1000/1000
Hot
Most Recent

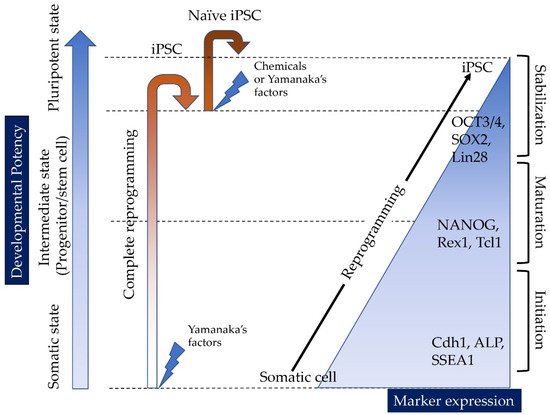
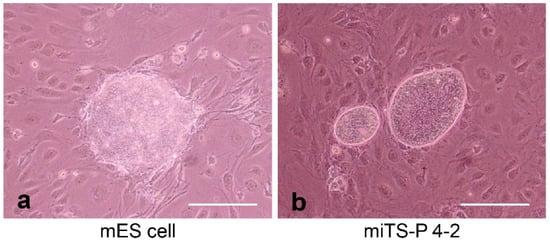
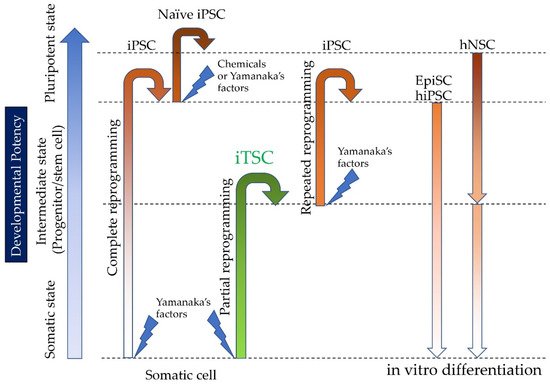
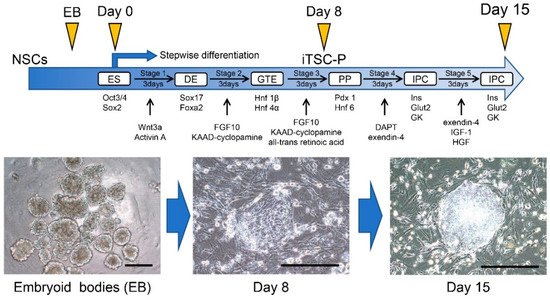
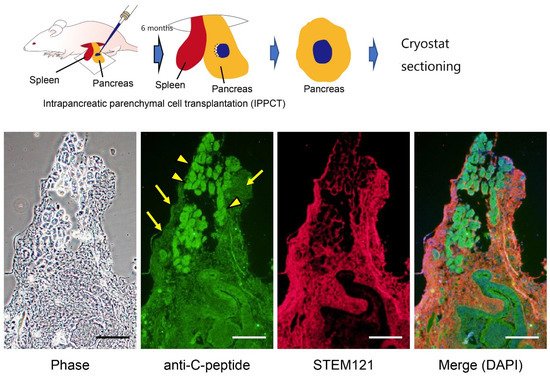
Induced tissue-specific stem cells (iTSCs) are partially reprogrammed cells which have an intermediate state, such as progenitors or stem cells. They originate from the de-differentiation of differentiated somatic cells into pluripotent stem cells, such as induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs), or from the differentiation of undifferentiated cells. They show a limited capacity to differentiate and a morphology similar to that of somatic cell stem cells present in tissues, but distinct from that of iPSCs and ESCs. iTSCs can be generally obtained 7 to 10 days after reprogramming of somatic cells with Yamanaka’s factors, and their fibroblast-like morphology remains unaltered. iTSCs can also be obtained directly from iPSCs cultured under conditions allowing cellular differentiation. In this case, to effectively induce iTSCs, additional treatment is required, as exemplified by the conversion of iPSCs into naïve iPSCs. iTSCs can proliferate continuously in vitro, but when transplanted into immunocompromised mice, they fail to generate solid tumors (teratomas), implying loss of tumorigenic potential. The low tendency of iTSCs to elicit tumors is beneficial, especially considering applications for regenerative medicine in humans. Several iTSC types have been identified, including iTS-L, iTS-P, and iTS-D, obtained by reprogramming hepatocytes, pancreatic cells, and deciduous tooth-derived dental pulp cells, respectively. This review provides a brief overview of iPSCs and discusses recent advances in the establishment of iTSCs and their possible applications in regenerative medicine.