
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pål Johansen | + 3956 word(s) | 3956 | 2021-06-11 08:53:53 | | | |
| 2 | Vivi Li | Meta information modification | 3956 | 2021-06-16 08:28:16 | | |
Video Upload Options
Photochemical internalization (PCI) is a further development of photodynamic therapy (PDT). In this report, we describe PCI as a potential tool for cellular internalization of chemotherapeutic agents or antigens and systematically review the ongoing research. One Phase-I clinical trial has been conducted, and it demonstrated that PCI-mediated bleomycin treatment was safe and identified tolerable doses of the photosensitizer disulfonated tetraphenyl chlorin (TPCS2a). Likewise, PCI was pre-clinically shown to mediate major histocompatibility complex (MHC) class I antigen presentation and generation of tumor-specific cytotoxic CD8+ T-lymphocytes (CTL) and cancer remission. A first clinical Phase I trial with the photosensitizer TPCS2a combined with human papilloma virus antigen (HPV) was recently completed and results are expected in 2020. Hence, photosensitizers and light can be used to mediate cytosolic delivery of endocytosed chemotherapeutics or antigens. While the therapeutic potential in cancer has been clearly demonstrated pre-clinically, further clinical trials are needed to reveal the true translational potential of PCI in humans.
1. Introduction
1.1. Cancer Therapy Development
1.2. Methods for Cytosolic Targeting
1.3. Photodynamic Therapy (PDT)
1.4. PCI—A Photosensitizer—And Light-Driven Technology for Cellular Internalization of Molecules
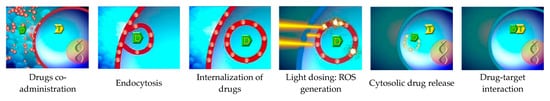
1.5. Photosensitizers in Use
| Name | Ex Wave-Length (nm) | Manufacturer | Application |
|---|---|---|---|
| FIRST GENERATION PHOTOSENSITIZERS | |||
| Porfimer sodium | 630 | Axcan Pharma | PDT of esophageal cancer, lung adenocarcinoma, and endobronchial cancer |
| SECOND GENERATION PHOTOSENSITIZERS/Prodrugs | |||
| 5-aminolaevulinic acid | 635 | DUSA Stabiopharma |
PDT of mild to moderate actinic keratosis Fluorescence guided resection of glioma |
| Methyl-aminolevulinic acid | 579–670 | Galderma | PDT of non-hyperkeratotic actinic keratosis and basal cell carcinoma |
| Temoporfin | 652 | Biolitec | PDT of advanced head and neck cancer |
| Talaporfin | 664 | Meiji Seika Novartis |
PDT of early centrally located lung cancer |
| Verteporfin | 690 | Novartis | PDT of age-related macular degeneration |
| Redaporfin | 749 | Luzitin | PDT of biliary tract cancer |
| PHOTOSENSITIZERS UNDER CLINICAL INVESTIGATIONS | |||
| Fotolon | 665 | Apocare Pharma | PDT of nasopharyngeal, sarcoma |
| Hexylaminolevulinate | 635 | Photocure | PDT of HPV-induced cervical precancerous lesions and non-muscle invasive bladder cancer |
| Radachlorin | 662 | Rada-pharma | PDT of skin cancer |
| Photochlor (HTTP) | 664 | Rosewell Park | PDT of head and neck cancer |
| Padeliporfin | 762 | Negma-Lerads | PDT of prostate cancer |
| Motexafin lutetium | 732 | Pharmacyclics | PDT of coronary artery disease |
| Rostaprofin | 664 | Miravant | PDT of age-related macular degeneration |
| Talaporfin | 664 | Meiji Seika | PDT of colorectal neoplasms, liver metastasis |
| Fimaporfin | 435 | PCI Biotech | PCI of cutaneous or sub-cutaneous malignancies, cholangiocarcinoma and PCI of vaccine antigens |
1.6. PCI in Immunotherapy
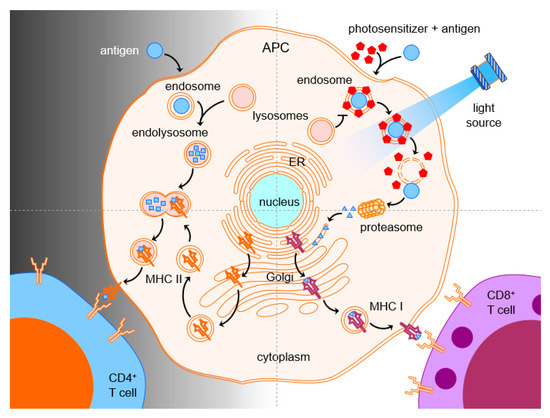
1.7. Cancer Vaccines
2. PCI of Cytotoxic Therapeutics
3. PCI in Immunotherapy
References
- Osipov, A.; Murphy, A.; Zheng, L. From immune checkpoints to vaccines: The past, present and future of cancer immunotherapy. Adv. Cancer Res. 2019, 143, 63–144.
- Falzone, L.; Salomone, S.; Libra, M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front. Pharmacol. 2018, 9, 1300.
- Agostinis, P.; Berg, K.; Cengel, K.A. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281.
- Melero, I.; Gaudernack, G.; Gerritsen, W.; Huber, C.; Parmiani, G.; Scholl, S.; Thatcher, N.; Wagstaff, J.; Zielinski, C.; Faulkner, I.; et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat. Rev. Clin. Oncol. 2014, 11, 509–524.
- Kvistborg, P.; Philips, D.; Kelderman, S.; Hageman, L.; Ottensmeier, C.; Joseph-Pietras, D.; Welters, M.J.P.; Van Der Burg, S.; Kapiteijn, E.; Michielin, O.; et al. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci. Transl. Med. 2014, 6, 1–10.
- Topalian, S.L.; Hodi, S.F.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N. Engl. J. Med. 2012, 366, 2443–2454.
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.M.; Hwu, W.-J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012, 366, 2455–2465.
- Palumbo, M.O.; Kavan, P.; Miller, W.H.; Panasci, L.; Assouline, S.; Johnson, N.; Cohen, V.; Patenaude, F.; Pollak, M.; Jagoe, R.T.; et al. Systemic cancer therapy: Achievements and challenges that lie ahead. Front. Pharmacol. 2013, 4, 1–9.
- Berg, K.; Weyergang, A.; Prasmickaite, L.; Bonsted, A.; Hogset, A.; Strand, M.-T.R.; Wagner, E.; Selbo, P.K. Photochemical Internalization (PCI): A Technology for Drug Delivery. In Photodynamic Therapy—Methods and Protocols, Methods in Molecular Biology; Gomer, C.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 133–145. ISBN 9781607616962.
- Larocca, C.; Schlom, J. Viral Vector-Based Therapeutic Cancer Vaccines. Cancer J. 2011, 17, 359–371.
- Choi, Y.; Chang, J. Viral vectors for vaccine applications. Clin. Exp. Vaccine Res. 2013, 2, 97.
- Fittipaldi, A.; Giacca, M. Transcellular protein transduction using the Tat protein of HIV-1. Adv. Drug Deliv. Rev. 2005, 57, 597–608.
- Tanaka, M.; Kato, A.; Satoh, Y.; Ide, T.; Sagou, K.; Kimura, K.; Hasegawa, H.; Kawaguchi, Y. Herpes Simplex Virus 1 VP22 Regulates Translocation of Multiple Viral and Cellular Proteins and Promotes Neurovirulence. J. Virol. 2012, 86, 5264–5277.
- Lindgren, M.; Hällbrink, M.; Prochiantz, A.; Langel, Ü. Cell-penetrating peptides. Trends Pharmacol. Sci. 2000, 21, 99–103.
- Arlen, P.M.; Kaufman, H.L.; DiPaola, R.S. Pox viral vaccine approaches. Semin. Oncol. 2005, 32, 549–555.
- Kass, E.; Schlom, J.; Thompson, J.; Guadagni, F.; Graziano, P.; Greiner, J.W. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Res. 1999, 59, 676–683.
- Ledermann, J.A.; Canevari, S.; Thigpen, T. Targeting the folate receptor: Diagnostic and therapeutic approaches to personalize cancer treatments. Ann. Oncol. 2015, 26, 2034–2043.
- US National Library of Medicine Study of Vintafolide (MK-8109, EC145) in Participants with Advanced Ovarian and Endometrial Cancers (MK-8109-007, EC-FV-02). Available online: (accessed on 13 October 2019).
- US National Library of Medicine Study of Vintafolide (MK-8109, EC145) in Participants with Progressive Adenocarcinoma of the Lung (MK-8109-008, EC-FV-03). Available online: (accessed on 13 October 2019).
- Hjálmsdóttir, Á.; Bühler, C.; Vonwil, V.; Roveri, M.; Håkerud, M.; Wäckerle-Men, Y.; Gander, B.; Johansen, P. Cytosolic Delivery of Liposomal Vaccines by Means of the Concomitant Photosensitization of Phagosomes. Mol. Pharm. 2016, 13, 320–329.
- Karlsen, K.; Korsholm, K.S.; Mortensen, R.; Ghiasi, S.M.; Andersen, P.; Foged, C.; Christensen, D. A stable nanoparticulate DDA/MMG formulation acts synergistically with CpG ODN 1826 to enhance the CD4+ T-cell response. Nanomedicine 2014, 9, 2625–2638.
- Bruno, C.; Waeckerle-Men, Y.; Håkerud, M.; Kündig, T.M.; Gander, B.; Johansen, P. Photosensitizer and Light Pave the Way for Cytosolic Targeting and Generation of Cytosolic CD8 T Cells Using PLGA Vaccine Particles. J. Immunol. 2015, 195, 166–173.
- Watarai, S.; Iwase, T.; Tajima, T.; Yuba, E.; Kono, K. Efficiency of pH-Sensitive Fusogenic Polymer-Modified Liposomes as a Vaccine Carrier. Sci. World J. 2013.
- Sanders, M.T.; Brown, L.E.; Deliyannis, G.; Pearse, M.J. ISCOMTM-based vaccines: The second decade. Immunol. Cell Biol. 2005, 83, 119–128.
- Olsen, C.E.; Berg, K.; Selbo, P.K.; Weyergang, A. Circumvention of resistance to photodynamic therapy in doxorubicin- resistant sarcoma by photochemical internalization of gelonin. Free Radic. Biol. Med. 2013, 65, 1300–1309.
- O’Rourke, C.; Hopper, C.; MacRobert, A.J.; Phillips, J.B.; Woodhams, J.H. Could clinical photochemical internalisation be optimised to avoid neuronal toxicity? Int. J. Pharm. 2017, 528, 133–143.
- Martínez-Jothar, L.; Beztsinna, N.; Van Nostrum, C.F.; Hennink, W.E.; Oliveira, S. Selective Cytotoxicity to HER2 Positive Breast Cancer Cells by Saporin-Loaded Nanobody-Targeted Polymeric Nanoparticles in Combination with Photochemical Internalization. Mol. Pharm. 2019, 16, 1633–1647.
- Norum, O.J.; Fremstedal, A.S.V.; Weyergang, A.; Golab, J.; Berg, K. Photochemical delivery of bleomycin induces T-cell activation of importance for curative effect and systemic anti-tumor immunity. J. Control. Release 2017, 268, 120–127.
- Stratford, E.W.; Bostad, M.; Castro, R.; Skarpen, E.; Berg, K.; Høgset, A.; Myklebost, O.; Selbo, P.K. Photochemical internalization of CD133-targeting immunotoxins efficiently depletes sarcoma cells with stem-like properties and reduces tumorigenicity. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4235–4243.
- Bostad, M.; Olsen, C.E.; Peng, Q.; Berg, K.; Høgset, A.; Selbo, P.K. Light-controlled endosomal escape of the novel CD133-targeting immunotoxin AC133-saporin by photochemical internalization—A minimally invasive cancer stem cell-targeting strategy. J. Control. Release 2015, 206, 37–48.
- Berstad, M.B.; Cheung, L.H.; Berg, K.; Peng, Q.; Fremstedal, A.S.V.; Patzke, S.; Rosenblum, M.G.; Weyergang, A. Design of an EGFR-targeting toxin for photochemical delivery: In vitro and in vivo selectivity and efficacy. Oncogene 2015, 34, 5582–5592.
- Eng, M.S.; Kaur, J.; Prasmickaite, L.; Engesæter, B.; Weyergang, A.; Skarpen, E.; Berg, K.; Rosenblum, M.G.; Mælandsmo, G.M.; Høgset, A.; et al. Enhanced targeting of triple-negative breast carcinoma and malignant melanoma by photochemical internalization of CSPG4-targeting immunotoxins. Photochem. Photobiol. Sci. 2018, 17, 539–551.
- Waeckerle-Men, Y.; Mauracher, A.; Håkerud, M.; Mohanan, D.; Kündig, T.M.; Høgset, A.; Johansen, P. Photochemical targeting of antigens to the cytosol for stimulation of MHC class-I-restricted T-cell responses. Eur. J. Pharm. Biopharm. 2013, 85, 34–41.
- Håkerud, M.; Waeckerle-Men, Y.; Selbo, P.K.; Kündig, T.M.; Høgset, A.; Johansen, P. Intradermal photosensitisation facilitates stimulation of MHC class-I restricted CD8 T-cell responses of co-administered antigen. J. Control. Release 2014, 174, 143–150.
- Håkerud, M.; Selbo, P.K.; Waeckerle-Men, Y.; Contassot, E.; Dziunycz, P.; Kündig, T.M.; Høgset, A.; Johansen, P. Photosensitisation facilitates cross-priming of adjuvant-free protein vaccines and stimulation of tumour-suppressing CD8 T cells. J. Control. Release 2015, 198, 10–17.
- Haug, M.; Brede, G.; Håkerud, M.; Nedberg, A.G.; Gederaas, O.A.; Flo, T.H.; Edwards, V.T.; Selbo, P.K.; Høgset, A.; Halaas, Ø. Photochemical internalization of peptide antigens provides a novel strategy to realize therapeutic cancer vaccination. Front. Immunol. 2018, 9, 1–14.
- Varypataki, E.M.; Hasler, F.; Waeckerle-Men, Y.; Vogel-Kindgen, S.; Høgset, A.; Kündig, T.M.; Gander, B.; Halin, C.; Johansen, P. Combined Photosensitization and Vaccination Enable CD8 T-Cell Immunity and Tumor Suppression Independent of CD4 T-Cell Help. Front. Immunol. 2019, 10, 1–12.
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 1–8.
- Agostinis, P.; Vantieghem, A.; Merlevede, W.; De Witte, P.A.M. Hypericin in cancer treatment: More light on the way. Int. J. Biochem. Cell Biol. 2002, 34, 221–241.
- Juarranz, Á.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic therapy of cancer. Basic principles and applications. Clin. Transl. Oncol. 2008, 10, 148–154.
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic photodynamic therapy: Basic principles, current clinical status and future directions. Cancers 2017, 9, 19.
- Castano, A.P.; Mroz, P.; Wu, M.X.; Hamblin, M.R. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc. Natl. Acad. Sci. USA 2008, 105, 5495–5500.
- Mroz, P.; Szokalska, A.; Wu, M.X.; Hamblin, M.R. Photodynamic therapy of tumors can lead to development of systemic antigen-specific immune response. PLoS ONE 2010, 5, 15194.
- Brodin, N.P.; Guha, C.; Tomé, W.A. Photodynamic therapy and its role in combined modality anticancer treatment. Technol. Cancer Res. Treat. 2015, 14, 355–368.
- Mroz, P.; Hashmi, J.T.; Huang, Y.Y.; Lange, N.; Hamblin, M.R. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev. Clin. Immunol. 2011, 7, 75–91.
- Garg, A.D.; Nowis, D.; Golab, J.; Agostinis, P. Photodynamic therapy: Illuminating the road from cell death towards anti-tumour immunity. Apoptosis 2010, 15, 1050–1071.
- Berg, K.; Selbo, P.K.; Prasmickaite, L.; Tjelle, T.E.; Sandvig, K.; Moan, J.; Gaudernack, G.; Fodstad, Ø.; Kjølsrud, S.; Anholt, H.; et al. Photochemical internalization: A novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999, 59, 1180–1183.
- Shin, D.; Christie, C.; Ju, D.; Nair, R.K.; Molina, S.; Berg, K.; Krasieva, T.B.; Madsen, S.J.; Hirschberg, H. Photochemical internalization enhanced macrophage delivered chemotherapy. Photodiagnosis Photodyn. Ther. 2018, 21, 156–162.
- Selbo, P.K.; Weyergang, A.; Høgset, A.; Norum, O.J.; Berstad, M.B.; Vikdal, M.; Berg, K. Photochemical internalization provides time- and space-controlled endolysosomal escape of therapeutic molecules. J. Control. Release 2010, 148, 2–12.
- Berg, K.; Folini, M.; Prasmickaite, L.; Selbo, P.; Bonsted, A.; Engesaeter, B.; Zaffaroni, N.; Weyergang, A.; Dietzea, A.; Maelandsmo, G.; et al. Photochemical Internalization: A New Tool for Drug Delivery. Curr. Pharm. Biotechnol. 2007, 8, 362–372.
- Berg, K.; Nordstrand, S.; Selbo, P.K.; Tran, D.T.T.; Angell-Petersen, E.; Høgset, A. Disulfonated tetraphenyl chlorin (TPCS 2a), a novel photosensitizer developed for clinical utilization of photochemical internalization. Photochem. Photobiol. Sci. 2011, 10, 1637–1651.
- Sultan, A.A.; Jerjes, W.; Berg, K.; Høgset, A.; Mosse, C.A.; Hamoudi, R.; Hamdoon, Z.; Simeon, C.; Carnell, D.; Forster, M.; et al. Disulfonated tetraphenyl chlorin (TPCS2a)-induced photochemical internalisation of bleomycin in patients with solid malignancies: A phase 1, dose-escalation, first-in-man trial. Lancet Oncol. 2016, 17, 1217–1229.
- US National Library of Medicine PCI Treatment/Gemcitabine & Chemotherapy vs. Chemotherapy Alone in Patients with Inoperable Extrahepatic Bile Duct Cancer (RELEASE). Available online: (accessed on 11 October 2019).
- Page, D.B.; Postow, M.A.; Callahan, M.K.; Allison, J.P.; Wolchok, J.D. Immune Modulation in Cancer with Antibodies. Annu. Rev. Med. 2014, 65, 185–202.
- Naidoo, J.; Page, D.B.; Wolchok, J.D. Immune modulation for cancer therapy. Br. J. Cancer 2014, 111, 2214–2219.
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546.
- US National Library of Medicine Phase 3 Study of Nivolumab or Nivolumab Plus Ipilimumab Versus Ipilimumab Alone in Previously Untreated Advanced Melanoma (CheckMate 067). Available online: (accessed on 14 October 2019).
- Zinkernagel, R.M. Immunological memory protective immunity. Cell. Mol. Life Sci. 2012, 69, 1635–1640.
- Van Der Burg, S.H.; Arens, R.; Ossendorp, F.; Van Hall, T.; Melief, C.J.M. Vaccines for established cancer: Overcoming the challenges posed by immune evasion. Nat. Rev. Cancer 2016, 16, 219–233.
- Mammas, I.N.; Sourvinos, G.; Zaravinos, A.; Spandidos, D.A. Vaccination against human papilloma virus (HPV): Epidemiological evidence of HPV in non-genital cancers. Pathol. Oncol. Res. 2011, 17, 103–119.
- Chemin, I. Evaluation of a hepatitis B vaccination program in Taiwan: Impact on hepatocellular carcinoma development. Futur. Oncol. 2010, 6, 21–23.
- Graff, J.N.; Chamberlain, E.D. Sipuleucel-T in the treatment of prostate cancer: An evidence-based review of its place in therapy. Core Evid. 2014, 10, 1–11.
- Høgset, A.; Prasmickaite, L.; Selbo, P.K.; Hellum, M.; Engesæter, B.; Bonsted, A.; Berg, K. Photochemical internalisation in drug and gene delivery. Adv. Drug Deliv. Rev. 2004, 56, 95–115.
- Perez, R.P.; Hamilton, T.C.; Ozols, R.F.; Young, R.C. Mechanisms and modulation of resistance to chemotherapy in ovarian cancer. Cancer 1993, 71, 1571–1580.
- Pron, G.; Mahrour, N.; Orlowski, S.; Tounekti, O.; Poddevin, B.; Belehradek, J.; Mir, L.M. Internalisation of the bleomycin molecules responsible for bleomycin toxicity: A receptor-mediated endocytosis mechanism. Biochem. Pharmacol. 1999, 57, 45–56.
- Sleijfer, S. Bleomycin-induced pneumonitis. Chest 2001, 120, 617–624.
- Berg, K.; Dietze, A.; Kaalhus, O.; Høgset, A. Site-specific drug delivery by photochemical internalization enhances the antitumor effect of bleomycin. Clin. Cancer Res. 2005, 11, 8476–8485.
- Weyergang, A.; Fremstedal, A.S.; Skarpen, E.; Peng, Q.; Mohamedali, K.A.; Eng, M.S.; Cheung, L.H.; Rosenblum, M.G.; Waltenberger, J.; Berg, K. Light-enhanced VEGF121/rGel: A tumor targeted modality with vascular and immune-mediated efficacy. J. Control. Release 2018, 288, 161–172.
- Liu, B.; Ma, W.; Jha, R.K.; Gurung, K. Cancer stem cells in osteosarcoma: Recent progress and perspective. Acta Oncol. 2011, 50, 1142–1150.
- Waldron, N.N.; Kaufman, D.S.; Oh, S.; Inde, Z.; Hexum, M.K.; Ohlfest, J.R.; Vallera, D.A. Targeting tumor-initiating cancer cells with dCD133KDEL shows impressive tumor reductions in a xenotransplant model of human head and neck cancer Nate. Mol. Cancer Ther. 2011, 10, 1829–1838.
- Smith, L.M.; Nesterova, A.; Ryan, M.C.; Duniho, S.; Jonas, M.; Anderson, M.; Zabinski, R.F.; Sutherland, M.K.; Gerber, H.P.; Van Orden, K.L.; et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br. J. Cancer 2008, 99, 100–109.
- Alewine, C.; Hassan, R.; Pastan, I. Advances in Anticancer Immunotoxin Therapy. Oncologist 2015, 20, 176–185.
- Li, M.; Liu, Z.S.; Liu, X.L.; Hui, Q.; Lu, S.Y.; Qu, L.L.; Li, Y.S.; Zhou, Y.; Ren, H.L.; Hu, P. Clinical targeting recombinant immunotoxins for cancer therapy. Onco. Targets. Ther. 2017, 10, 3645–3665.
- Selbo, P.K.; Sivam, G.; Fodstad, Y.; Sandvig, K.; Berg, K. In vivo documentation of photochemical internalization, a novel approach to site specific cancer therapy. Int. J. Cancer 2001, 92, 761–766.
- University College London Safety Study of Amphinex Based Photochemical Internalisation (PCI) of Bleomycin in Patients with Cutaneous Cancer. Available online: (accessed on 27 September 2019).
- Zanetti, M. Tapping CD4 T Cells for Cancer Immunotherapy: The Choice of Personalized Genomics. J. Immunol. 2015, 194, 2049–2056.
- Gajewski, T.F.; Meng, Y.; Blank, C.; Brown, I.; Kacha, A.; Kline, J.; Harlin, H. Immune resistance orchestrated by the tumor microenvironment. Immunol. Rev. 2006, 213, 131–145.
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K.; et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800.
- Schwartz, R.H. T Cell Anergy. Annu. Rev. Immunol. 2003, 21, 305–334.
- Ochsenbein, A.F. Immunological ignorance of solid tumors. In Springer Seminars in Immunopathology; Springer: Berlin, Germany, 2005; Volume 27, pp. 19–35.
- Cebon, J. Perspective: Cancer vaccines in the era of immune checkpoint blockade. Mamm. Genome 2018, 29, 703–713.
- Xia, A.; Zhang, Y.; Xu, J.; Yin, T.; Lu, X.J. T Cell Dysfunction in Cancer Immunity and Immunotherapy. Front. Immunol. 2019, 10, 1719.
- US National Library of Medicine Study to Assess Safety, Tolerability and Immune Response of Fimaporfin-induced Photochemical Internalisation of Antigen/Adjuvant. Available online: (accessed on 28 October 2019).
- Selbo, P.K.; Janetzki, S.; Welters, M.J.P.; Håkerud, M.; Nedberg, A.G.; Edwards, V.T.; Olivecrona, H.; van der Burg, S.H.; Otterhaug, T.; Hogset, A. 109P Phase I clinical study for validation of fimaporfin-based photochemical internalisation: A novel technology for enhancing cellular immune responses important for therapeutic effect of peptide-and protein-based vaccines. Ann. Oncol. 2019, 30.




