
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppe Mannino | + 1949 word(s) | 1949 | 2021-05-24 10:36:16 | | | |
| 2 | Camila Xu | Meta information modification | 1949 | 2021-05-24 11:35:01 | | | | |
| 3 | Camila Xu | Meta information modification | 1949 | 2021-05-24 11:35:58 | | |
Video Upload Options
Anthocyanidins are colored molecules having medium-size and belonging to the class of flavonoids.
1. Anthocyanidins and Anthocyanins
1.1. Chemical Structures and Classification
Anthocyanidins are colored molecules having medium-size and belonging to the class of flavonoids [1]. Actually, 25 different anthocyanidins are known (Figure 1), that differ from each other for the presence of hydroxyl (−OH) and methoxy (−OCH3) groups bound at the scaffold core (Figure 1) [2]. Consequently, anthocyanidins are grouped into 3-hydroxyanthocyanidins, 3-deoxyanthocyanidins, and O-methylated anthocyanidins. Cyanidin (Cy), Delphinidin (Dp), Pelargonidin (Pg), three among the non-methylated anthocyanidins, are the most common in nature. In particular, it was estimated that 50% of plants producing anthocyanidins have Cy, 12% have Dp, and 10% have Pg [3][4]. Peonidin (Pn), Malvidin (Mv), and Petunidin (Pt), belonging to the methylated anthocyanidins, can be also easily found in plants [3][4].
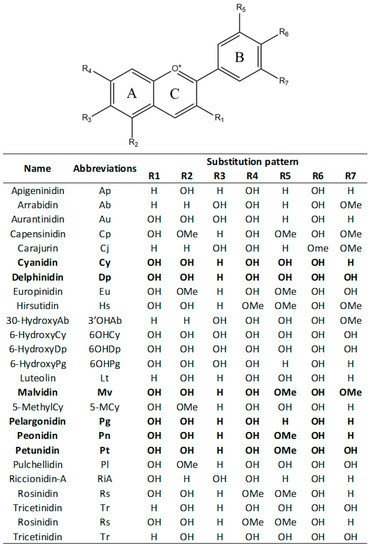
Figure 1. Chemical scaffold of anthocyanin compounds and their relative substituents. In the table, the most common anthocyanidins are reported in bold.
In most of the cases, anthocyanidins are bounded with sugar moieties, forming the corresponding anthocyanins. Glycosylation is achieved enzymatically following the adding of the sugar portion at the 3rd and/or 5th position (R1 and/or R2 subsistent of the chemical structure displayed in Figure 1 of the scaffold [5][6]. As a consequence of the glycosylation, anthocyanins have an increased water solubility and stability with respect to the related anthocyanidins [6]. Despite the most common glycosylation process involves the condensation of monosaccharides such as glucose, galactose, rhamnose, arabinose, rutinose and xylose, also disaccharides and trisaccharides may be attached in some cases [1]. Finally, anthocyanins may be also often acylated with organic acids such as p-coumaric, caffeic, and ferulic acids via ester bonds usually to the 3-position of the sugar moiety [1][5]. Consequently, to date more than 500 different anthocyanins that differ not only for the glycosylation pattern of the scaffold, but also for the presence and position of aliphatic or aromatic carboxylates are reported. In spite of their great structure variability, the anthocyanins most distributed in plants are those originated by Cy, Dp, and Pg. They are present in 80% of the leaves, 69% of the fruits, and 50% of the colored flowers [7][3][4][8][9]. On the other hand, anthocyanins formed by Pt, Mv, and Pn, are limitedly distributed [7][3][4][8][9][10].
The conjugated bonds in the chemical scaffold are one of the responsible factors for the light absorption at about 500 nm [5][11]. However, also the type of substituents present in the benzyl ring, local pH, state of aggregation and complexation with other inorganic and organic molecules may contribute to color variation. In particular, it has been observed that anthocyanins may display almost the chromatic scale [5][11][12].
2. Biosynthesis
Anthocyanidins and anthocyanins are almost exclusively produced by plants, in a branch of the phenylpropanoid pathway that is also involved in the biosynthesis of other flavonoids [13][14] (Figure 2). The enzymes involved in biosynthesis of anthocyanidins are localized in the endoplasmic reticulum, organized into a multi-enzyme complex named flavonoid metabolon [13][14]. The precursor for the synthesis of all flavonoids is the phenylalanine. This amino acid marks the branch point of primary and secondary metabolism from which the phenyl-propanoid pathway can lead to the synthesis of all phenolic compounds [13]. As first step of the pathway, phenylalanine is converted by phenylalanine ammonia-lyase (PAL) in cinnamic acid, which is then further transformed into coumaric acid by the action of cinnamic acid 4-hydroxylase (C4H). Following the activation catalyzed by the 4-coumarate-CoA ligase (4CL), 4-coumaryl-CoA reacts with three molecules of malonyl-CoA in a reaction catalyzed by chalcone synthase (CHS). This reaction allows the formation of 4-hydroxychalcone (ex. naringenin chalcone) and it marks the start of the flavonoid biosynthetic pathway. The 4-hydroxychalcone is transformed into the respective 7,3′,5′,trihydroxyl-flavone (ex. naringenin) by the action of chalcone isomerase (CHI). Afterwards, flavanone 3-hydroxylase (F3H) oxidizes 7,3′,5′,trihydroxyl-flavone into flavonol-form (ex. dihydrokaempferol). Then, dihydrokaempferol is transformed into dihydromyricetin or dihydroquercetin by the action of flavonoid 3′-hydroxylase (F3′H) or flavonoid 3′,5′-hydroxylase (F3′5′H), respectively. In order to convert the three hydroflavonols into anthocyanidins, the combined action of dihydroflavonol-4-reductase (DFR) and anthocyanidin synthase (ANS) is required. The first enzyme yields to the formation of the leucoanthocyanidins, meanwhile the second one catalyzes the 2-oxoglutaratedependent oxidation of each leucoanthocyanidin into 2-flavan-3,4-diol. These latter compounds spontaneously evolve to the respective anthocyanidins [15][16].
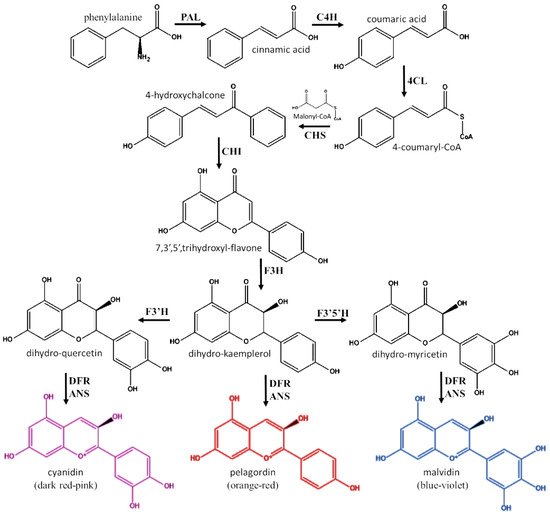
Figure 2. Biochemical pathway for the synthesis of anthocyanidins. PAL: phenylalanine ammonia-lyase; C4H: cinnamic acid 4-hydroxylase; 4CL: 4-coumarate-CoA ligase; CHS: chalcone synthase; CHI: chalcone isomerase; F3H: flavanone 3-hydroxylase; F3′H: flavonoid 3′-hydroxylase; F3′5′H: flavonoid 3′,5′-hydroxylase; DFR: dihydroflavonol reductase; ANS: anthocyanidin synthase (ANS).
After their synthesis, anthocyanins are transported to the plant vacuole through vesicle trafficking pathway that may involve, or not, Golgi apparatus [17]. In vacuole, anthocyanidins are converted into the more stable form by the action of UDP-glucose flavonoid 3-O-glucosyltransferase (UF3GT) or UDP-glucose flavonoid 5-O-glucosyltransferase (UF5GT). These two enzymes add a sugar moiety respectively at the 3rd and/or 5th position (R1 and/or R2 subsistent of the chemical structure displayed in Figure 1 of the chemical scaffold [5][6][15]. Finally, the glucoside form of anthocyanidins may be further modified in many species by glycosylation, methylation, acylation, or condensation with other organic molecules [15][16].
3. Role in Plants
Anthocyanins are one of the major groups of natural pigments and they are responsible for colors of many leaves, flowers, and fruits [18]. In the past the physiological role of anthocyanins in plants was exclusively ascribed to improve the reproductive success by facilitating communication between plants and pollinators or seed-dispersers [19]. On the other hand, in order to justify the occurrence of anthocyanins also in plant districts different from flowers and fruits, it was mistakenly assumed that they could be an incidental consequence of the flavonoid pathway [20]. Indeed, the intermediate compounds dihydrokaempferol, dihymyricetin, and dihyquercetin may alternatively be oxidized into respective flavon-3-ols by flavonol synthase (FLS) as well as used for the production of anthocyanins (Figure 3) [5][8]. However, it was shown that some parts of the plants devoid of immediate signaling function contained a considerable amount of these flavonoids [5][21][22]. On the other hand, anthocyanins have specific histological localization, and their accumulation patterns do not match those of other pigments [5][23].
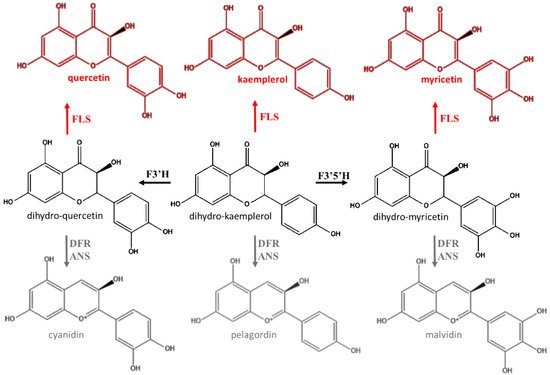
Figure 3. Alternative biochemical pathway to anthocyanin synthesis. FLS: flavonoid synthase; F3′H: flavonoid 3′-hydroxylase; F3′5′H: flavonoid 3′,5′-hydroxylase; DFR: dihydroflavonol reductase; ANS: anthocyanidin synthase (ANS).
For these reasons, recently, anthocyanin role in plants was questioned. To date, it is well-known that these molecules are involved in several defensive processes, including the screen role against UV-B [24][25][26][27][28] and plant protection against high light intensities [25][28][29]. However, light stresses are not the only abiotic stress in which anthocyanins seem to play a key role. Indeed, thanks to their high antioxidant capacity, these flavonoids are involved in all those responses that contrast oxidative stress induced by heat conditions [30][31] and water and nutrient deficit [30][32][33]. Moreover, anthocyanins are also involved in response to biotic stresses, such as mechanical damage due to herbivore attack [34][35][36], insect infestation or fungal infection [37][38][39]. Table 1 reports the main abiotic and biotic stress conditions in which variations of the total content of anthocyanins were observed.
Table 1. Documented plant responses to abiotic and biotic stresses that involves anthocyanins.
| Condition | Specie | References | |
|---|---|---|---|
| Abiotic Stress | Heat Stress | Ipomoea batatas | [22][40][41] |
| Daucus carota | [42] | ||
| Rosa hybrida | [43] | ||
| Solanum melongena | [44][45][46][47] | ||
| Saccharum officinarum | [48][49] | ||
| Camellia sinensis | [50] | ||
| Sorghum vulgare | [51] | ||
| Vitis vinifera | [52] | ||
| Oryza glaberrima | [53] | ||
| Actinidia deliciosa | [54] | ||
| Arabidopsis thaliana | [55] | ||
| Quercus suber | [56] | ||
| Light Stress | Solanum melongena | [57][58][59] | |
| Phalaenopsis aphrodite | [60] | ||
| Silene littorea | [24] | ||
| Arabidopsis thaliana | [26][61][62][63][64] | ||
| Chrysanthemum morifolium | [65] | ||
| Begonia semperflorens | [66] | ||
| Brassica campestris | [67] | ||
| Perilla frutescens | [68][69] | ||
| Lonicera japonica | [70] | ||
| Actinidia deliciosa | [54] | ||
| Malus domestica | [29][71] | ||
| Water Stress | Camellia sinensis | [72] | |
| Vitis vinifera | [30][73] | ||
| Hibiscus sabdariffa | [74] | ||
| Malus domestica | [75] | ||
| Fragaria ananassa | [76] | ||
| Ocimum basilicum | [33] | ||
| Sorghum vulgare | [77] | ||
| Oryza sativa | [78] | ||
| Punica granatum | [32] | ||
| Salt Stress | Arabidopsis thaliana | [79][80][81] | |
| Nicotiana tabaccum | [82] | ||
| Hibiscus rosasinensis | [83] | ||
| Fragaria chiloensis | [84] | ||
| Oryza sativa | [85] | ||
| Solanum tuberosum | [86] | ||
| Biotic Stress | Insect Attack | Arabidopsis thaliana | [34][35][36][87] |
| Gossypium arboreum | [88] | ||
| Solanum tuberosum | [89] | ||
| Sorghum halepense | [90] | ||
| Fragaria ananassa | [91] | ||
| Vaccinium myrtillus | [92] | ||
| Fungi Attack | Arabidopsis thaliana | [39][93][94] | |
| Oryza sativa | [78][85] | ||
| Fragaria ananassa | [38][95][96] | ||
Beyond the involvement of anthocyanins to contrast the oxidative stress conditions related to abiotic and biotic menaces, anthocyanins seem to be also able to contribute to the physiological processes during non-stress conditions, such as the elevation of leaf temperature [70][97]; transport of nutrients and monosaccharides [98][99][100]; and regulation of osmotic balance [30][84]. Table 2 reports the main plant physiological pathways in which anthocyanins are involved.
Table 2. Documented plant physiological processes in which anthocyanins are involved.
| Plant Physiological Role | Specie | References |
|---|---|---|
| Elevation of Leaf Temperature | Several species | [100][101][102][103][104][105] |
| Lactuca sativa | [106] | |
| Arabidopsis thaliana | [107][108] | |
| Galax urceolata | [109] | |
| Senescence | Several species | [110][111][112][113] |
| Populus euramericana | [114] | |
| Arabidopsis thaliana | [115][116] | |
| Brassica oleracea | [117] | |
| Actinidia deliciosa | [118] | |
| Torenia fournieri | [119] | |
| Transportation of Monosaccharides | Several Species | [98][99][100] |
| Zea mays | [120][121] | |
| Vitis vinifera | [122][123][124] | |
| Regulation of Osmotic Balance | Several species | [104][125][126] |
| Xerophyta viscosa | [127] | |
| Vitis vinifera | [128][129][130] | |
| Fragaria ananassa | [131] | |
| Populus deltoides | [132] | |
| Arabidopsis thaliana | [55][108] | |
| Craterostigma wilmsii | [127] | |
| Camouflage | Several Species | [102][133][134][135][136][137][138][139] |
| Theobroma cacao | [100] | |
| Mangifera indica | [100] | |
| Enhancing of Light Absorption | Several Species | [105][134][140][141][142] |
| Theobroma cacao | [100] | |
| Zea mays | [143] | |
| Mangifera indica | [100] |
4. Distribution in Edible Sources and Contribution in Human Diet
Fruits and vegetables are the only edible sources from which it is possible assuming anthocyanin compounds [4][144]. Although among the fruits the anthocyanin content is very variable, generally the level of anthocyanins in fruits is much higher than in vegetables [145]. The lowest anthocyanin content per 100 g of fresh weight was recorded for grapefruit [146][147], date [148], and fig [149], meanwhile some berries, such as cranberry [7], chokeberry [150], huckleberry [151], blueberry [152], raspberry [153][154], and bilberry [155][156] shows the highest one. Concerning vegetables, the most reach in anthocyanidins and anthocyanins are red cabbage [157][158][159], purple cabbage [160], and purple potato [40][161]. However, total anthocyanin content in fruits and vegetables considerably varies among the different genera and cultivars, and it is strongly affected by different light, temperature, and agronomic factors [162]. Figure 4 shows the cluster distribution of anthocyanins in plant kingdom according to the anthocyanin content reported in Phenol-Explorer Online Database [163][164][165]. For this analysis, Euclidean distances were calculated by using the average linkage method.
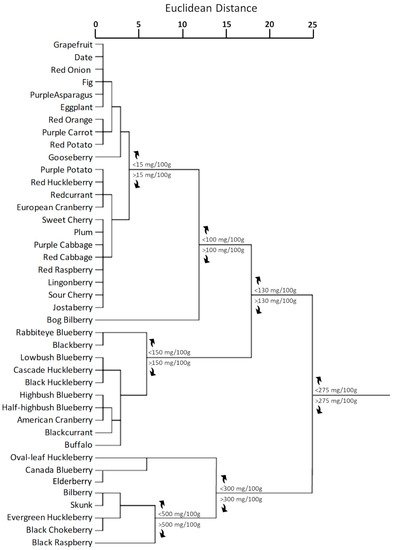
Figure 4. Cluster distribution of anthocyanins in plant kingdom based on the total anthocyanin content according to Phenol-Explorer Database [163][164][165]. Euclidean distances were calculated with average linkage method. Statistical analysis and graphical representation were made using SPSS v. 24 software. The cluster was generated by using SPSS ver.24 statistical software.
In the recent years, some flowers were proposed as alternative edible sources of phytochemicals. In order to be included in human diet, flowers have to be non-toxic and innocuous [166][167]. Indeed, flowers may contain toxic substances, including hemaglutinnins, oxalic acid, cyanogenic glycosides, or alkaloids and cause severe damage to the consumers [166]. However, many flowers can be considered safe, and therefore can be consumed as food. Although flowers are little known as edible sources, they have been used for over 500 years in Europe and China as herbal medicine [168]. Actually, they are mainly used for enhancing the aesthetic value of foods, as evidenced by the increasing number of edible flower cookbooks, culinary magazine articles, and dedicated television segments [169][170]. Despite edible flowers are still considered a niche product, they are gaining attention due to their exotic aroma and textures, delicate flavor, attractive color and phytochemical composition [171]. In particular, edible flowers are a potential source of several bioactive compounds, including anthocyanins [170][171][172]. Among them, begonia (Begonia tuberhybride), tagete (Tagetes patula), mini rose (Rosa chinensis), mini daisy (Bellis annua), litoria (Clitoria ternatea), cosmos (Cosmos sulphureus), and cravine (Dianthus chinensis) are the most known and commercialized [171].
Apart from their origins and physiological roles in plants, anthocyanidins and anthocyanins seem to play important roles in human health and well-being [7][5][156]. Indeed, their intaking through the consumption of foods rich in these flavonoid compounds seems to be linked to an improvement of the redox balance thanks to their high scavenging and reducing activities [7][162][173]. On the other hand, interesting properties, such as antitumor, antiatherogenic, antiviral, and anti-inflammatory effects, decrease of capillary permeability and fragility, inhibition of platelet aggregation and immune stimulation were reported [174]. The positive effects ascribed to the consumption of fruits and vegetables rich in anthocyanidins and anthocyanins are not limited to the gastro-intestinal tract. Indeed, anthocyanins resisting to gastric digestion may be absorbed in the stomach via bilitranslocase-mediated mechanism [175][176][177][178], or in the intestine through a mechanism involving the sodium–glucose co-transporter as suggested for other flavonoids [176][177][178][179][180].
References
- Brouillard, R. Chemical Structure of Anthocyanins; Academic Press: New York, NY, USA, 1982; Volume 1.
- Jordheim, M. Basic Anthocyanin Chemistry and Dietary Sources; CRC Press: Boca Raton, FL, USA, 2013; pp. 13–90. ISBN 978-1-4398-9471-2.
- Lawrence, W.J.C.; Price, J.R.; Robinson, G.M.; Robinson, R. The distribution of anthocyanins in flowers, fruits and leaves. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1939, 230, 149–178.
- Timberlake, C.F.; Bridle, P. Distribution of Anthocyanins in Food Plants; Academic Press: New York, NY, USA, 1982.
- Dini, C.; Zaro, M.J.; Viña, S.Z. Bioactivity and functionality of anthocyanins: A review. Curr. Bioact. Compd. 2018, 15, 507–523.
- Tang, B.; Li, L.; Hu, Z.; Chen, Y.; Tan, T.; Jia, Y.; Xie, Q.; Chen, G. Anthocyanin accumulation and transcriptional regulation of anthocyanin biosynthesis in purple pepper. J. Agric. Food Chem. 2020, 68, 12152–12163.
- Mannino, G.; Di Stefano, V.; Lauria, A.; Pitonzo, R.; Gentile, C. Vaccinium macrocarpon (cranberry)-based dietary supplements: Variation in mass uniformity, proanthocyanidin dosage and anthocyanin profile demonstrates quality control standard needed. Nutrients 2020, 12, 992.
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J. Plant Biochem. Physiol. 2017, 5, 1–9.
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99.
- Crozier, A.; Yokota, T.; Jaganath, I.B.; Marks, S.; Saltmarsh, M.; Clifford, M.N. Secondary Metabolites in Fruits, Vegetables, Beverages and Other Plant-based Dietary Components. In Plant Secondary Metabolites; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 208–302.
- Markakis, P. Anthocyanins as Food Colors; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 0323157904.
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871.
- Holton, T.A.; Cornish, E.C. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 1995, 7, 1071.
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749.
- Yonekura-Sakakibara, K.; Nakayama, T.; Yamazaki, M.; Saito, K. Modification and stabilization of anthocyanins. In Anthocyanins; Springer: Berlin, Germany, 2008; pp. 169–190.
- Passeri, V.; Koes, R.; Quattrocchio, F.M. New Challenges for the design of high value plant products: Stabilization of anthocyanins in plant vacuoles. Front. Plant Sci. 2016, 7, 153.
- Poustka, F.; Irani, N.G.; Feller, A.; Lu, Y.; Pourcel, L.; Frame, K.; Grotewold, E. A trafficking pathway for anthocyanins overlaps with the endoplasmic reticulum-to-vacuole protein-sorting route in Arabidopsis and contributes to the formation of vacuolar inclusions. Plant Physiol. 2007, 145, 1323–1335.
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Stanys, V.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From plant pigments to health benefits at mitochondrial level. Crit. Rev. Food Sci. Nutr. 2020, 60, 3352–3365.
- Rudall, P.J. Colourful cones: How did flower colour first evolve? J. Exp. Bot. 2020, 71, 759–767.
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains—Characteristics, biosynthesis, processing, and stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289.
- Rodrigues, R.F.; da Silva, P.F.; Shimizu, K.; Freitas, A.A.; Kovalenko, S.A.; Ernsting, N.P.; Quina, F.H.; Maçanita, A. Ultrafast internal conversion in a model anthocyanin-polyphenol complex: Implications for the biological role of anthocyanins in vegetative tissues of plants. Chemistry 2009, 15, 1397–1402.
- Fogelman, E.; Oren-Shamir, M.; Hirschberg, J.; Mandolino, G.; Parisi, B.; Ovadia, R.; Tanami, Z.; Faigenboim, A.; Ginzberg, I. Nutritional value of potato (Solanum tuberosum) in hot climates: Anthocyanins, carotenoids, and steroidal glycoalkaloids. Planta 2019, 249, 1143–1155.
- Steyn, W.J.J.; Wand, S.J.E.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361.
- Del Valle, J.C.; Buide, M.L.M.L.; Whittall, J.B.; Valladares, F.; Narbona, E. UV radiation increases phenolic compound protection but decreases reproduction in Silene littorea. PLoS ONE 2020, 15, e0231611.
- Foyer, C.H.; Lelandais, M.; Kunert, K.J. Photooxidative stress in plants. Physiol. Plant. 1994, 92, 696–717.
- Zheng, X.-T.T.; Yu, Z.-C.C.; Tang, J.-W.W.; Cai, M.-L.L.; Chen, Y.-L.L.; Yang, C.-W.W.; Chow, W.S.; Peng, C.-L.L. The major photoprotective role of anthocyanins in leaves of Arabidopsis thaliana under long-term high light treatment: Antioxidant or light attenuator? Photosynth. Res. 2020, 1–6.
- Barker, D.H.; Seaton, G.G.R.; Robinson, S.A. Internal and external photoprotection in developing leaves of the CAM plant Cotyledon orbiculata. Plant Cell Environ. 1997, 20, 617–624.
- Ko, S.-S.; Jhong, C.-M.; Lin, Y.-J.; Wei, C.-Y.; Lee, J.-Y.; Shih, M.-C. Blue light mediates chloroplast avoidance and enhances photoprotection of vanilla orchid. Int. J. Mol. Sci. 2020, 21, 8022.
- Merzlyak, M.N.; Chivkunova, O.B. Light-stress-induced pigment changes and evidence for anthocyanin photoprotection in apples. J. Photochem. Photobiol. B Biol. 2000, 55, 155–163.
- Zarrouk, O.; Brunetti, C.; Egipto, R.; Pinheiro, C.; Genebra, T.; Gori, A.; Lopes, C.M.; Tattini, M.; Chaves, M.M. Grape ripening is regulated by deficit irrigation/elevated temperatures according to cluster position in the canopy. Front. Plant Sci. 2016, 7, 1640.
- De Leonardis, A.M.; Fragasso, M.; Beleggia, R.; Ficco, D.B.M.; de Vita, P.; Mastrangelo, A.M. Effects of heat stress on metabolite accumulation and composition, and nutritional properties of durum wheat grain. Int. J. Mol. Sci. 2015, 16, 30382–30404.
- Mena, P.; Galindo, A.; Collado-González, J.; Ondoño, S.; García-Viguera, C.; Ferreres, F.; Torrecillas, A.; Gil-Izquierdo, A. Sustained deficit irrigation affects the colour and phytochemical characteristics of pomegranate juice. J. Sci. Food Agric. 2013, 93, 1922–1927.
- Luna, M.C.; Bekhradi, F.; Ferreres, F.; Jordán, M.J.; Delshad, M.; Gil, M.I. Effect of water stress and storage time on anthocyanins and other phenolics of different genotypes of fresh sweet basil. J. Agric. Food Chem. 2015, 63, 9223–9231.
- Qi, T.; Song, S.; Ren, Q.; Wu, D.; Huang, H.; Chen, Y.; Fan, M.; Peng, W.; Ren, C.; Xie, D. The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 2011, 23, 1795–1814.
- Song, S.; Qi, T.; Fan, M.; Zhang, X.; Gao, H.; Huang, H.; Wu, D.; Guo, H.; Xie, D. The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet. 2013, 9, e1003653.
- Nakata, M.; Mitsuda, N.; Herde, M.; Koo, A.J.K.; Moreno, J.E.; Suzuki, K.; Howe, G.A.; Ohme-Takagi, M. A bHLH-type transcription factor, aba-inducible bhlh-type transcription factor/ja-associated myc2-like1, acts as a repressor to negatively regulate jasmonate signaling in arabidopsis. Plant Cell 2013, 25, 1641–1656.
- Khonkhaeng, B.; Cherdthong, A. Improving nutritive value of purple field corn residue and rice straw by culturing with white-rot fungi. J. Fungi 2020, 6, 69.
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria x ananassa var. Selva) in conditions of reduced fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225.
- Wang, L.; Wang, B.; Yu, H.; Guo, H.; Lin, T.; Kou, L.; Wang, A.; Shao, N.; Ma, H.; Xiong, G.; et al. Transcriptional regulation of strigolactone signalling in Arabidopsis. Nature 2020, 583, 277–281.
- Sakatani, M.; Suda, I.; Oki, T.; Kobayashi, S.; Kobayashi, S.; Takahashi, M. Effects of purple sweet potato anthocyanins on development and intracellular redox status of bovine preimplantation embryos exposed to heat shock. J. Reprod. Dev. 2007, 53, 605–614.
- Liu, Y.; Lin-Wang, K.; Espley, R.V.; Wang, L.; Li, Y.; Liu, Z.; Zhou, P.; Zeng, L.; Zhang, X.; Zhang, J. StMYB44 negatively regulates anthocyanin biosynthesis at high temperatures in tuber flesh of potato. J. Exp. Bot. 2019, 70, 3809–3824.
- Commisso, M.; Toffali, K.; Strazzer, P.; Stocchero, M.; Ceoldo, S.; Baldan, B.; Levi, M.; Guzzo, F. Impact of phenylpropanoid compounds on heat stress tolerance in carrot cell cultures. Front. Plant Sci. 2016, 7, 1439.
- Dela, G.; Or, E.; Ovadia, R.; Nissim-Levi, A.; Weiss, D.; Oren-Shamir, M. Changes in anthocyanin concentration and composition in ‘Jaguar’rose flowers due to transient high-temperature conditions. Plant Sci. 2003, 164, 333–340.
- Wu, X.; Zhang, S.; Liu, X.; Shang, J.; Zhang, A.; Zhu, Z.; Zha, D. Chalcone synthase (CHS) family members analysis from eggplant (Solanum melongena L.) in the flavonoid biosynthetic pathway and expression patterns in response to heat stress. PLoS ONE 2020, 15, e0226537.
- Zhang, S.; Zhang, A.; Wu, X.; Zhu, Z.; Yang, Z.; Zhu, Y.; Zha, D. Transcriptome analysis revealed expression of genes related to anthocyanin biosynthesis in eggplant (Solanum melongena L.) under high-temperature stress. BMC Plant Biol. 2019, 19, 1–13.
- Wu, X.; Zhang, A.; Zhu, Z.; Yao, J.; Zha, D.; Li, X. Effects of high-temperature stress on active oxygen metabolism, anthocyanin content and its main synthases in eggplant peel. Acta Agric. Jiangxi 2018, 30, 1–5.
- Zhang, A.; Zhu, Z.; Shang, J.; Zhang, S.; Shen, H.; Wu, X.; Zha, D. Transcriptome profiling and gene expression analyses of eggplant (Solanum melongena L.) under heat stress. PLoS ONE 2020, 15, e0236980.
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228.
- Shao, L.; Shu, Z.; Sun, S.; Peng, C.; Wang, X.; Lin, Z. Antioxidation of anthocyanins in photosynthesis under high temperature stress. J. Integr. Plant Biol. 2007, 49, 1341–1351.
- Shen, J.; Zhang, D.; Zhou, L.; Zhang, X.; Liao, J.; Duan, Y.; Wen, B.; Ma, Y.; Wang, Y.; Fang, W.; et al. Transcriptomic and metabolomic profiling of Camellia sinensis L. cv. “Suchazao” exposed to temperature stresses reveals modification in protein synthesis and photosynthetic and anthocyanin biosynthetic pathways. Tree Physiol. 2019, 39, 1583–1599.
- Chopra, R.; Burow, G.; Burke, J.J.; Gladman, N.; Xin, Z. Genome-wide association analysis of seedling traits in diverse Sorghum germplasm under thermal stress. BMC Plant Biol. 2017, 17, 12.
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (vitis vinifera) fruit. BMC Plant Biol. 2014, 14, 108.
- Zhang, X.; Rerksiri, W.; Liu, A.; Zhou, X.; Xiong, H.; Xiang, J.; Chen, X.; Xiong, X. Transcriptome profile reveals heat response mechanism at molecular and metabolic levels in rice flag leaf. Gene 2013, 530, 185–192.
- Yu, M.; Man, Y.; Wang, Y. Light- and temperature-induced expression of an R2R3-MYB gene regulates anthocyanin biosynthesis in red-fleshed kiwifruit. Int. J. Mol. Sci. 2019, 20, 5228.
- Kim, S.; Hwang, G.; Lee, S.; Zhu, J.-Y.; Paik, I.; Nguyen, T.T.; Kim, J.; Oh, E. High ambient temperature represses anthocyanin biosynthesis through degradation of HY5. Front. Plant Sci. 2017, 8, 1787.
- Correia, B.; Rodriguez, J.L.; Valledor, L.; Almeida, T.; Santos, C.; Cañal, M.J.; Pinto, G. Analysis of the expression of putative heat-stress related genes in relation to thermotolerance of cork oak. J. Plant Physiol. 2014, 171, 399–406.
- Li, J.; Ren, L.; Gao, Z.; Jiang, M.; Liu, Y.; Zhou, L.; He, Y.; Chen, H. Combined transcriptomic and proteomic analysis constructs a new model for light-induced anthocyanin biosynthesis in eggplant (Solanum melongena L.). Plant. Cell Environ. 2017, 40, 3069–3087.
- Jiang, M.; Ren, L.; Lian, H.; Liu, Y.; Chen, H. Novel insight into the mechanism underlying light-controlled anthocyanin accumulation in eggplant (Solanum melongena L.). Plant Sci. 2016, 249, 46–58.
- Li, J.; He, Y.-J.; Zhou, L.; Liu, Y.; Jiang, M.; Ren, L.; Chen, H. Transcriptome profiling of genes related to light-induced anthocyanin biosynthesis in eggplant (Solanum melongena L.) before purple color becomes evident. BMC Genom. 2018, 19, 1–12.
- Ko, S.-S.; Jhong, C.-M.; Shih, M.-C. Blue light acclimation reduces the photoinhibition of phalaenopsis aphrodite (moth Orchid). Int. J. Mol. Sci. 2020, 21, 6167.
- Koyama, T.; Sato, F. The function of ETHYLENE RESPONSE FACTOR genes in the light-induced anthocyanin production of Arabidopsis thaliana leaves. Plant Biotechnol. 2018, 35, 87–91.
- Lotkowska, M.E.; Tohge, T.; Fernie, A.R.; Xue, G.-P.; Balazadeh, S.; Mueller-Roeber, B. The Arabidopsis transcription factor MYB112 promotes anthocyanin formation during salinity and under high light stress. Plant Physiol. 2015, 169, 1862–1880.
- Woo, N.S.; Gordon, M.J.; Graham, S.R.; Rossel, J.B.; Badger, M.R.; Pogson, B.J. A mutation in the purine biosynthetic enzyme ATASE2 impacts high light signalling and acclimation responses in green and chlorotic sectors of Arabidopsis leaves. Funct. Plant Biol. 2011, 38, 401–419.
- Pfab, A.; Breindl, M.; Grasser, K.D. The Arabidopsis histone chaperone FACT is required for stress-induced expression of anthocyanin biosynthetic genes. Plant Mol. Biol. 2018, 96, 367–374.
- Hong, Y.; Li, M.; Dai, S. iTRAQ-based protein profiling provides insights into the mechanism of light-induced anthocyanin biosynthesis in chrysanthemum (Chrysanthemum × morifolium). Genes 2019, 10, 1024.
- Wang, J.; Guo, M.; Li, Y.; Wu, R.; Zhang, K. High-throughput transcriptome sequencing reveals the role of anthocyanin metabolism in begonia semperflorens under high light stress. Photochem. Photobiol. 2018, 94, 105–114.
- Zhu, H.; Li, X.; Zhai, W.; Liu, Y.; Gao, Q.; Liu, J.; Ren, L.; Chen, H.; Zhu, Y. Effects of low light on photosynthetic properties, antioxidant enzyme activity, and anthocyanin accumulation in purple pak-choi (Brassica campestris ssp. Chinensis Makino). PLoS ONE 2017, 12, e0179305.
- Miki, S.; Wada, K.C.; Takeno, K. A possible role of an anthocyanin filter in low-intensity light stress-induced flowering in Perilla frutescens var. crispa. J. Plant Physiol. 2015, 175, 157–162.
- Wada, K.C.; Kondo, H.; Takeno, K. Obligatory short-day plant, Perilla frutescens var. crispa can flower in response to low-intensity light stress under long-day conditions. Physiol. Plant. 2010, 138, 339–345.
- Carpenter, K.L.; Keidel, T.S.; Pihl, M.C.; Hughes, N.M. Support for a photoprotective function of winter leaf reddening in nitrogen-deficient individuals of Lonicera japonica. Molecules 2014, 19, 17810–17828.
- Steyn, W.J.; Wand, S.J.E.; Jacobs, G.; Rosecrance, R.C.; Roberts, S.C. Evidence for a photoprotective function of low-temperature-induced anthocyanin accumulation in apple and pear peel. Physiol. Plant. 2009, 136, 461–472.
- Cheruiyot, E.K.; Mumera, L.M.; Ng’etich, W.K.; Hassanali, A.; Wachira, F. Polyphenols as potential indicators for drought tolerance in tea (Camellia sinensis L.). Biosci. Biotechnol. Biochem. 2007, 71, 707310505.
- Griesser, M.; Weingart, G.; Schoedl-Hummel, K.; Neumann, N.; Becker, M.; Varmuza, K.; Liebner, F.; Schuhmacher, R.; Forneck, A. Severe drought stress is affecting selected primary metabolites, polyphenols, and volatile metabolites in grapevine leaves (Vitis vinifera cv. Pinot noir). Plant Physiol. Biochem. 2015, 88, 17–26.
- Hinojosa-Gómez, J.; San Martín-Hernández, C.; Heredia, J.B.; León-Félix, J.; Osuna-Enciso, T.; Muy-Rangel, M.D. Anthocyanin induction by drought stress in the calyx of roselle cultivars. Molecules 2020, 25, 1555.
- Faghih, S.; Zamani, Z.; Fatahi, R.; Liaghat, A. Effects of deficit irrigation and kaolin application on vegetative growth and fruit traits of two early ripening apple cultivars. Biol. Res. 2019, 52, 43.
- Adak, N.; Gubbuk, H.; Tetik, N. Yield, quality and biochemical properties of various strawberry cultivars under water stress. J. Sci. Food Agric. 2018, 98, 304–311.
- Kamali, S.; Mehraban, A. Nitroxin and arbuscular mycorrhizal fungi alleviate negative effects of drought stress on Sorghum bicolor yield through improving physiological and biochemical characteristics. Plant Signal. Behav. 2020, 15, 1813998.
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Phisalaphong, M.; Singh, H.P.; Cha-Um, S. Promoting water deficit tolerance and anthocyanin fortification in pigmented rice cultivar (Oryza sativa L. subsp. indica) using arbuscular mycorrhizal fungi inoculation. Physiol. Mol. Biol. Plants Int. J. Funct. Plant Biol. 2019, 25, 821–835.
- Loubser, J.; Hills, P. The application of a commercially available citrus-based extract mitigates moderate NaCl-Stress in arabidopsis thaliana plants. Plants 2020, 9, 1010.
- Jeong, C.Y.; Lee, W.J.; Truong, H.A.; Trịnh, C.S.; Jin, J.Y.; Kim, S.; Hwang, K.Y.; Kang, C.-S.; Moon, J.-K.; Hong, S.-W.; et al. Dual role of SND1 facilitates efficient communication between abiotic stress signalling and normal growth in Arabidopsis. Sci. Rep. 2018, 8, 10114.
- Van Oosten, M.J.; Sharkhuu, A.; Batelli, G.; Bressan, R.A.; Maggio, A. The Arabidopsis thaliana mutant air1 implicates SOS3 in the regulation of anthocyanins under salt stress. Plant Mol. Biol. 2013, 83, 405–415.
- Naing, A.H.; Park, K.I.; Ai, T.N.; Chung, M.Y.; Han, J.S.; Kang, Y.-W.; Lim, K.B.; Kim, C.K. Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biol. 2017, 17, 65.
- Trivellini, A.; Gordillo, B.; Rodríguez-Pulido, F.J.; Borghesi, E.; Ferrante, A.; Vernieri, P.; Quijada-Morín, N.; González-Miret, M.L.; Heredia, F.J. Effect of salt stress in the regulation of anthocyanins and color of hibiscus flowers by digital image analysis. J. Agric. Food Chem. 2014, 62, 6966–6974.
- Garriga, M.; Retamales, J.B.; Romero-Bravo, S.; Caligari, P.D.S.; Lobos, G.A. Chlorophyll, anthocyanin, and gas exchange changes assessed by spectroradiometry in Fragaria chiloensis under salt stress. J. Integr. Plant Biol. 2014, 56, 505–515.
- Tisarum, R.; Theerawitaya, C.; Samphumphuang, T.; Polispitak, K.; Thongpoem, P.; Singh, H.P.; Cha-Um, S. Alleviation of salt stress in upland rice (Oryza sativa L. ssp. indica cv. Leum Pua) using arbuscular mycorrhizal fungi inoculation. Front. Plant Sci. 2020, 11, 348.
- Cheng, Y.-J.; Kim, M.-D.; Deng, X.-P.; Kwak, S.-S.; Chen, W. Enhanced salt stress tolerance in transgenic potato plants expressing IbMYB1, a sweet potato transcription factor. J. Microbiol. Biotechnol. 2013, 23, 1737–1746.
- Appel, H.M.; Cocroft, R.B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 2014, 175, 1257–1266.
- Li, X.; Ouyang, X.; Zhang, Z.; He, L.; Wang, Y.; Li, Y.; Zhao, J.; Chen, Z.; Wang, C.; Ding, L. Over-expression of the red plant gene R1 enhances anthocyanin production and resistance to bollworm and spider mite in cotton. Mol. Genet. Genom. 2019, 294, 469–478.
- Chen, S.; Lin, I.W.; Chen, X.; Huang, Y.; Chang, S.; Lo, H.; Lu, H.; Yeh, K. Sweet potato NAC transcription factor, Ib NAC 1, upregulates sporamin gene expression by binding the SWRE motif against mechanical wounding and herbivore attack. Plant J. 2016, 86, 234–248.
- Costa-Arbulú, C.; Gianoli, E.; Gonzáles, W.L.; Niemeyer, H.M. Feeding by the aphid Sipha flava produces a reddish spot on leaves of Sorghum halepense: An induced defense. J. Chem. Ecol. 2001, 27, 273–283.
- Fischer, T.C.; Mirbeth, B.; Rentsch, J.; Sutter, C.; Ring, L.; Flachowsky, H.; Habegger, R.; Hoffmann, T.; Hanke, M.; Schwab, W. Premature and ectopic anthocyanin formation by silencing of anthocyanidin reductase in strawberry (Fragaria × ananassa). New Phytol. 2014, 201, 440–451.
- Koski, T.-M.; Kalpio, M.; Laaksonen, T.; Sirkiä, P.M.; Kallio, H.P.; Yang, B.; Linderborg, K.M.; Klemola, T. Effects of insect herbivory on bilberry production and removal of berries by frugivores. J. Chem. Ecol. 2017, 43, 422–432.
- Liu, Y.; Li, M.; Li, T.; Chen, Y.; Zhang, L.; Zhao, G.; Zhuang, J.; Zhao, W.; Gao, L.; Xia, T. Airborne fungus-induced biosynthesis of anthocyanins in Arabidopsis thaliana via jasmonic acid and salicylic acid signaling. Plant Sci. 2020, 300, 110635.
- Venneman, J.; Vandermeersch, L.; Walgraeve, C.; Audenaert, K.; Ameye, M.; Verwaeren, J.; Steppe, K.; Van Langenhove, H.; Haesaert, G.; Vereecke, D.; et al. Respiratory CO(2) combined with a blend of volatiles emitted by endophytic serendipita strains strongly stimulate growth of arabidopsis implicating auxin and cytokinin signaling. Front. Plant Sci. 2020, 11, 544435.
- Todeschini, V.; AitLahmidi, N.; Mazzucco, E.; Marsano, F.; Gosetti, F.; Robotti, E.; Bona, E.; Massa, N.; Bonneau, L.; Marengo, E.; et al. Impact of beneficial microorganisms on strawberry growth, fruit production, nutritional quality, and volatilome. Front. Plant Sci. 2018, 9, 1611.
- Parada, J.; Valenzuela, T.; Gómez, F.; Tereucán, G.; García, S.; Cornejo, P.; Winterhalter, P.; Ruiz, A. Effect of fertilization and arbuscular mycorrhizal fungal inoculation on antioxidant profiles and activities in Fragaria ananassa fruit. J. Sci. Food Agric. 2019, 99, 1397–1404.
- Teixeira, C.S.P.; Lucas, R.J.; Olykan, S.T.; Moot, D.J. Causes of leaf reddening in subterranean clover cultivars. New Zeal. J. Agric. Res. 2020, 63, 315–331.
- Matile, P. Biochemistry of Indian summer: Physiology of autumnal leaf coloration. Exp. Gerontol. 2000, 35, 145–158.
- Wagner, R.J.; Wagner, A.B.; Howard, R.A. The ecology of an elfin forest in Puerto Rico, 9. Chemical studies of colored leaves. J. Arnold Arbor. 1969, 50, 556–565.
- Lee, D.W.; Brammeier, S.; Smith, A.P. The selective advantages of anthocyanins in developing leaves of mango and cacao. Biotropica 1987, 19, 40–49.
- Sturgeon, K.B.; Mitton, J.B. Cone color polymorphism associated with elevation in white fir, Abies concolor, in southern Colorado. Am. J. Bot. 1980, 67, 1040–1045.
- McClure, J.W. Physiology and functions of flavonoids. In The Flavonoids; Springer: Berlin, Germany, 1975; pp. 970–1055.
- Saunders, J.A.; McClure, J.W. The distribution of flavonoids in chloroplasts of twenty five species of vascular plants. Phytochemistry 1976, 15, 809–810.
- Chalker-Scott, L.; Chalker-Scott, L. Environmental significance of anthocyanins in plant stress responses. Photochem. Photobiol. 1999, 70, 1–9.
- Weger, H.G.; Silim, S.N.; Guy, R.D. Photosynthetic acclimation to low temperature by western red cedar seedlings. Plant. Cell Environ. 1993, 16, 711–717.
- Simko, I.; Hayes, R.J.; Furbank, R.T. Non-destructive phenotyping of lettuce plants in early stages of development with optical sensors. Front. Plant Sci. 2016, 7, 1985.
- Wingler, A.; Tijero, V.; Müller, M.; Yuan, B.; Munné-Bosch, S. Interactions between sucrose and jasmonate signalling in the response to cold stress. BMC Plant Biol. 2020, 20, 176.
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585.
- Hughes, N.M.; Neufeld, H.S.; Burkey, K.O. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol. 2005, 168, 575–587.
- Wen, B.; Xiao, W.; Mu, Q.; Li, D.; Chen, X.; Wu, H.; Li, L.; Peng, F. How does nitrate regulate plant senescence? Plant Physiol. Biochem. PPB 2020, 157, 60–69.
- Masclaux-Daubresse, C. Autophagy controls carbon, nitrogen, and redox homeostasis in plants. Autophagy 2016, 12, 896–897.
- Thomas, H.; Huang, L.; Young, M.; Ougham, H. Evolution of plant senescence. BMC Evol. Biol. 2009, 9, 163.
- Hoch, W.A.; Zeldin, E.L.; McCown, B.H. Physiological significance of anthocyanins during autumnal leaf senescence. Tree Physiol. 2001, 21, 1–8.
- Tallis, M.J.; Lin, Y.; Rogers, A.; Zhang, J.; Street, N.R.; Miglietta, F.; Karnosky, D.F.; De Angelis, P.; Calfapietra, C.; Taylor, G. The transcriptome of populus in elevated CO reveals increased anthocyanin biosynthesis during delayed autumnal senescence. New Phytol. 2010, 186, 415–428.
- Dhar, N.; Caruana, J.; Erdem, I.; Subbarao, K.V.; Klosterman, S.J.; Raina, R. The Arabidopsis senescence-associated gene 13 regulates dark-induced senescence and plays contrasting roles in defense against bacterial and fungal pathogens. Mol. Plant. Microbe. Interact. 2020, 33, 754–766.
- Masclaux-Daubresse, C.; Clément, G.; Anne, P.; Routaboul, J.-M.; Guiboileau, A.; Soulay, F.; Shirasu, K.; Yoshimoto, K. Stitching together the multiple dimensions of autophagy using metabolomics and transcriptomics reveals impacts on metabolism, development, and plant responses to the environment in Arabidopsis. Plant Cell 2014, 26, 1857–1877.
- Bárcena, A.; Martínez, G.; Costa, L. Low intensity light treatment improves purple kale (Brassica oleracea var. sabellica) postharvest preservation at room temperature. Heliyon 2019, 5, e02467.
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 426.
- Nagira, Y.; Ikegami, K.; Koshiba, T.; Ozeki, Y. Effect of ABA upon anthocyanin synthesis in regenerated torenia shoots. J. Plant Res. 2006, 119, 137–144.
- Botha, C.E.J.; Cross, R.H.M.; Van Bel, A.J.E.; Peter, C.I. Phloem loading in the sucrose-export-defective (SXD-1) mutant maize is limited by callose deposition at plasmodesmata in bundle sheath—Vascular parenchyma interface. Protoplasma 2000, 214, 65–72.
- Russin, W.A.; Evert, R.F.; Vanderveer, P.J.; Sharkey, T.D.; Briggs, S.P. Modification of a specific class of plasmodesmata and loss of sucrose export ability in the sucrose export defective1 maize mutant. Plant Cell 1996, 8, 645–658.
- Castellarin, S.D.; Gambetta, G.A.; Wada, H.; Shackel, K.A.; Matthews, M.A. Fruit ripening in Vitis vinifera: Spatiotemporal relationships among turgor, sugar accumulation, and anthocyanin biosynthesis. J. Exp. Bot. 2011, 62, 4345–4354.
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 2014, 65, 821–832.
- Boss, P.K.; Davies, C. Molecular biology of sugar and anthocyanin accumulation in grape berries. In Molecular Biology & Biotechnology of the Grapevine; Springer: Berlin, Germany, 2001; pp. 1–33.
- Chalker-Scott, L. Do anthocyanins function as osmoregulators in leaf tissues? Adv. Bot. Res. 2002, 37, 104–129.
- Hughes, N.M.; Reinhardt, K.; Feild, T.S.; Gerardi, A.R.; Smith, W.K. Association between winter anthocyanin production and drought stress in angiosperm evergreen species. J. Exp. Bot. 2010, 61, 1699–1709.
- Sherwin, H.W.; Farrant, J.M. Protection mechanisms against excess light in the resurrection plants Craterostigma wilmsii and Xerophyta viscosa. Plant Growth Regul. 1998, 24, 203–210.
- Roby, G.; Harbertson, J.F.; Adams, D.A.; Matthews, M.A. Berry size and vine water deficits as factors in winegrape composition: Anthocyanins and tannins. Aust. J. Grape Wine Res. 2004, 10, 100–107.
- Hirasuna, T.J.; Shuler, M.L.; Lackney, V.K.; Spanswick, R.M. Enhanced anthocyanin production in grape cell cultures. Plant Sci. 1991, 78, 107–120.
- Suzuki, M. Enhancement of anthocyanin accumulation by high osmotic stress and low pH in grape cells (Vitis hybrids). J. Plant Physiol. 1995, 147, 152–155.
- Sato, K.; Nakayama, M.; Shigeta, J. Culturing conditions affecting the production of anthocyanin in suspended cell cultures of strawberry. Plant Sci. 1996, 113, 91–98.
- Tholakalabavi, A.; Zwiazek, J.J.; Thorpe, T.A. Osmotically-stressed poplar cell cultures: Anthocyanin accumulation, deaminase activity, and solute composition. J. Plant Physiol. 1997, 151, 489–496.
- Janzen, D.H. New Horizons in the Biology of Plant Defenses; Cmabridge University Press: Cambridge, UK, 1979.
- Gould, K.S.; Kuhn, D.N.; Lee, D.W.; Oberbauer, S.F. Why leaves are sometimes red. Nature 1995, 378, 241–242.
- Feild, T.S.; Lee, D.W.; Holbrook, N.M. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol. 2001, 127, 566–574.
- Hamilton, W.D.; Brown, S.P. Autumn tree colours as a handicap signal. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 1489–1493.
- Coley, P.D.; Aide, T.M. Red coloration of tropical young leaves: A possible antifungal defence? J. Trop. Ecol. 1989, 5, 293–300.
- Stone, B.C. Protective Coloration of Young Leaves in Certain Malaysian Palms. Biotropica 1979, 11, 26.
- Givnish, T.J. Leaf mottling: Relation to growth form and leaf phenology and possible role as camouflage. Funct. Ecol. 1990, 463–474.
- Lee, D.W. The spectral distribution of radiation in two neotropical rainforests. Biotropica 1987, 19, 161–166.
- Close, D.C.; Beadle, C.L. The ecophysiology of foliar anthocyanin. Bot. Rev. 2003, 69, 149–161.
- Neill, S.; Gould, K.S. Optical properties of leaves in relation to anthocyanin concentration and distribution. Can. J. Bot. 2000, 77, 1777–1782.
- Pietrini, F.; Iannelli, M.A.; Massacci, A. Anthocyanin accumulation in the illuminated surface of maize leaves enhances protection from photo-inhibitory risks at low temperature, without further limitation to photosynthesis. Plant. Cell Environ. 2002, 25, 1251–1259.
- Mannino, G.; Perrone, A.; Campobenedetto, C.; Schittone, A.; Margherita Bertea, C.; Gentile, C. Phytochemical profile and antioxidative properties of Plinia trunciflora fruits: A new source of nutraceuticals. Food Chem. 2020, 307, 125515.
- Mazza, G. Anthocyanins in Fruits, Vegetables, and Grains; CRC Press: Boca Raton, FL, USA, 2018; ISBN 135108660X.
- Aadil, R.M.; Zeng, X.-A.; Jabbar, S.; Nazir, A.; Mann, A.A.; Khan, M.K.I.; Ramzan, A. Quality evaluation of grapefruit juice by thermal and high pressure processing treatment. Pak. J. Agric. Res. 2017, 30, 249–257.
- Chong, M.F.F.; MacDonald, R.; Lovegrove, J.A. Fruit polyphenols and CVD risk: A review of human intervention studies. Br. J. Nutr. 2010, 104, S28–S39.
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of antioxidant activity, anthocyanins, carotenoids, and phenolics of three native fresh and sun-dried date (Phoenix dactylifera L.) varieties grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599.
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723.
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)–A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634.
- Francis, F.J.; Harborne, J.B. Anthocyanins of the garden huckleberry, Solanum guineese. J. Food Sci. 1966, 31, 524–528.
- Morissette, A.; Kropp, C.; Songpadith, J.-P.; Junges Moreira, R.; Costa, J.; Mariné Casadó, R.; Pilon, G.; Varin, T.V.; Dudonné, S.; Boutekrabt, L. Blueberry proanthocyanidins and anthocyanins improve metabolic health through a gut microbiota-dependent mechanism in diet-induced obese mice. Am. J. Physiol. Metab. 2020, 318, E965–E980.
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560.
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405.
- Zhang, Z.; Kou, X.; Fugal, K.; McLaughlin, J. Comparison of HPLC methods for determination of anthocyanins and anthocyanidins in bilberry extracts. J. Agric. Food Chem. 2004, 52, 688–691.
- Vigliante, I.; Mannino, G.; Maffei, M.E. OxiCyan®, a phytocomplex of bilberry (Vaccinium myrtillus) and spirulina (Spirulina platensis), exerts both direct antioxidant activity and modulation of ARE/Nrf2 pathway in HepG2 cells. J. Funct. Foods 2019, 61, 103508.
- Wiczkowski, W.; Szawara-Nowak, D.; Topolska, J. Red cabbage anthocyanins: Profile, isolation, identification, and antioxidant activity. Food Res. Int. 2013, 51, 303–309.
- McDougall, G.J.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red cabbage–stability to simulated gastrointestinal digestion. Phytochemistry 2007, 68, 1285–1294.
- Yuan, Y.; Chiu, L.-W.; Li, L. Transcriptional regulation of anthocyanin biosynthesis in red cabbage. Planta 2009, 230, 1141.
- He, Q.; Zhang, Z.; Zhang, L. Anthocyanin accumulation, antioxidant ability and stability, and a transcriptional analysis of anthocyanin biosynthesis in purple heading Chinese cabbage (Brassica rapa L. ssp. pekinensis). J. Agric. Food Chem. 2016, 64, 132–145.
- Han, K.-H.; Sekikawa, M.; Shimada, K.; Hashimoto, M.; Hashimoto, N.; Noda, T.; Tanaka, H.; Fukushima, M. Anthocyanin-rich purple potato flake extract has antioxidant capacity and improves antioxidant potential in rats. Br. J. Nutr. 2006, 96, 1125–1134.
- Mannino, G.; Gentile, C.; Maffei, M.E. Chemical partitioning and DNA fingerprinting of some pistachio (Pistacia vera L.) varieties of different geographical origin. Phytochemistry 2019, 160, 40–47.
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024.
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Knox, C.; Llorach, R.; Eisner, R.; Cruz, J.; Neveu, V.; Wishart, D.; Manach, C.; et al. Phenol-Explorer 2.0: A major update of the phenol-explorer database integrating data on polyphenol metabolism and pharmacokinetics in humans and experimental animals. Database 2012, 2012, bas031.
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070.
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.B.; Periago, M.J. Nutritional composition and antioxidant capacity in edible flowers: Characterisation of phenolic compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2015, 16, 805–822.
- Chaiyasut, C.; Sivamaruthi, B.S.; Pengkumsri, N.; Sirilun, S.; Peerajan, S.; Chaiyasut, K.; Kesika, P. Anthocyanin profile and its antioxidant activity of widely used fruits, vegetables, and flowers in Thailand. Asian J. Pharm. Clin. Res. 2016, 9, 218–224.
- Zheng, J.; Meenu, M.; Xu, B. A systematic investigation on free phenolic acids and flavonoids profiles of commonly consumed edible flowers in China. J. Pharm. Biomed. Anal. 2019, 172, 268–277.
- Acikgoz, F.E. Edible flowers. J. Exp. Agric. Int. 2017, 1–5.
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50.
- De Morais, J.S.; Sant’Ana, A.S.; Dantas, A.M.; Silva, B.S.; Lima, M.S.; Borges, G.C.; Magnani, M. Antioxidant activity and bioaccessibility of phenolic compounds in white, red, blue, purple, yellow and orange edible flowers through a simulated intestinal barrier. Food Res. Int. 2020, 131, 109046.
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants–A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569.
- Jakobek, L.; Šeruga, M.; Medvidović-Kosanović, M.; Novak, I. Anthocyanin content and antioxidant activity of various red fruit juices. Dtsch. Leb. 2007, 103, 58–64.
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phyther. Res. 2016, 30, 1265–1286.
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003, 544, 210–213.
- Hribar, U.; Poklar Ulrih, N. The metabolism of anthocyanins. Curr. Drug Metab. 2014, 15, 3–13.
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508.
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520.
- Kay, C.D. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutr. Res. Rev. 2006, 19, 137–146.
- Blaschek, W. Natural products as lead compounds for sodium glucose cotransporter (SGLT) inhibitors. Planta Med. 2017, 83, 985–993.




