Food ingestion triggers several physiological responses in the digestive system, including the release of gastrointestinal hormones from enteroendocrine cells that are involved in appetite signalling. Disturbed regulation of gut hormone release may affect energy homeostasis and contribute to obesity.
- obesity
- gastrointestinal hormones
- nutrient sensing
- circadian clock
- gastric bypass surgery
1. Introduction
2. Strategies for the Management of Obesity: Role of Gut Hormones
2.1. Diet-Induced Weight Loss
Caloric restriction induces weight loss in obese individuals and restores the preprandial rise in ghrelin plasma levels [41]. Evidence from animal studies suggests that this increase in ghrelin levels may resensitize the brain and overcome ghrelin resistance to induce rebound weight gain [122]. Thus, ghrelin may act as a survival hormone to prevent further weight loss during a negative energy balance.
The effect of caloric restriction on plasma motilin levels has not been studied and has been hampered by the fact that motilin does not exist in rodents [29].
In obese patients, lower postprandial levels of GLP-1 and PYY were observed along with increased appetite scores, following an 8-week low-energy intake diet and a 2–3 week refeeding period [123]. Similarly, reductions in leptin, PYY and CCK were observed following a weight loss program with a very low energy diet which was accompanied by an increase in subjective appetite scores [124,125]. Importantly, one year after initial weight reduction, levels did not revert to levels recorded before weight loss, suggesting that alterations in gut hormone levels may facilitate regain of lost weight [124].
Interest in prebiotic supplementation with oligofructose or inulin for weight management stems from studies in rodents that reported reductions in body weight and altered gut hormone levels [126,127]. Prebiotic fibers are fermented by the gut microbiota to short chain fatty acids (SCFAs) that act on enteroendocrine cells via FFAR2 or FFAR3 to affect gut hormone release [106]. In a randomized, double-blind placebo controlled trial, oligofructose supplementation for 12 weeks reduced body weight in overweight and obese adults [128]. Ghrelin levels were reduced and PYY, but not GLP-1 levels were increased. Targeted delivery of the SCFA propionate to the colon of overweight patients with an inulin-propionate ester reduced energy intake and increased postprandial plasma PYY and GLP-1 levels in overweight patients [129]. Supplementation for 24 weeks reduced weight gain and prevented the deterioration in insulin sensitivity observed in the inulin control group. However, the rise in PYY and GLP-1 levels was not observed in the long-term study, indicating that desensitization may have occurred. A recent randomized clinical trial investigated the impact of modulation of the microbiome with isoenergetic diets that differed in their concentrations of prebiotics. The high-fiber diet selectively promoted a group of SCFA producers as the major active producers. When the SCFA producers were present in greater diversity and abundance, the improvement in haemoglobin A1c levels was greater, possibly reflecting in part increased GLP-1 production [130]. Evidence of crosstalk between the gut microbiome is also derived from studies with administration of Akkermansia muciniphila, known to prevent diet-induced obesity [131]. This commensal bacterium increased levels of 2-acylglycerols, endogenous cannabinoids, known to stimulate GLP-1 levels via GPR119 [132].
2.2. Roux-en-Y Gastric Bypass Surgery Restores the Gut Hormone Balance
 A Roux-en-Y gastric bypass (RYGB) surgery, where the pouch of the stomach is bypassed to the small intestine, is an effective way of inducing and maintaining weight loss in morbidly obese patients. After RYGB surgery, the contact of nutrients with much of the stomach and duodenum is bypassed, resulting in a rapid delivery of undigested nutrients to the jejunum. This rerouting has been shown to affect the expression of nutrient sensors in the gut that together with other intestinal adaptations, such as changes in morphology and altered bacterial fermentation, contribute to alterations in gut hormone profiles [133,134,135].
A Roux-en-Y gastric bypass (RYGB) surgery, where the pouch of the stomach is bypassed to the small intestine, is an effective way of inducing and maintaining weight loss in morbidly obese patients. After RYGB surgery, the contact of nutrients with much of the stomach and duodenum is bypassed, resulting in a rapid delivery of undigested nutrients to the jejunum. This rerouting has been shown to affect the expression of nutrient sensors in the gut that together with other intestinal adaptations, such as changes in morphology and altered bacterial fermentation, contribute to alterations in gut hormone profiles [133,134,135].
Indeed, the reported weight loss with ensuing improvement in glucose homeostasis in patients undergoing RYGB surgery or sleeve gastrectomy is associated with elevated postprandial PYY and GLP-1 levels, even one year after surgery [136,137]. CCK-secreting cells are mainly located in the bypassed duodenum. In two studies, where the effect of RYGB on CCK was investigated, a faster and higher peak response towards a meal was found [137,138]. In addition, there is a possible association between the higher plasma levels of these satiety hormones and the reduced food reward system in patients after a RYGB surgery, these patients exhibit a modified behavioral and brain reward response to food [139,140].
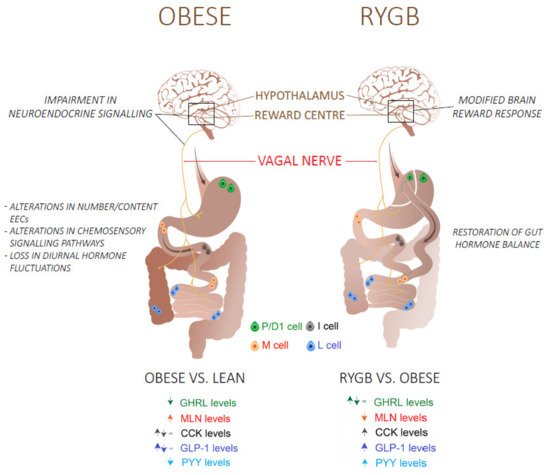
The reported effects of RYGB surgery on plasma ghrelin levels are inconsistent with a decrease, no change or an increase reported [136]. The size of the created pouch and difficulties inherent to the measurement of biological active octanoylated ghrelin levels have contributed to this. It is therefore unlikely that ghrelin is responsible for the post-surgical metabolic improvements. Regarding the other orexigenic hormone, motilin, Deloose et al. reported decreased motilin plasma levels in parallel with hedonic hunger scores after RYGB [44]. Figure 1 summarizes the differences in gut hormone levels in obese individuals before and after RYGB surgery.
Figure 1. An overview of the mechanisms and the differences in fasting (GHRL, MLN) and postprandial (CCK, GLP-1, PYY) gut hormone plasma levels in obese/type 2 diabetes patients before and after a Roux-en-Y gastric bypass (RYGB) surgery. Abbreviations: GHRL: Ghrelin; MLN: Motilin; CCK: Cholecystokinin; GLP-1: glucagon-like peptide 1; peptide YY.
2.3. Combination Therapy
GGLP-1R agonists are used widely to treat T2DM. Liraglutide, which is administered once a day, was until now the only GLP-1 receptor (GLP-1R) agonist to be approved for weight management [31][141]. Recently, Semaglutide, a long acting GLP-1R agonist, has proven to be effective in weight management as an adjunct to lifestyle by inducing 14.9% weight loss from baseline in overweight and obese individuals [32][142]. Combined agonism, mostly by combining GLP-1 analogues with other food intake-inhibiting and/or glucose-lowering hormones, may cause a synergistic pharmacological action in obese individuals and patients with T2DM. Therefore, combination therapy is currently considered as the way to go to mimic the beneficial effects of RYGB surgery in a non-surgical manner [33][143]. Table 2 gives an overview of several combinations with GLP-1R analogues that are currently in clinical trial.
| Combination Therapy |
|---|
| Physiological Effect |
|---|
| Drug Candidates |
|---|
| GLP-1–GIP | Insulinotropic effect Decrease food intake cardiovascular protection |
Drug | Company | Status |
| Tirzepatide | Eli Lilly | Phase II | ||
| GLP-1–GCG | Insulinotropic effect cardiovascular protection Decrease food intake Increase energy expenditure |
Drug | Company | Status |
| Cotadutide | Astrazeneca | Phase II | ||
| Efinopegdutide | Hanmi Pharmaceuticals | Phase II | ||
| GLP-1–GCG-GIP | Insulinotropic effect Increase energy expenditure cardiovascular protection Decrease food intake |
Drug | Company | Status |
| MAR423 | Novo-nordisk/Marcadia | Phase I | ||
| HM15211 | Hanmi Pharmacueticals | Phase II |
Glucagon-like-peptide 1 (GLP-1), glucose-dependent insulinotropic peptide (GIP), glucagon (GCG).
Table 2. An overview of several combination therapies with GLP-1R agonists that are currently in clinical trials.
2.3.1. GLP-1 and GIP
2.3.1. GLP-1 and GIP
Glucose-dependent insulinotropic peptide (GIP) is an incretin hormone that is secreted by K-cells in response to nutrients to stimulate insulin secretion through activation of GIP receptors on pancreatic beta cells, and acts as a blood glucose stabilising hormone by regulating insulin and glucagon secretion [34][35][144,145]. GIP also exerts direct actions on lipid metabolism, promoting lipogenesis and weight gain, and GIPR agonists have been demonstrated to exacerbate the postprandial glucagon excursion in individuals with T2DM [36][146]. Therefore, GIP receptor (GIPR) antagonists were initially developed to induce weight loss and to control glycaemia levels in obesity and individuals with T2DM [37][147]. Even though individuals with T2DM have a decreased insulinotropic effect of GIP, due to impaired responsiveness by beta cells, the loss of GIP has been shown to enhance GLP-1R activity [38][39][55,148]. Evidence suggests that GIPR agonism can also positively impact body weight. A recent study showed that injection of a peripherally long acting, selective mouse GIPR agonist in DIO mice, lowered body weight due to reduced food intake [40][149]. Therefore, dual agonism of GLP-1R, which exerts glycaemic control, and GIPR represents a strategy in treating obesity and T2DM. Coadministration of the selective GIP receptor agonist, ZP4165, together with the GLP-1R agonist, liraglutide, in DIO mice resulted in superior body weight loss and improved blood glucose and plasma cholesterol levels [41][150]. Currently, tirzepatide, a dual-incretin peptide from Eli Lilly, has reached multi-dose clinical trials and shows promise in the treatment of obesity and T2DM [151].7.3.2. [42]GLP-1 and GCG
The use of glucagon (GCG) with GLP-1 may intuitively appear contradictory since it antagonizes the effect of insulin and increases glucose levels, evoking hyperglycaemia. Nevertheless, glucagon also induces thermogenesis, increases energy expenditure and has hypolipidemic effects, which are beneficial for weight management in obese individuals [152]. Moreover, while chronic GCG stimulation exhibits glucose intolerance, acute GCG agonism at a lower dose, which is not able to evoke hyperglycaemia, enhances glucose tolerance and improves insulin sensitivity [153]. This suggests the use of GLP1-GCG dual agonists in not only obesity, but also in T2DM. Many preclinical studies have demonstrated the body weight and glucose lowering effects of GLP-1R/GCGR agonists. For example, a single high-dose or multiple low-dose injections of a GLP-1R/GCGR dual agonist induced body weight loss which was associated with increased energy expenditure and thermogenesis [154]. However, the effect of GLP-1R/GCGR dual agonists on body weight in human studies has not yet been found as effective as in animal studies. Cotadutide, a novel dual agonist by AstraZeneca, demonstrated superior results in body weight reduction relative to the GLP-1R agonist liraglutide during preclinical studies in DIO mice and normal weight cynomolgus monkeys [155]. Currently, results from Phase II clinical trials with cotadutide demonstrated beneficial effects on blood glucose levels, changes in liver fat and glycogen stores in patients with T2DM [156].
Oxyntomodulin (OXM) is a naturally occurring GLP1R/GCGR dual agonist that is secreted by L-cells after food intake to induce satiety and increase energy expenditure [157]. As native OXM has a very short half-life due to degradation by DPP4 and fast renal clearance, OXM analogues are being developed as a therapeutic candidate to treat obesity and T2DM. Recently, a PEGylated analogue showed a 27.1% body weight reduction at a high dose in DIO mice, which was significantly higher than the weight loss effect with liraglutide [158].
7.3.3. GLP-1 and PYY3-36
The combination of GLP-1 analogue with PYY3-36 mainly has a role in body weight management. Co-infusion of PYY3-36 and GLP-1 reduced energy intake by 30% compared to placebo in overweight men, which was not achieved when a mono-infusion was administered of PYY3-36 or GLP-1 [159]. In addition, co-administration of PYY3-36 with oxyntomodulin reduced energy intake by 42.7% in overweight and obese volunteers, and the effect was more pronounced than when either hormone was infused separately [160]. No drugs are yet in clinical trials for combinations with PYY3-36.
2.3.24. GLP-1 and , GCG
2.3.3. GLP-1 and GIPYY3-36
The combination of three gut hormones, triagonists, have emerged as new way of inducing multiple metabolic improvements. An acylated GLP-1 analogueR/GCGR/GIPR triagonist exerted in vivo and in vitro receptor activity in rodents with PYY3-36superior metainly has a role inbolic effects, improved glycaemic control and body weight management. Co-infusion of PYY3-36loss, relative to their co-agonists [161]. HM15211 (Hanmi Pharmaceuticals) is a triagonist with high GCG activity for obesity treatment and a banlanced GLP-1 reduced energy intake by 30% compared to placebo in overand GIP activity, to neutralize the hyperglycemic risk of GCG. Preclinical studies with HM15211 have shown improved weight men, which was not achieved when a mono-infusion was administered of PYY3-36 oloss, reduced liver fat and possibly inflammation, and may be effective for the treatment of non-alcoholic fatty liver disease as well [162]. HM15211 is currently in phase II clinical trials with a 30% reduction of liver GLP-1 [50]. In faddition, co-administration of PYY3-36 in comparison to placebo after a wi12-month oxyntomodtreatment [163].
Multin reduced energy intake by 42.7% in overweight-agonists are the next generation of therapies to treat patients with T2DM and obese volunteers, and thity. They avoid the adverse effect was more pronounced than when either hormone was infused separately [51]s of surgery (malnutrition, post-prandial hypoglycaemia, bowel obstruction, etc.) and GLP1R agonists (gastrointestinal symptoms). No drMugs are yet in clinical trials for combinations with PYY3-36lti-agonists can therefore be a solution for these individuals as a way to manage body weight.
2.3.4. GLP-1, GCG and GIP
3. Conclusions
Gut hormones are important players in the regulation of appetite. Obesity has a clear impact on fasted and meal-related fluctuations in gut hormone release but the effect on some hormones remains controversial. The mechanisms involved are complex and multifactorial, relating to changes in the number/content of EECs, effect of age and gender, alterations in nutrients’ sensing mechanisms that regulate postprandial responses, alterations in diurnal fluctuations, and may also involve alterations in the central responsiveness to gut hormones. Further exploration of the crosstalk between the gut microbiome and EECs is of interest. Restoring the disordered gut hormone balance in obesity by targeting nutrient sensors in selective regions of the gut or by combined administration of gut peptide mimetics represent a major potential therapeutic targets to improve the prevention and management of obesity.
