperature In European Beech Forests. Ecol. Indic. 2012, 23, 323–331.
- Seibold, S.; Bässler, C.; Brandl, R.; Büche, B.; Szallies, A.; Thorn, S.; Ulyshen, M.D.; Müller, J.; Baraloto, C. Microclimate and Habitat Heterogeneity As The Major Drivers of Beetle Diversity In Dead Wood. J. Appl. Ecol. 2016, 53, 934–943.
- Přívětivý, T.; Adam, D.; Vrška, T. Decay Dynamics of Abies Alba And Picea Abies Deadwood in Relation to Environmental Conditions. For. Ecol. Manag. 2018, 427, 250–259.
- Hararuk, O.; Kurz, W.A.; Didion, M. Dynamics of Dead Wood Decay In Swiss Forests. For. Ecosyst. 2020, 7.
- Bouget, C.; Larrieu, L.; Brin, A. Key Features For Saproxylic Beetle Diversity Derived From Rapid Habitat Assessment In Temperate Forests. Ecol. Indic. 2014, 36, 656–664.
- Kraus, D.; Krumm, F. Integrative Approaches as an Opportunity for the Conservation of Forest Biodiversity; European Forest Institute: Joensuu, Finland, 2013; 284p.
- Lassauce, A.; Lieutier, F.; Bouget, C. Woodfuel Harvesting And Biodiversity Conservation in Temperate Forests: Effects of Logging Residue Characteristics On Saproxylic Beetle Assemblages. Biol. Conserv. 2012, 147, 204–212.
- Macagno, A.L.M.; Hardersen, S.; Nardi, G.; Lo Giudice, G.; Mason, F. Measuring Saproxylic Beetle Diversity In Small And Medium Diameter Dead Wood: The "Grab-And-Go" Method. Eur. J. Èntomol. 2015, 112, 510–519.
- Puletti, N.; Canullo, R.; Mattioli, W.; Gawryś, R.; Corona, P.; Czerepko, J. A Dataset Of Forest Volume Deadwood Estimates For Europe. Ann. For. Sci. 2019, 76, 68.
- Paillet, Y.; Bergès, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.-J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity Differences between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conserv. Biol. 2010, 24, 101–112.
- Thorn, S.; Bässler, C.; Brandl, R.; Burton, P.J.; Cahall, R.; Campbell, J.L.; Castro, J.; Choi, C.-Y.; Cobb, T.; Donato, D.C.; et al. Impacts of salvage logging on biodiversity: A meta-analysis. J. Appl. Ecol. 2018, 55, 279–289.
- Fridman, J.; Walheim, M. Amount, structure, and dynamics of dead wood on managed forestland in Sweden. For. Ecol. Manag. 2000, 131, 23–36.
- Siitonen, I. Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol. Bull. 2001, 49, 11–41.
- Christensen, M.; Hahn, K.; Mountford, E.P.; Ódor, P.; Standovár, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, S.; Meyer, P.; Winter, S.; et al. Dead wood in European beech (Fagus sylvatica) forest reserves. For. Ecol. Manag. 2005, 210, 267–282.
- Vašíček, J. (Ed.) Národní Inventarizace Lesů v České Republice 2001–2004; ÚHUL: Brandýs nad Labem, Czech Republic, 2007.
- Kučera, M.; Adolt, R. (Eds.) Národní inventarizace lesů v České republice—výsledky druhého cyklu 2011–2015 [online]. Vydání první. Brandýs nad Labem: Ústav pro hospodářskou úpravu lesů Brandýs nad Labem. 2019. Available online: (accessed on 20 June 2021).
- Bujoczek, L.; Bujoczek, M.; Zięba, S. How much, why and where? Deadwood in forest ecosystems: The case of Poland. Ecol. Indic. 2021, 121, 107027.
- Haeler, E.; Bergamini, A.; Blaser, S.; Ginzler, C.; Hindenlang, K.; Keller, C.; Kiebacher, T.; Kormann, U.G.; Scheidegger, C.; Schmidt, R.; et al. Saproxylic species are linked to the amount and isolation of dead wood across spatial scales in a beech forest. Landsc. Ecol. 2021, 36, 89–104.
- Jonsell, M.; Widenfalk, L.; Hellqvist, S. Substrate specificity among Diptera in decaying bioenergy wood: Can they be conserved by the same measures as are currently applied to beetles? Biodivers. Conserv. 2020, 29, 2623–2662.
- Ettwein, A.; Korner, P.; Lanz, M.; Lachat, T.; Kokko, H.; Pasinelli, G. Habitat selection of an old-growth forest specialist in managed forests. Anim. Conserv. 2020, 23, 547–560.
- Dudley, N.; Vallauri, D. Restoration of Deadwood as a Critical Microhabitat in Forest Landscapes. For. Restor. Landsc. 2006, 203–207.
- Seibold, S.; Brandl, R.; Buse, J.; Hothorn, T.; Schmidl, J.; Thorn, S.; Müller, J. Association of extinction risk of saproxylic beetles with ecological degradation of forests in Europe. Conserv. Biol. 2015, 29, 382–390.
- Kappes, H.; Topp, W. Coleoptera from dead wood in a managed broadleaved forest in Central Europe. Biodivers. Conserv. 2004, 13, 1905–1924.
- Brin, A.; Brustel, H.; Jactel, H. Species variables or environmental variables as indicators of forest biodiversity: A case study using saproxylic beetles in Maritime pine plantations. Ann. For. Sci. 2009, 66, 306.
- Müller, J.; Bußler, H.; Bense, U.; Brustel, H.; Flechtner, G.; Fowles, A.; Kahlen, M.; Möller, G.; Mühle, H.; Schmidl, J.; et al. Urwald relict species—Saproxylic beetles indicating structural qualities and habitat tradition. Wald. Online 2005, 2, 106–113.
- Buse, J.; Ranius, T.; Assmann, T. An Endangered Longhorn Beetle Associated with Old Oaks and Its Possible Role as an Ecosystem Engineer. Conserv. Biol. 2008, 22, 329–337.
- Cizek, L.; Schlaghamerský, J.; Bořucký, J.; Hauck, D.; Helešic, J. Range expansion of an endangered beetle: Alpine Longhorn Rosalia alpina (Coleoptera: Cerambycidae) spreads to the lowlands of Central Europe. EÈntomol. Fenn. 2009, 20, 200–206.
- Eckelt, A.; Müller, J.; Bense, U.; Brustel, H.; Bußler, H.; Chittaro, Y.; Cizek, L.; Frei, A.; Holzer, E.; Kadej, M.; et al. “Primeval forest relict beetles” of Central Europe: A set of 168 umbrella species for the protection of primeval forest remnants. J. Insect Conserv. 2017, 22, 15–28.
- Kostanjsek, F.; Sebek, P.; Baranova, B.; Jelaska, L.S.; Riedl, V.; Cizek, L. Size matters! Habitat preferences of the wrinkled bark beetle, Rhysodes sulcatus, the relict species of European primeval forests. Insect Conserv. Divers. 2018, 11, 545–553.
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur. J. For. Res. 2007, 127, 1–22.
- Kirby, K.; Reid, C.; Thomas, R.; Goldsmith, F. Preliminary estimates of fallen dead wood and standing dead trees in managed and unmanaged forests in Britain. J. Appl. Ecol. 1998, 35, 148–155.
- Hardersen, S.; Macagno, A.L.M.; Chiari, S.; Audisio, P.; Gasparini, P.; Giudice, G.L.; Nardi, G.; Mason, F. Forest management, canopy cover and geographical distance affect saproxylic beetle communities of small-diameter beech deadwood. For. Ecol. Manag. 2020, 467, 118152.
- Schiegg, K. Saproxylic insect diversity of beech: Limbs are richer than trunks. For. Ecol. Manag. 2001, 149, 295–304.
- Dufour-Pelletier, S.; Tremblay, J.A.; Hébert, C.; Lachat, T.; Ibarzabal, J. Testing the Effect of Snag and Cavity Supply on Deadwood-Associated Species in a Managed Boreal Forest. Forests 2020, 11, 424.
- Jonsell, M.; Weslien, J. Felled or standing retained wood—it makes a difference for saproxylic beetles. For. Ecol. Manag. 2003, 175, 425–435.
- Jonsell, M.; Nittérus, K.; Stighäll, K. Saproxylic beetles in natural and man-made deciduous high stumps retained for conservation. Biol. Conserv. 2004, 118, 163–173.
- Lindhe, A.; Lindelöw, Å.; Åsenblad, N. Saproxylic Beetles in Standing Dead Wood Density in Relation to Substrate Sun-exposure and Diameter. Biodivers. Conserv. 2005, 14, 3033–3053.
- Berg, A.; Ehnström, B.; Gustafsson, L.; Hallingbäck, T.; Jonsell, M.; Weslien, J. Threatened plant, animal, and fungus species in Swedish forests—Distribution andhabitat associations. Conserv. Biol. 1994, 8, 718–731.
- Bouget, C.; Nusillard, B.; Pineau, X.; Ricou, C. Effect of deadwood position on saproxylic beetles in temperate forests and conservation interest of oak snags. Insect Conserv. Divers. 2011, 5, 264–278.
- Paillet, Y.; Archaux, F.; Boulanger, V.; Debaive, N.; Fuhr, M.; Gilg, O.; Gosselin, F.; Guilbert, E. Snags and large trees drive higher tree microhabitat densities in strict forest reserves. For. Ecol. Manag. 2017, 389, 176–186.
- Rosenvald, R.; Lõhmus, P.; Rannap, R.; Remm, L.; Rosenvald, K.; Runnel, K.; Lõhmus, A. Assessing long-term effectiveness of green-tree retention. For. Ecol. Manag. 2019, 448, 543–548.
- Vuidot, A.; Paillet, Y.; Archaux, F.; Gosselin, F. Influence of tree characteristics and forest management on tree microhabitats. Biol. Conserv. 2011, 144, 441–450.
- Taylor, S.L.; MacLean, D.A. Dead wood dynamics in declining balsam fir and spruce stands in New Brunswick, Canada. Can. J. For. Res. 2007, 37, 750–762.
- Vacek, S.; Vacek, Z.; Bílek, L.; Hejcmanová, P.; Štícha, V.; Remeš, J. The dynamics and structure of dead wood in natural spruce-beech forest stand—A 40 year case study in the Krkonoše National Park. Dendrobiol. 2015, 73, 21–32.
- Harmon, M.E.; Fasth, B.G.; Yatskov, M.; Kastendick, D.; Rock, J.; Woodall, C.W. Release of coarse woody detritus-related carbon: A synthesis across forest biomes. Carbon Balance Manag. 2020, 15, 1.
- Sverdrup-Thygeson, A.; Skarpaas, O.; Ødegaard, F. Hollow oaks and beetle conservation: The significance of the surroundings. Biodivers. Conserv. 2009, 19, 837–852.
- Pilskog, H.E.; Birkemoe, T.; Framstad, E.; Sverdrup-Thygeson, A. Effect of Habitat Size, Quality, and Isolation on Functional Groups of Beetles in Hollow Oaks. J. Insect Sci. 2016, 16, 26.
- Parmain, G.; Bouget, C. Large solitary oaks as keystone structures for saproxylic beetles in European agricultural landscapes. Insect Conserv. Divers. 2018, 11, 100–115.
- Hort, L.; Vrška, T. Podíl odumřelého dřeva v pralesovitých útvarech ČR. In Význam a Funkce Odumřelého Dřeva v Lesních Porostech: Česká Lesnická Společnost Pobočka Pro Silva Bohemica; VRŠKA, T., Ed.; Vydala Správa Národního parku Podyjí: Znojmo, Czechia, 1999; pp. 75–87. ISBN 80-238-4739-2.
- Larrieu, L.; Cabanettes, A.; Gouix, N.; Burnel, L.; Bouget, C.; Deconchat, M. Post-harvesting dynamics of the deadwood profile: The case of lowland beech-oak coppice-with-standards set-aside stands in France. Eur. J. For. Res. 2019, 138, 239–251.
- Oettel, J.; Lapin, K.; Kindermann, G.; Steiner, H.; Schweinzer, K.-M.; Frank, G.; Essl, F. Patterns and drivers of deadwood volume and composition in different forest types of the Austrian natural forest reserves. For. Ecol. Manag. 2020, 463, 118016.
- Floren, A.; Müller, T.; Dittrich, M.; Weiss, M.; Linsenmair, K.E. The influence of tree species, stratum and forest management on beetle assemblages responding to deadwood enrichment. For. Ecol. Manag. 2014, 323, 57–64.
- Jacobsen, R.M.; Burner, R.C.; Olsen, S.L.; Skarpaas, O.; Sverdrup-Thygeson, A. Near-natural forests harbor richer saproxylic beetle communities than those in intensively managed forests. For. Ecol. Manag. 2020, 466, 118124.
- Schiegg, K. Effects of dead wood volume and connectivity on saproxylic insect species diversity. Écoscience 2000, 7, 290–298.
- Schiegg, K. Are the saproxylic beetle species characteristic of high dead wood connectivity? Ecography 2000, 23, 579–587.
- Škorpík, M. Odumřelé dřevo jako mikrobiotop významných druhů hmyzu. In Význam a Funkce Odumřelého Dřeva v Lesních Porostech: Česká Lesnická Společnost Pobočka Pro Silva Bohemica; VRŠKA, T., Ed.; Vydala Správa Národního parku Podyjí: Znojmo, Czechia, 1999; pp. 107–119. ISBN 80-238-4739-2.
- Kraigher, H.; Jurc, D.; Kalan, P.; Kutnar, L.; Levanič, T.; Rupel, M.; Smolej, I. Beech coarse woody debris characteristics in two virgin forest reserves in southern Slovenia. Zb. Gozdarstva Lesar. 2002, 69, 91–134.
- Storaunet, K.O.; Rolstad, J. Time since death and fall of Norway spruce logs in old-growth and selectively cut boreal forest. Can. J. For. Res. 2002, 32, 1801–1812.
- Zielonka, T. When does dead wood turn into a substrate for spruce replacement? J. Veg. Sci. 2006, 17, 739–746.
- Lombardi, F.; Cherubini, P.; Lasserre, B.; Tognetti, R.; Marchetti, M. Tree rings used to assess time since death of deadwood of different decay classes in beech and silver fir forests in the central Apennines (Molise, Italy). Can. J. For. Res. 2008, 38, 821–833.
- Šamonil, P.; Antolík, L.; Svoboda, M.; Adam, D. Dynamics of windthrow events in a natural fir-beech forest in the Carpathian mountains. For. Ecol. Manag. 2009, 257, 1148–1156.
- Šebková, B.; Šamonil, P.; Janík, D.; Adam, D.; Král, K.; Vrška, T.; Hort, L.; Unar, P. Spatial and volume patterns of an unmanaged submontane mixed forest in Central Europe: 160 years of spontaneous dynamics. For. Ecol. Manag. 2011, 262, 873–885.
- Herrmann, S.; Kahl, T.; Bauhus, J. Decomposition dynamics of coarse woody debris of three important central European tree species. For. Ecosyst. 2015, 2, 106.
- Zumr, V.; Remeš, J. Saproxylic beetles as an indicator of forest biodiversity and the influence of forest management on their crucial life attributes: Review. Rep. For. Res. 2020, 65, 242–257.
- Míchal, I. Ponechávání odumřelého dřeva z hlediska péče o biologickou rozmanitost. In Význam a Funkce Odumřelého Dřeva v Lesních Porostech: Česká Lesnická Společnost Pobočka Pro Silva Bohemica; VRŠKA, T., Ed.; Vydala Správa Národního parku Podyjí: Znojmo, Czechia, 1999; pp. 9–19. ISBN 80-238-4739-2.
- Philipp S. Duncker; Susana Barreiro; Geerten M. Hengeveld; Torgny Lind; William L. Mason; Slawomir Ambrozy; Heinrich Spiecker; Classification of Forest Management Approaches: A New Conceptual Framework and Its Applicability to European Forestry. Ecology and Society 2012, 17, 1, 10.5751/es-05262-170451.
More
Information
Subjects:
Forestry
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
2.2K
Entry Collection:
Environmental Sciences
Revision:
1 time
(View History)
Update Date:
03 Jul 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




