
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mona Farhadipour | + 2129 word(s) | 2129 | 2021-06-03 12:13:24 | | | |
| 2 | Amina Yu | + 74 word(s) | 2203 | 2021-06-11 03:37:01 | | | | |
| 3 | Amina Yu | -17 word(s) | 2186 | 2021-06-15 10:14:21 | | |
Video Upload Options
Food ingestion triggers several physiological responses in the digestive system, including the release of gastrointestinal hormones from enteroendocrine cells that are involved in appetite signalling. Disturbed regulation of gut hormone release may affect energy homeostasis and contribute to obesity.
1. Introduction
2. Strategies for the Management of Obesity: Role of Gut Hormones
2.1. Diet-Induced Weight Loss
2.2. Roux-en-Y Gastric Bypass Surgery Restores the Gut Hormone Balance
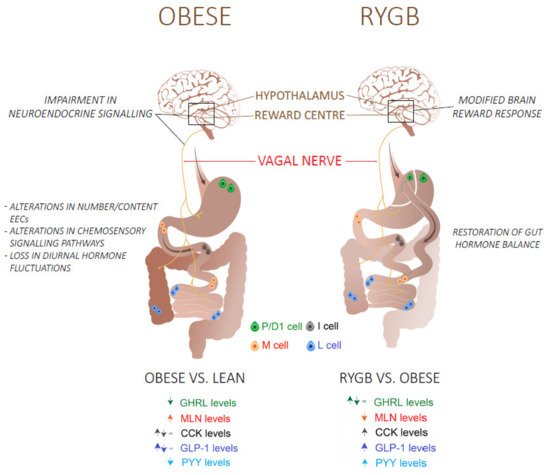
Figure 1. An overview of the mechanisms and the differences in fasting (GHRL, MLN) and postprandial (CCK, GLP-1, PYY) gut hormone plasma levels in obese/type 2 diabetes patients before and after a Roux-en-Y gastric bypass (RYGB) surgery. Abbreviations: GHRL: Ghrelin; MLN: Motilin; CCK: Cholecystokinin; GLP-1: glucagon-like peptide 1; peptide YY.
2.3. Combination Therapy
GGLP-1R agonists are used widely to treat T2DM. Liraglutide, which is administered once a day, was until now the only GLP-1 receptor (GLP-1R) agonist to be approved for weight management [31]. Recently, Semaglutide, a long acting GLP-1R agonist, has proven to be effective in weight management as an adjunct to lifestyle by inducing 14.9% weight loss from baseline in overweight and obese individuals [32]. Combined agonism, mostly by combining GLP-1 analogues with other food intake-inhibiting and/or glucose-lowering hormones, may cause a synergistic pharmacological action in obese individuals and patients with T2DM. Therefore, combination therapy is currently considered as the way to go to mimic the beneficial effects of RYGB surgery in a non-surgical manner [33]. Table 2 gives an overview of several combinations with GLP-1R analogues that are currently in clinical trial.
| Combination Therapy | Physiological Effect | Drug Candidates | ||
|---|---|---|---|---|
| GLP-1–GIP | Insulinotropic effect Decrease food intake cardiovascular protection |
Drug | Company | Status |
| Tirzepatide | Eli Lilly | Phase II | ||
| GLP-1–GCG | Insulinotropic effect cardiovascular protection Decrease food intake Increase energy expenditure |
Drug | Company | Status |
| Cotadutide | Astrazeneca | Phase II | ||
| Efinopegdutide | Hanmi Pharmaceuticals | Phase II | ||
| GLP-1–GCG-GIP | Insulinotropic effect Increase energy expenditure cardiovascular protection Decrease food intake |
Drug | Company | Status |
| MAR423 | Novo-nordisk/Marcadia | Phase I | ||
| HM15211 | Hanmi Pharmacueticals | Phase II | ||
Glucagon-like-peptide 1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), glucagon (GCG).
2.3.1. GLP-1 and GIP
Glucose-dependent insulinotropic peptide (GIP) is an incretin hormone that is secreted by K-cells in response to nutrients to stimulate insulin secretion through activation of GIP receptors on pancreatic beta cells, and acts as a blood glucose stabilising hormone by regulating insulin and glucagon secretion [34][35]. GIP also exerts direct actions on lipid metabolism, promoting lipogenesis and weight gain, and GIPR agonists have been demonstrated to exacerbate the postprandial glucagon excursion in individuals with T2DM [36]. Therefore, GIP receptor (GIPR) antagonists were initially developed to induce weight loss and to control glycaemia levels in obesity and individuals with T2DM [37]. Even though individuals with T2DM have a decreased insulinotropic effect of GIP, due to impaired responsiveness by beta cells, the loss of GIP has been shown to enhance GLP-1R activity [38][39]. Evidence suggests that GIPR agonism can also positively impact body weight. A recent study showed that injection of a peripherally long acting, selective mouse GIPR agonist in DIO mice, lowered body weight due to reduced food intake [40]. Therefore, dual agonism of GLP-1R, which exerts glycaemic control, and GIPR represents a strategy in treating obesity and T2DM. Coadministration of the selective GIP receptor agonist, ZP4165, together with the GLP-1R agonist, liraglutide, in DIO mice resulted in superior body weight loss and improved blood glucose and plasma cholesterol levels [41]. Currently, tirzepatide, a dual-incretin peptide from Eli Lilly, has reached multi-dose clinical trials and shows promise in the treatment of obesity and T2DM [42].2.3.2. GLP-1 and GCG
2.3.3. GLP-1 and PYY3-36
The combination of GLP-1 analogue with PYY3-36 mainly has a role in body weight management. Co-infusion of PYY3-36 and GLP-1 reduced energy intake by 30% compared to placebo in overweight men, which was not achieved when a mono-infusion was administered of PYY3-36 or GLP-1 [50]. In addition, co-administration of PYY3-36 with oxyntomodulin reduced energy intake by 42.7% in overweight and obese volunteers, and the effect was more pronounced than when either hormone was infused separately [51]. No drugs are yet in clinical trials for combinations with PYY3-36.
2.3.4. GLP-1, GCG and GIP
3. Conclusions
Gut hormones are important players in the regulation of appetite. Obesity has a clear impact on fasted and meal-related fluctuations in gut hormone release but the effect on some hormones remains controversial. The mechanisms involved are complex and multifactorial, relating to changes in the number/content of EECs, effect of age and gender, alterations in nutrients’ sensing mechanisms that regulate postprandial responses, alterations in diurnal fluctuations, and may also involve alterations in the central responsiveness to gut hormones. Further exploration of the crosstalk between the gut microbiome and EECs is of interest. Restoring the disordered gut hormone balance in obesity by targeting nutrient sensors in selective regions of the gut or by combined administration of gut peptide mimetics represent a major potential therapeutic targets to improve the prevention and management of obesity.
References
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002, 346, 1623–1630.
- Briggs, D.I.; Lockie, S.H.; Wu, Q.; Lemus, M.B.; Stark, R.; Andrews, Z.B. Calorie-restricted weight loss reverses high-fat diet-induced ghrelin resistance, which contributes to rebound weight gain in a ghrelin-dependent manner. Endocrinology 2013, 154, 709–717.
- He, J.; Irwin, D.M.; Chen, R.; Zhang, Y.-P. Stepwise loss of motilin and its specific receptor genes in rodents. J. Mol. Endocrinol. 2010, 44, 37–44.
- Sloth, B.; Due, A.; Larsen, T.M.; Holst, J.J.; Heding, A.; Astrup, A. The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period. Br. J. Nutr. 2009, 101, 1846–1858.
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604.
- Chearskul, S.; Delbridge, E.; Shulkes, A.; Proietto, J.; Kriketos, A. Effect of weight loss and ketosis on postprandial cholecystokinin and free fatty acid concentrations. Am. J. Clin. Nutr. 2008, 87, 1238–1246.
- Rastelli, M.; Cani, P.D.; Knauf, C. The gut microbiome influences host endocrine functions. Endocr. Rev. 2019, 40, 1271–1284.
- Steensels, S.; Cools, L.; Avau, B.; Vancleef, L.; Farré, R.; Verbeke, K.; Depoortere, I. Supplementation of oligofructose, but not sucralose, decreases high-fat diet induced body weight gain in mice independent of gustducin-mediated gut hormone release. Mol. Nutr. Food Res. 2017, 61, 61.
- Steensels, S.; Depoortere, I. Chemoreceptors in the Gut. Ann. Rev. Physiol. 2018, 80, 117–141.
- Parnell, J.A.; Reimer, R.A. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 2009, 89, 1751–1759.
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754.
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156.
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113.
- Ekberg, J.H.; Hauge, M.; Kristensen, L.V.; Madsen, A.N.; Engelstoft, M.S.; Husted, A.-S.; Sichlau, R.; Egerod, K.L.; Timshel, P.; Kowalski, T.J.; et al. GPR119, a Major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40). Endocrinology 2016, 157, 4561–4569.
- Steensels, S.; Lannoo, M.; Avau, B.; Laermans, J.; Vancleef, L.; Farré, R.; Verbeke, K.; Depoortere, I. The role of nutrient sensing in the metabolic changes after gastric bypass surgery. J. Endocrinol. 2017, 232, 363–376.
- Seeley, R.J.; Berridge, K.C. The hunger games. Cell 2015, 160, 805–806.
- Peiris, M.; Aktar, R.; Raynel, S.; Hao, Z.; Mumphrey, M.B.; Berthoud, H.-R.; Blackshaw, L.A. Effects of obesity and gastric bypass surgery on nutrient sensors, endocrine cells, and mucosal innervation of the mouse colon. Nutrients 2018, 10, 1529.
- Moffett, R.C.; Docherty, N.G.; le Roux, C.W. The altered enteroendocrine reportoire following roux-en-Y-gastric bypass as an effector of weight loss and improved glycaemic control. Appetite 2021, 156, 104807.
- Peterli, R.; E Steinert, R.; Woelnerhanssen, B.; Peters, T.; Christoffel-Courtin, C.; Gass, M.; Kern, B.; Von Fluee, M.; Beglinger, C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: A randomized, prospective trial. Obes. Surg. 2012, 22, 740–748.
- Foschi, D.; Corsi, F.; Pisoni, L.; Vago, T.; Bevilacqua, M.; Asti, E.; Righi, I.; Trabucchi, E. Plasma cholecystokinin levels after vertical banded gastroplasty: Effects of an acidified meal. Obes. Surg. 2004, 14, 644–647.
- Goldstone, A.P.; Miras, A.; Scholtz, S.; Jackson, S.; Neff, K.J.; Pénicaud, L.; Geoghegan, J.; Chhina, N.; Durighel, G.; Bell, J.D.; et al. Link between increased satiety gut hormones and reduced food reward after gastric bypass surgery for obesity. J. Clin. Endocrinol. Metab. 2016, 101, 599–609.
- Orellana, E.R.; Covasa, M.; Hajnal, A. Neuro-hormonal mechanisms underlying changes in reward related behaviors following weight loss surgery: Potential pharmacological targets. Biochem. Pharmacol. 2019, 164, 106–114.
- Deloose, E.; Janssen, P.; Lannoo, M.; Van Der Schueren, B.; Depoortere, I.; Tack, J. Higher plasma motilin levels in obese patients decrease after Roux-en-Y gastric bypass surgery and regulate hunger. Gut 2016, 65, 1110–1118.
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102.
- Wilding, J.P.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.; Wadden, T.A.; et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 2021, 384, 989.
- Brandt, S.J.; Müller, T.D.; DiMarchi, R.D.; Tschöp, M.H.; Stemmer, K. Peptide-based multi-agonists: A new paradigm in metabolic pharmacology. J. Intern. Med. 2018, 284, 581–602.
- Christensen, M.B.; Calanna, S.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Glucose-dependent insulinotropic polypeptide: Blood glucose stabilizing effects in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E418–E426.
- Christensen, M.B. Glucose-dependent insulinotropic polypeptide: Effects on insulin and glucagon secretion in humans. Dan. Med. J. 2016, 63, 63.
- Chia, C.W.; Carlson, O.D.; Kim, W.; Shin, Y.K.; Charles, C.P.; Kim, H.S.; Melvin, D.L.; Egan, J.M. Exogenous glucose-dependent insulinotropic polypeptide worsens post prandial hyperglycemia in type 2 diabetes. Diabetes 2009, 58, 1342–1349.
- Campbell, J.E. Targeting the GIPR for obesity: To agonize or antagonize? Potential mechanisms. Mol. Metab. 2021, 46, 101139.
- Nauck, M.A.; Heimesaat, M.M.; Orskov, C.; Holst, J.J.; Ebert, R.; Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Investig. 1993, 91, 301–307.
- Calanna, S.; Christensen, M.; Holst, J.J.; Laferrère, B.; Gluud, L.L.; Vilsbøll, T.; Knop, F.K. Secretion of glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes: Systematic review and meta-analysis of clinical studies. Diabetes Care 2013, 36, 3346–3352.
- Mroz, P.A.; Finan, B.; Gelfanov, V.; Yang, B.; Tschöp, M.H.; DiMarchi, R.D.; Perez-Tilve, D. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol. Metab. 2019, 20, 51–62.
- Nørregaard, P.K.; Deryabina, M.A.; Tofteng Shelton, P.; Fog, J.U.; Daugaard, J.R.; Eriksson, P.O.; Larsen, L.F.; Jessen, L. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes Obes. Metab. 2018, 20, 60–68.
- Hartman, M.L.; Sanyal, A.J.; Loomba, R.; Wilson, J.M.; Nikooienejad, A.; Bray, R.; Karanikas, C.A.; Duffin, K.L.; Robins, D.A.; Haupt, A. Effects of novel dual GIP and GLP-1 receptor agonist tirzepatide on biomarkers of nonalcoholic steatohepatitis in patients with type 2 diabetes. Diabetes Care 2020, 43, 1352–1355.
- Kleinert, M.; Sachs, S.; Habegger, K.M.; Hofmann, S.M.; Müller, T.D. Glucagon regulation of energy expenditure. Int. J. Mol. Sci. 2019, 20, 5407.
- Kim, T.; Holleman, C.L.; Nason, S.; Arble, D.M.; Ottaway, N.; Chabenne, J.; Loyd, C.; Kim, J.-A.; Sandoval, D.; Drucker, D.J.; et al. Hepatic glucagon receptor signaling enhances insulin-stimulated glucose disposal in rodents. Diabetes 2018, 67, 2157–2166.
- Day, J.W.; Ottaway, N.; Patterson, J.; Gelfanov, V.; Smiley, D.; Gidda, J.; Findeisen, H.; Bruemmer, D.; Drucker, D.J.; Chaudhary, N.; et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat. Chem. Biol. 2009, 5, 749–757.
- Henderson, S.J.; Konkar, A.; Hornigold, D.C.; Trevaskis, J.L.; Jackson, R.; Fredin, M.F.; Jansson-Löfmark, R.; Naylor, J.; Rossi, A.; Bednarek, M.A.; et al. Robust anti-obesity and metabolic effects of a dual GLP-1/glucagon receptor peptide agonist in rodents and non-human primates. Diabetes Obes. Metab. 2016, 18, 1176–1190.
- Laker, R.C. Cotadutide (MEDI0382): A dual receptor agonist with glucagon-like peptide-1 and glucagon activity, modulates hepatic glycogen and fat content. Presented at 80th Scientific Sessions of the American Diabetes Association, Relocated from Chicago to Cyberspace, Chicago, IL, USA, 12–16 June 2020; Available online: (accessed on 1 March 2021).
- Holst, J.J.; Albrechtsen, N.J.; Gabe, M.B.N.; Rosenkilde, M.M. Oxyntomodulin: Actions and role in diabetes. Peptides 2018, 100, 48–53.
- Ma, T.; Huo, S.; Xu, B.; Li, F.; Wang, P.; Liu, Y.; Lei, H. A novel long-acting oxyntomodulin analogue eliminates diabetes and obesity in mice. Eur. J. Med. Chem. 2020, 203, 112496.
- Schmidt, J.B.; Gregersen, N.T.; Pedersen, S.D.; Arentoft, J.L.; Ritz, C.; Schwartz, T.W.; Holst, J.J.; Astrup, A.; Sjödin, A. Effects of PYY3-36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1248–E1256.
- Field, B.C.; Wren, A.M.; Peters, V.; Baynes, K.C.; Martin, N.M.; Patterson, M.; Alsaraf, S.; Amber, V.; Wynne, K.; Ghatei, M.A.; et al. PYY3-36 and oxyntomodulin can be additive in their effect on food intake in overweight and obese humans. Diabetes 2010, 59, 1635–1639.
- Finan, B.; Yang, B.; Ottaway, N.; Smiley, D.L.; Ma, T.; Clemmensen, C.; Chabenne, J.; Zhang, L.; Habegger, K.M.; Fischer, K.; et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nat. Med. 2015, 21, 27–36.
- Kim, J.K. Therapeutic efficacy of a novel long-acting GLP-1/GIP/Glucagon triple agonist (HM15211) in NASH and fibrosis animal models. In Proceedings of the EASD annual Meeting, Berlin, Germany, 3 October 2018.
- Hanmi Pharmaceutical Company Ltd. Study to Evaluate Efficacy, Safety and Tolerability of HM15211 in Subjects. 2021. Available online: (accessed on 1 March 2021).




