
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shuai Zhang | + 1415 word(s) | 1415 | 2021-05-07 11:39:11 | | | |
| 2 | Rita Xu | Meta information modification | 1415 | 2021-05-14 05:17:08 | | |
Video Upload Options
Catheter-associated urinary tract infections (CAUTIs) are among the leading nosocomial infections in the world and have led to the extensive study of various strategies to prevent infection. However, despite an abundance of anti-infection materials having been studied over the last forty-five years, only a few types have come into clinical use, providing an insignificant reduction in CAUTIs. Marine resources have emerged as an unexplored area of opportunity offering huge potential in discovering novel bioactive materials to combat human diseases. To date, some marine microbial-derived materials have exhibited potent antimicrobial, antiadhesive and antibiofilm activity against a broad spectrum of uropathogens (including multidrug-resistant pathogens) that could be potentially used in urinary catheters to eradicate CAUTIs.
1. Introduction
Urinary catheters are hollow, partially flexible tubes that are designed to drain urine from the bladder. The earliest use of urinary catheters can be traced back to the third century B.C., but the modern indwelling catheter, called the ‘Foley’ catheter, was designed by Frederick B. Foley in the mid-1930s [1]. To date, over 100 million urinary catheters are used worldwide per year since catheterization rates remain high at 20% in non-intensive care units and 61% in intensive care units (ICUs) [2]. Despite the care taken to avoid contamination, catheters are still susceptible to infections as they provide direct access for uropathogens from the outside environment into the urinary tract, impairing local host defence mechanisms of the bladder [3][4]. Opportunistic uropathogens (Table 1) are mainly faecal or skin microbiota from the patients that can enter the bladder through the catheter lumen (34%) or along the catheter–urethral interface (66%) causing infections and complications, such as encrustation, bladder stones, bacteriuria, pyelonephritis, septicaemia and endotoxic shock (Figure 1) [3][5][6][7]. According to the European Centre for Disease Prevention and Control (ECDC), catheter-associated urinary tract infections (CAUTIs) account for 27% of all hospital-acquired infections in developed countries, and over 1 million cases occur in the USA and Europe [1][8]. In the UK, CAUTIs cost the NHS GBP 1–2.5 billion and account for approximately 2100 deaths annually [9].
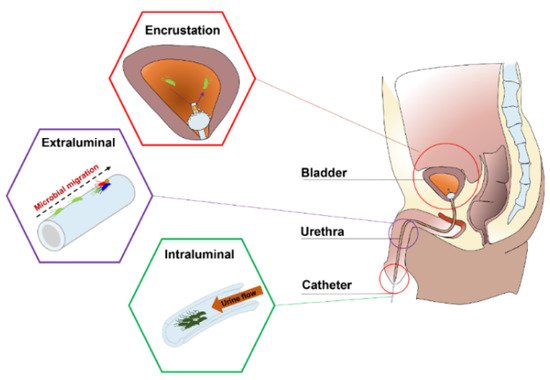
Figure 1. Anatomical cross-section of the renal system in a male showing CAUTIs.
Depending on clinical indications, the duration of catheterization may be short- (<7 days) or long-term (>28 days). Early work showed that 10–50% of patients undergoing short-term catheterization developed bacteriuria, and all patients undergoing long-term catheterization became infected, regardless of whether the catheter system was open or closed [10]. As a basic survival strategy, bacteria that encounter a catheter surface submerged in urine become attached within minutes [11]. These attached bacteria begin to phenotypically change, producing extracellular polymeric substances (EPS) (mainly exopolysaccharides and proteins) that allow the emerging biofilm community to develop a complex, three-dimensional structure within hours. Once established, the biofilms are very difficult to eradicate and exhibit a high tolerance to antibiotics and other biocidal treatments, making them a continuous focus for infections that can only be eliminated by the constant removal of the catheters [3][7][12][13][14][15]. Furthermore, in the presence of urease-positive bacteria (e.g., Proteus mirabilis), urease catalyses the hydrolysis of urea into carbon dioxide and ammonia, which increases the urine pH, leading to the precipitation of calcium and magnesium phosphate crystals (encrustation) and the formation of crystalline biofilms on the catheter, which eventually results in the complete blockage of the catheter [7][16][17]. Previous attempts to prevent CAUTIs include improving sterile techniques to inhibit the access of microbes into the urinary tract and limiting microbial accumulation on the catheter surface by intermittent catheterization. However, clinical evidence shows that these efforts do not lead to a noticeable reduction in CAUTIs [15]. Therefore, developing novel urinary catheters with antibiofilm and antiencrustation properties remains the most direct and promising strategy for this significant clinical problem.
Table 1. Common pathogens causing CAUTI.
| Short-Term Catheterization | Type | References |
| Escherichia coli | GN bacterium | [18] |
| Serratia spp. | GN bacterium | [19] |
| Staphylococcus epidermidis | GP bacterium | [20] |
| Enterococcus spp. | GP bacterium | [21] |
| Bacillus subtilis | GP bacterium | [3] |
| Long-Term Catheterization | Type | References |
| Providencia aeruginosa | GN bacterium | [1] |
| Proteus mirabilis | GN bacterium | [7] |
| Providencia stuartii | GN bacterium | [22] |
| Morganella morganii | GN bacterium | [6] |
| Klebsiella pneumoniae | GN bacterium | [23] |
| Staphylococcus aureus | GP bacterium | [24] |
| Candida spp. | Fungus | [25] |
2. Current Anti-Infection Strategies against CAUTIs and Challenges
Current commercial urinary catheters can be generally classified as standard and antimicrobial catheters (Figure 2). Owing to their superior malleability, several materials used for making standard catheters include polyvinyl chloride (PVC), polyurethane (PU), silicone and latex [20]. Of these, silicone has emerged as the material of choice for urinary catheters due to distinct advantages, including excellent biocompatibility, no allergic reactions, superior chemical and thermal stability and good mechanical strength [2][26]. Morris et al. [27] compared the antiencrustation performance of 18 types of catheter and found that all-silicone urinary catheters took the longest time to encrust and block. The main reason for this lies in that the all-silicone catheter has a wider lumen, allowing a faster urine flow, which prevents the accumulation of crystalline deposits. However, recent studies demonstrated that there was no significant difference between the development of infections or bacterial adherence on silicone catheters as compared to other types of catheters [20][26][28][29].
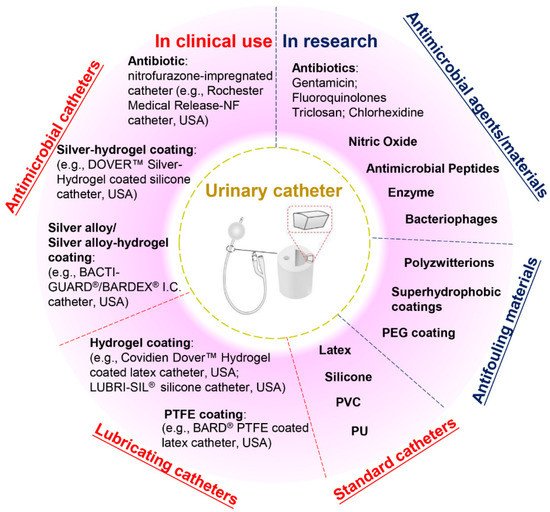
Figure 2. Classification of urinary catheters in clinical use and recently reported antimicrobial and antifouling materials for the prevention of CAUTIs.
Given that bacterial adhesion is the critical step in the progression of biofilm formation, numerous attempts have been made to endow the catheters with antiadhesive or antimicrobial properties, or both. Antiadhesive coatings are designed to prevent microbial adhesion through mechanisms of steric repulsion, electrostatic repulsion or low surface energy instead of killing the microbes [15]. To date, hydrogel- and polytetrafluoroethylene (PTFE)-coated catheters are commercially available, but clinical studies demonstrate that their efficacies against CAUTI are insignificant when compared with standard catheters [2][4][26][30]. Despite their lubricating features that may help improve patient comfort, hydrogel- or PTFE-coated catheters are only suitable for short- or medium-term catheterization (<28 days). Antimicrobial coatings are characterized by bactericidal or bacteriostatic activities that protect the catheters from microbial adhesion and migration. Recent reviews of commercial antimicrobial catheters identified two main strategies used: silver-based coatings and antibiotic impregnation [1][7][31]. However, all these catheters have only been reported to yield positive results for short-term application [7][20][32].
Silver is among the few FDA-approved antimicrobial materials for urinary catheter coatings, and its antimicrobial activity is associated with the release of silver ions. To date, silver has been applied in catheter coatings in the forms of bulk silver, silver alloy (silver/gold or palladium) and silver-hydrogels. Silver is very prone to oxidation in aqueous conditions, and the release of silver ions from these coatings often undergoes an initial burst-release phase followed by a slow-release phase. In clinical trials, the long-term antimicrobial efficacy of these silver-based coatings has proven limited [1][28][33]. In this scenario, attempts have been made to introduce silver nanoparticles into catheter coatings to attain enhanced antimicrobial efficacy. However, concerns have also been raised about the potential toxicity towards patients due to the fast and excessive release of silver ions [33][34]. In comparison, despite studies suggesting that the overuse of antibiotics may result in the development of antibiotic resistance, certain types of antibiotic-impregnated catheters have been proven to be more effective than silver-based antimicrobial catheters in preventing CAUTIs [3][20][35][36][37]. For example, nitrofurazone-impregnated catheters are commercially available, and studies comparing silver-alloy coated catheters, silver-hydrogel coated catheters, and nitrofurazone-impregnated catheters have found that nitrofurazone could effectively reduce the risk of symptomatic CAUTI and bacteriuria in short-term catheterization by impairing bacterial adherence and planktonic growth, while silver-based catheters have only demonstrated minimal effects [28][38]. Pickard et al. [33] compared the ability of silver-alloy-coated catheters and nitrofural-impregnated catheters for the reduction in incidence of symptomatic CAUTIs in adults requiring short-term catheterization via a clinical model, and the results demonstrated that nitrofural-impregnated catheters were more effective than the silver-alloy-coated catheters. In addition, impregnating antibiotics into catheters provides a cost-effective strategy to manufacture antimicrobial catheters, as this can be achieved by simply submerging swollen catheters in antibiotic-containing solutions for antibiotic encapsulation [31]. However, there are still certain concerns about using nitrofurazone in urinary catheters, such as patient discomfort [38] and the potential risks of developing tumours [39] and resistance in bacteria. This has hindered research in this field, and there is a growing demand for developing new antibiotics instead.
Apart from silver and antibiotics, recent research has also focused on a variety of antifouling materials (e.g., poly(ethylene glycol) (PEG) and polyzwitterions) [2][40] and biocidal materials (e.g., nitric oxide, antimicrobial peptides, enzymes and bacteriophages) [41][42][43][44] for urinary catheter coatings and has reported with varying levels of success, which offers potential for complete protection against CAUTIs. However, the promising performance of anti-infection coatings under laboratory conditions has not been translated into clinical success to date. Therefore, exploring novel anti-infection materials for urinary catheters will remain a current and broad interest in the upcoming decade.
References
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40.
- Andersen, M.J.; Flores-Mireles, A.L. Urinary catheter coating modifications: The race against catheter-associated infections. Coatings 2020, 10, 23.
- Jacobsen, S.M.; Stickler, D.J.; Mobley, H.L.; Shirtliff, M.E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 2008, 21, 26–59.
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced antibacterial and antiadhesive activities of silver-PTFE nanocomposite coating for urinary catheters. ACS Biomater. Sci. Eng. 2019, 5, 2804–2814.
- Stickler, D.J. Clinical complications of urinary catheters caused by crystalline biofilms: Something needs to be done. J. Intern. Med. 2014, 276, 120–129.
- Cortese, Y.J.; Wagner, V.E.; Tierney, M.; Devine, D.; Fogarty, A. Review of catheter-associated urinary tract infections and in vitro urinary tract models. J. Heal. Eng. 2018, 14, 1–16.
- Ramstedt, M.; Ribeiro, I.A.C.; Bujdakova, H.; Mergulhao, F.J.M.; Jordao, L.; Thomsen, P.; Alm, M.; Burmolle, M.; Vladkova, T.; Can, F.; et al. Evaluating efficacy of antimicrobial and antifouling materials for urinary tract medical devices: Challenges and recommendations. Macromol. Biosci. 2014, 19, e1800384.
- Saint, S.; Gaies, E.; Fowler, K.E.; Harrod, M.; Krein, S.L. Introducing a catheter-associated urinary tract infection (CAUTI) prevention guide to patient safety (GPS). Am. J. Infect. Control 2014, 42, 548–550.
- Milo, S.; Nzakizwanayo, J.; Hathaway, H.J.; Jones, B.V.; Jenkins, A.T.A. Emerging medical and engineering strategies for the prevention of long-term indwelling catheter blockage. Proc. Inst. Mech. Eng. Part H 2019, 233, 68–83.
- Stickler, D.J. Bacterial biofilms and the encrustation of urethral catheters. Biofouling 1996, 9, 13.
- Dunne, W.M., Jr. Bacterial adhesion: Seen any good biofilms lately? Clin. Microbiol. Rev. 2002, 15, 155–166.
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890.
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332.
- Hooton, T.M.; Bradley, S.F.; Cardenas, D.D.; Colgan, R.; Geerlings, S.E.; Rice, J.C.; Saint, S.; Schaeffer, A.J.; Tambayh, P.A.; Tenke, P.; et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International clinical practice guidelines from the Infectious Diseases Society of America. CID 2010, 50, 625–663.
- Yu, K.; Lo, J.C.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81.
- Stickler, D.; Ganderton, L.; King, J.; Nettleton, J.; Winters, C. Proteus mirabilis biofilms and the encrustation of urethral catheters. Urol. Res. 1993, 21, 407–411.
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. Superhydrophobic coatings for urinary catheters to delay bacterial biofilm formation and catheter-associated urinary tract infection. ACS Appl. Bio Mater. 2020, 3, 282–291.
- Pelling, H.; Nzakizwanayo, J.; Milo, S.; Denham, E.L.; MacFarlane, W.M.; Bock, L.J.; Sutton, J.M.; Jones, B.V. Bacterial biofilm formation on indwelling urethral catheters. Lett. Appl. Microbiol. 2019, 68, 277–293.
- Efthimiou, I.; Skrepetis, K. Prevention of Catheter-Associated Urinary Tract Infections. Recent Advances in the Field of Urinary Tract Infections; IntechOpen: London, UK, 2013; Available online: (accessed on 20 September 2020).
- Al-Qahtani, M.; Safan, A.; Jassim, G.; Abadla, S. Efficacy of anti-microbial catheters in preventing catheter associated urinary tract infections in hospitalized patients: A review on recent updates. J. Infect. Public Health 2019, 12, 760–766.
- Guiton, P.S.; Hung, C.S.; Hancock, L.E.; Caparon, M.G.; Hultgren, S.J. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect. Immun. 2010, 78, 4166–4175.
- Johnson, J.R.; Johnston, B.; Kuskowski, M.A. In vitro comparison of nitrofurazone- and silver alloy-coated foley catheters for contact-dependent and diffusible inhibition of urinary tract infection-associated microorganisms. Antimicrob. Agents Chemother. 2012, 56, 4969–4972.
- Peng, D.; Li, X.; Liu, P.; Luo, M.; Chen, S.; Su, K.; Zhang, Z.; He, Q.; Qiu, J.; Li, Y. Epidemiology of pathogens and antimicrobial resistance of catheter-associated urinary tract infections in intensivecare units: A systematic review and meta-analysis. Am. J. Infect. Control 2018, 46, e81–e90.
- Walker, J.N.; Flores-Mireles, A.L.; Pinkner, C.L.; Schreiber, H.L.I.; Joens, M.S.; Park, A.M.; Potretzke, A.M.; Bauman, T.M.; Pinkner, J.S.; Fitzpatrick, J.; et al. Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract. Proc. Natl. Acad. Sci. USA 2017, 114, E8721–E8730.
- Fisher, L.E.; Hook, A.L.; Ashraf, W.; Yousef, A.; Barrett, D.A.; Scurr, D.J.; Chen, X.; Smith, E.F.; Fay, M.; Parmenter, C.D.; et al. Biomaterial modification of urinary catheters with antimicrobials to give long-term broadspectrum antibiofilm activity. J. Control. Release 2015, 202, 57–64.
- Lawrence, E.L.; Turner, I.G. Materials for urinary catheters: A review of their history and development in the UK. Med. Eng. Phys. 2005, 27, 443–453.
- Morris, N.S.; Stickler, D.J.; Winters, C. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br. J. Urol. 1997, 80, 58–63.
- Desai, D.G.; Liao, K.S.; Cevallos, M.E.; Trautner, B.W. Silver or nitrofurazone impregnation of urinary catheters has a minimal effect on uropathogen adherence. J. Urol. 2010, 184, 2565–2571.
- Cohen, A.B.; Dagli, M.; Stavropoulos, S.W.J.; Mondschein, J.I.; Soulen, M.C.; Shlansky-Goldberg, R.D.; Solomon, J.A.; Chittams, J.L.; Trerotola, S.O. Silicone and polyurethane tunneled linfusion catheters: A comparison of durability and breakage rates. J. Vasc. Interv. Radiol. 2011, 22, 638–641.
- Kazmierska, K.A.; Thompson, R.; Morris, N.; Long, A.; Ciach, T. In vitro multicompartmental bladder model for assessing blockage of urinary catheters: Effect of hydrogel coating on dynamics of Proteus mirabilis growth. Urology 2010, 76, e515–e520.
- Liu, L.; Shi, H.; Yu, H.; Yan, S.; Luan, S. The recent advances in surface antibacterial strategies for biomedical catheters. Biomater. Sci. 2020, 8, 4095–4108.
- Trautner, B.W.; Hull, R.A.; Darouiche, R.O. Prevention of catheter-associated urinary tract infection. Curr. Opin. Infect. Dis. 2005, 18, 37–41.
- Pickard, R.; Lam, T.; MacLennan, G.; Starr, K.; Kilonzo, M.; McPherson, G.; Gillies, K.; McDonald, A.; Walton, K.; Buckley, B.; et al. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: A multicentre randomised controlled trial. Lancet 2012, 380, 1927–1935.
- Zhang, S.; Liang, X.; Gadd, G.M.; Zhao, Q. A sol-gel based silver nanoparticle/polytetrafluorethylene (AgNP/PTFE) coating with enhanced antibacterial and anti-corrosive properties. Appl. Surf. Sci. 2021, 535, 147675.
- Wu, K.; Yang, Y.; Zhang, Y.; Deng, J.; Lin, C. Antimicrobial activity and cytocompatibility of silver nanoparticles coated catheters via a biomimetic surface functionalization strategy. Int. J. Nanomed. 2015, 10, 7241–7252.
- Feneley, R.C.L.; Hopley, I.B.; Wells, P.N.T. Urinary catheters: History, current status, adverse events and research agenda. J. Med. Eng. Technol. 2015, 39, 459–470.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318.
- Lam, T.B.; Omar, M.I.; Fisher, E.; Gillies, K.; MacLennan, S. Types of indwelling urethral catheters for short-term catheterisation in hospitalised adults. Cochrane Database Syst. Rev. 2014, 23, CD004013.
- Hiraku, Y.; Sekine, A.; Nabeshi, H.; Midorikawa, K.; Murata, M.; Kumagai, Y.; Kawanishi, S. Mechanism of carcinogenesis induced by a veterinary antimicrobial drug, nitrofurazone, via oxidative DNA damage and cell proliferation. Cancer Lett. 2004, 215, 141–150.
- Sankar, S.; Rajalakshmi, T. Application of poly ethylene glycol hydrogel to overcome latex urinary catheter related problems. Biofactors 2007, 30, 217–225.
- Carlsson, S.; Weitzberg, E.; Wiklund, P.; Lundberg, J.O. Intravesical nitric oxide delivery for prevention of catheter-associated urinary tract infections. Antimicrob. Agents Chemother. 2005, 49, 2352–2355.
- Li, X.; Li, P.; Saravanan, R.; Basu, A.; Mishra, B.; Lim, S.H.; Su, X.; Tambyah, P.A.; Leong, S.S. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014, 10, 258–266.
- Lehman, S.M.; Donlan, R.M. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob. Agents Chemother. 2015, 59, 1127–1137.
- Thallinger, B.; Brandauer, M.; Burger, P.; Sygmund, C.; Ludwig, R.; Ivanova, K.; Kun, J.; Scaini, D.; Burnet, M.; Tzanov, T.; et al. Cellobiose dehydrogenase functionalized urinary catheter as novel antibiofilm system. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1448–1456.




