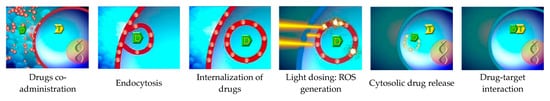
2. PCI of Cytotoxic Therapeutics
PDT kills tumorous tissue by means of photosensitizer and light. In contrast, photosensitizer and light in PCI is not primarily used to kill tumor cells but as a vehicle for specific and intracellular delivery of anti-cancer drugs. By existing data, PCI has proven to be a promising method for targeting therapeutic molecules to tumor cells for the purpose of specific killing. A wide range of drugs have been tested, e.g., macromolecular proteins, peptides, nucleic acids, and synthetic polymers, but also low-molecular weight chemotherapeutic drugs [
47,
49,
50,
72]. The method of PCI of cytotoxic therapeutics is especially applicable to drugs where the therapeutic target is intracellular and the PCI mediating cytosolic delivery of drugs has poor access to their cytosolic target.
One potential application of the PCI of cytotoxic therapeutics is to overcome drug resistance, which is one of the major challenges to reach effective cancer treatment. Up to 50% of malignant tumors are intrinsically resistant to chemotherapy [
73], with the additional problem of attained resistance after repetitive drug administration. In this case, PDT represents an alternative treatment method that is usually not associated with resistance. However, PDT is often tissue and cell unspecific and, therefore, mostly applicable for superficial and solid tumors (). By contrast, the combination of PDT and chemotherapy in PCI has been suggested to enable PDT-guided delivery of chemotherapeutic drugs to specific tumor cells, and, thereby, overcome the problem of resistance [
50]. For example, the chemotherapeutic drug bleomycin is approved for treatment of testicular carcinomas, lymphomas, head and neck cancers, and other non-melanoma skin cancers, but is known to become trapped in intracellular compartments after administration, which consequently leads to the need for higher therapeutic doses [
74]. This is associated with an increased risk of pneumonitis and subsequent lung fibrosis [
75]. However, PCI of bleomycin enhanced cytotoxicity compared to bleomycin alone both in vitro [
26] and in vivo [
28], which suggests improved bio-distribution and organ specificity with reduced resistance. By consequence, PCI can enable reduction of the dose needed to achieve a therapeutic effect and thereby reducing non-specific, adverse events [
76].
The use of PDT for cell-targeted delivery of immunotoxins, such as the ribosome-inactivating proteins saporin and gelonin, have also been investigated with PCI. Immunotoxins were coupled with specific cell-targeting proteins that can bind to CSPG4 [
32], to CD133-expressing cancer stem cells [
29,
30], to EGFR [
27,
31], or to VEGFR [
63]. Targeting of CD133-expressing cancer stem cells is based on the knowledge that a small population of stem-like cancer cells are often resistant to traditional chemotherapies, where the consequence is tumor relapse and metastasis [
77]. Unfortunately, the clinical success of CD133-directed immunotoxins has been compromised by the potential harm on normal stem cells that also express CD133 [
78,
79,
80,
81]. However, the PCI of CD133-directed immunotoxins seems to pose a potential solution to normal stem cell toxicity by increasing tumor-cell selectivity [
29,
30]. Systemically administered CD133-directed immunotoxins were found to localize predominantly in the tumor tissue, with no detection in normal tissue except in the kidney and the liver [
30]. Hence, PCI may reduce the frequency of drug administrations, which by consequence may reduce treatment-associated AEs and resistance. Since the efficacy of conventional cancer therapies are often limited by a dose-dependent toxicity [
50,
72,
82], PCI may represent a rational and promising approach for chemotherapeutic targeting and killing of drug-therapy or multi-therapy-resistant cancer cells. PCI of bleomycin was safe in patients with squamous cell carcinoma or other advanced or recurrent malignancies of the head and neck, torso, and upper limbs [
52,
83]. A pivotal Phase-II study is currently recruiting patients with inoperable bile duct cancer to assess effectiveness of PCI of gemcitabine complemented by systemic gemcitabine/cisplatin chemotherapy compared to gemcitabine/cisplatin alone [
53].
3. PCI in Immunotherapy
The immune system can play an important role in fighting cancer cells. Immunotherapy, such as checkpoint-inhibition, cytokine therapy, adoptive cell transfer therapy, and therapeutic vaccines all have the potential to induce immune responses that can surveil tumor, suppress growth of or kill cancerous cells, and give the patient a long-lasting immunity that may prevent remissions. However, cancer cells can interfere with the immune system in many ways. In this case, the potential immune-suppressive tumor micro environment (TME) may represent a significant challenge for effective anti-tumor therapies. The TME can be seen as an environment generated by various interactions between cancer cells and immune cells. Cancer cells, as they develop and grow, exploit immune-regulatory mechanisms by interacting with immune cells such as regulatory T and B cells, DCs, and myeloid-derived suppressor cells. Tumors can downregulate protein p53 or other tumor suppressors, downregulate MHC class I or co-stimulatory molecules on APCs, attract immunosuppressive leucocytes, activate CTLA4, PD1, or other co-inhibitory receptors on T cells [
4,
59,
84,
85]. The tumor-cell mediated activation of co-inhibitory receptors on T cells directly interferes with T-cell mediated tumor destruction [
86], whereas the lack of co-stimulation can lead to T-cell anergy [
87]. Additionally, immune escape due to self-tolerance of tumor antigens makes it difficult to target the immune system, notably T cells [
88].
While adjuvant immunotherapy with checkpoint inhibitors have found wide application during the last decade, therapeutic cancer vaccination has proven more laborious and less effective. Cancer vaccination aims to stimulate tumor-specific immune responses against delivered antigens. In order to achieve this, one has to overcome hurdles, such as the correct selection of antigens among the plethora of heterogeneously expressed and genetically unstable tumor antigens [
4,
89] and the use of appropriate adjuvants. To avoid mechanisms of central tolerance in the thymus, it is important to choose immunogenic antigens for vaccination [
59]. In this case, neoantigens and viral antigens are not subject to self-tolerance mechanisms and could be used for stronger anti-tumor T-cell responses than regular tumor antigens. Since tumor neoantigens are usually patient-specific, they typically require personalized vaccines, whereas tumor-specific viral antigens could be used for off-the-shelf vaccines.
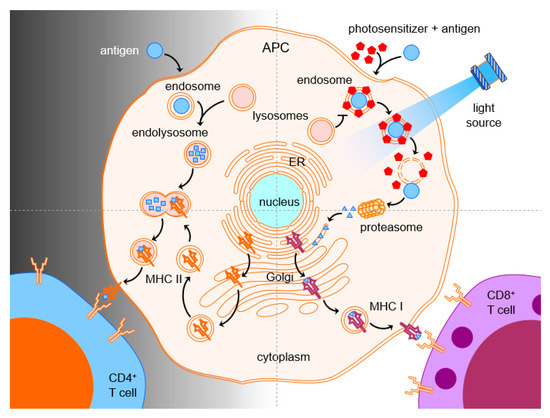
Cytotoxic T cells and natural killer (NK) cells have shown to make important immunological contributions in fighting tumors. In order for therapeutic vaccines to trigger the generation of tumor-specific CTLs, the MHC class I pathway of antigen presentation needs to be accessed. However, vaccine antigens end up in phagolysosomes of APCs and are presented in the context of MHC class II, which leads to stimulation of CD4 rather than CD8 T cells. One possible approach for CTL activation is to shuffle the antigen across the plasma membrane and, thereby, avoid endocytosis altogether. Another way has been obtained by triggering endosomal escape of the internalized antigen subsequent to the endocytosis or phagocytosis of antigens into APCs. The combination of antigens with a photosensitizer and light can facilitate cytosolic release of the endocytosed antigen by disruption of the endosomal membrane. The now cytosolic antigen can be processed by proteasomes and presented via MHC class I pathway for stimulation of CD8 T-cell responses, which, therefore, overcame the problem of the CD8 deficit after vaccination ().
Successful stimulation of tumor-specific immunity by PCI has been demonstrated in several mouse models of cancer. PCI mediated induction of antigen-specific CD8 T-cell proliferation and IFN-γ production [
20,
22,
33,
34,
35,
36], prevention of tumor grafting [
34,
35], suppression of tumor growth, and improved progression-free survival in mice [
35,
37]. Studies have demonstrated the mechanism of antigen and photosensitizer uptake in APCs and that, upon application of light, the antigen is released from endosomes or even phagolysosomes into the cytosol [
34,
35,
36]. While it has been recognized that the generation of primary CD8 T-cell responses to non-inflammatory antigens typically require MHC class II-restricted CD4 T helper cells, Varypataki et al. demonstrated that CD8 T-cell responses and their ability to control tumor growth after PCI-based vaccination were not impaired in MHC class-II and CD4 T-cell deficient mice [
37]. In order to verify the significance of the data, further tumor models will be needed, and future findings may have clinical importance with regard to the fact that many tumor patients are treated with CD4 T-cell-sensitive immunosuppressive agents [
90]. Antigen-specific CD8 T-cell responses could also be generated autologously in mice after prior PCI-mediated loading of the antigen to DCs in vitro [
33]. In light of the autologous vaccine Sipuleucel-T, which is the only FDA-approved and therapeutic cancer vaccine, it would be interesting to follow up on this technique with other models, e.g., DNA or mRNA treated DCs or DCs treated with tumor antigens. This would enable us to conclude on the true potential of PCI-based autologous vaccination. In the above mentioned report [
33], the DCs were treated with the model antigen OVA, which is a strong antigen, while tumor antigens are typically weak.
A Phase-I clinical trial was completed on 27 August, 2019 on the safety of photochemical internalization of a large immunogenic protein (KLH) and two smaller and less immunogenic peptides (HPV) in healthy volunteers [
91]. The primary objective was to study the incidence of AEs after a single administration of the photosensitizer and light. The first results thereof were presented at the ESMO Immuno-Oncology Congress in December 2019 [
92]. The induction of HPV-specific immune response in blood showed an increase in the number of healthy donors with HPV-specific CD4+ and CD8+ T-cell responses to PCI-based vaccination compared to baseline levels. Further details and results of the study are expected to be released. However, additional Phase-II and III trials will be needed to investigate the translational potential of current pre-clinical and anecdotal clinical reports.
Therapeutic benefits of anti-cancer vaccines in development are inconclusive. Even with optimized antigen selection and delivery, tumor-intrinsic evasive actions, as well as the lack of understanding of the tumor-microenvironment, pose unforeseeable obstacles. A deeper understanding of the interactions between the immune system and cancer cells will be inevitable for treatment optimization. One possible approach to overcome the immunosuppressant TME has been suggested to be the combination of cancer vaccines and other immunotherapies such as checkpoint inhibition, cytotoxic agents, or classical chemotherapies [
59]. Therapeutic vaccination could help prime the immune system to recognize tumor antigens or individualized neoantigens, and the effect of established cancer therapies could, therefore, be improved [
36]. Another obstacle for future clinical trials is expected to be the possible shift from self-tolerance to autoimmunity, triggered by immunotherapy combined with local inflammation following treatment. So far, no such effects have been observed with PCI, but, since autoimmunity typically develops slowly, a further assessment will be required. However, the risk of autoimmune reactions is not limited to cancer vaccination, but to all immune-stimulating procedures. Photochemical internalization, as a technology with the potential to enhance therapeutic cancer vaccines, can be seen as a promising tool to optimize anti-cancer immunotherapy.