1000/1000
Hot
Most Recent

Although the beneficial effects of nuts on cardiometabolic diseases have been well established, little is known about the effects of nuts on age-related diseases. Given that age-related diseases share many biological pathways with cardiometabolic diseases, it is plausible that diets rich in nuts might be beneficial in ameliorating age-related conditions. Overall, the currently available evidence suggests that nut consumption, particularly when consumed as part of a healthy diet or over a prolonged period, is associated with positive outcomes such as longer telomere length, reduced risk of sarcopenia, and better cognition in older adults.
Human life expectancy has increased globally, and the increment rate has been more rapid in industrialised countries [1]. Greater life expectancy can be largely attributed to medical advancements, which significantly reduces mortality rates [2]. Due to increased longevity, healthy ageing and better quality of life are becoming more important among older adults [3]. The quality of life of older adults is multidimensional and depends on: (a) individual factors, e.g., satisfaction with one own’s physical/mental health, functional capacity (autonomy), emotional comfort, spirituality, and financial security; and (b) environmental factors, e.g., social interaction, network, and support [4].
The focus of this review is on how nut intake either consumed alone or as part of the dietary pattern can improve the quality of life of older adults. We propose that nuts may improve the quality of life of older adults through the promotion of better health, cognitive function, and functional capacity in this population, as depicted in a conceptual framework below (Figure 1). This framework is based on the premise that nuts, which are high in essential nutrients, improve diet quality and the overall nutritional status of older adults (see previous review [5]). As outlined in this framework, better nutrition and diet quality will, in turn, improve the health, wellbeing, and the quality of life of older adults.
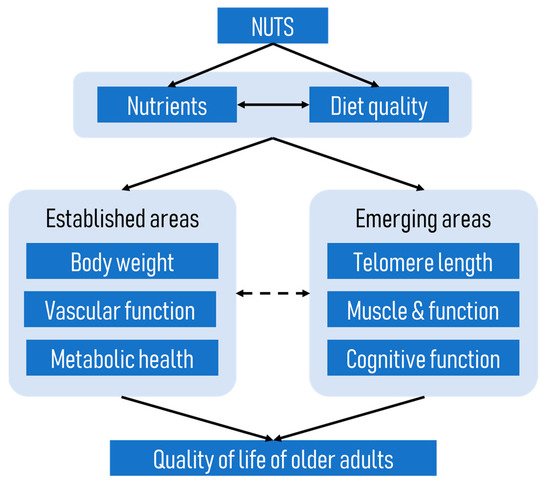
Figure 1. A conceptual framework of how nuts improve the quality of life of older adults.
A number of previous reviews have highlighted the benefits of nuts on body weight regulation [6][7], improved vascular function, and prevention of cancer [8] and metabolic diseases such as cardiovascular disease [9][10] and type 2 diabetes mellitus [10][11], which are prevalent among older individuals; therefore, these aspects will not be addressed again in this review. Instead, we will explore other emerging areas such as the potential effects of nuts on telomere length, muscle and function, and cognitive function of older adults. These aspects are of importance because telomere length has been shown to be an important indicator of ageing [12], while optimal physical and cognitive function will allow older adults to live independently as long as possible. It is also important to note that the emerging areas discussed in this review are not independent of the well-established areas shown in Figure 1. For example, telomere length has been linked to the onset of several age-related diseases [13], and vascular function has been shown to influence cognitive function [14].
Regular nut consumption is associated with a reduced risk of chronic diseases [15][16], including biomarkers of these age-related diseases [10][17][18]. The effects of nut consumption on telomere length have gained attention as one possible mechanism whereby nuts may reduce age-related diseases [19]. Telomeres are caps which protect the ends of chromosomes, and their lengths are an indicator of biological age. They protect DNA from oxidative damage, allowing the cell to divide normally. The length of telomeres is carefully controlled by a variety of proteins, including the enzyme telomerase, which promotes telomere length and stability [20]. Telomere length is shortened with each cell division, with a loss of around 50–200 bases [21]. Eventually, the telomere will reach a length that is associated with cell apoptosis [22]. Shortening of telomeres is negatively associated with cell longevity, and has been associated with disorders such as cancer, cardiovascular disease, neurodegenerative diseases, hypertension, and type 2 diabetes [23][24].
Although increasing age is strongly correlated with shorter telomere length, the variability in the rate of telomere shortening—independent of chronological age—suggests that other factors are important. Several modifiable lifestyle factors have been implicated in the rate of telomere shortening. For example, telomere length has been positively associated with greater fruit and vegetable intakes, and higher levels of physical activity; and negatively associated with higher saturated fat and meat consumption intakes, adiposity, and smoking [25][26][27][28][29][30]. The following sections review the evidence for the association of nut consumption and telomere length to determine whether this may partly explain the reduction in age-related diseases observed with regular nut intake.
There are several potential mechanisms whereby nuts may exert a positive effect on telomere length and cellular senescence. A number of nutrients have been implicated as having an important role in DNA methylation and integrity due to their antioxidant properties [31]. These nutrients include isoflavanoids, folate, vitamin E, and polyunsaturated fatty acids (PUFAs). Although nuts differ in a number of individual nutrients, they are all rich sources of antioxidant nutrients and unsaturated fatty acids. For example, peanuts and hazelnuts are particularly good sources of folate, whereas walnuts, Brazil nuts, and pine nuts are good sources of PUFA. It has been suggested that telomere length is a marker of oxidative stress [32]. Previous studies have shown that regular nut consumption is associated with reductions in some, but not all, markers of oxidative stress and inflammation [33][34]. In addition, Cannudas et al. [35] showed reduced oxidative damage of DNA with the consumption of pistachio nuts over four months.
To examine the association between nut consumption and telomere length, we reviewed one prospective study and two intervention studies that specifically examined the independent effect of nut consumption on telomere length. We also reviewed fifteen cross-sectional analyses, two prospective analyses, and two intervention studies that included or emphasised nuts as part of a dietary pattern.
One study from a large nationally representative sample examined the association between nut consumption and telomere length [19]. Tucker et al., using 24 h recall data from the National Health and Nutrition Examination Survey (NHANES) 1999–2002 (n = 5582), found a positive association between the consumption of nuts and seeds and telomere length. The association was linear (after adjustment), with each 1% of total energy derived from nuts and seeds associated with a length which was 4.5 base pairs longer. To give some perspective to these results, the authors calculated that using an estimated age-related rate of shortening of telomeres of 15.4 base pairs per year, adults of the same age who consumed 5% of total energy from nuts and seeds had around one- to two-thirds less cell ageing compared to non-consumers.
Observational studies that have examined the association between telomere length and diet quality or dietary patterns that include or emphasise nut consumption have been performed in a range of different ethnic groups and countries, including Korea [27], China [36][37], Spain [38][39], Hong Kong [40], Italy [41], the United States [25][42][43][44], Iran [45], Australia [46], and Finland [47]. Of the fifteen observational studies, thirteen used a cross-sectional design [25][36][37][38][39][40][41][42][43][44][45][46], one study [27] used a prospective design, and one study included both a cross-sectional and prospective analysis [47]. Details of these studies can be found in Table 1. Although the dietary indices used in these studies do not allow us to identify the independent effects of nuts, nuts were a food group which comprised the healthy component of these indices.
Table 1. Nut consumption and telomeres.
| Author, Year | Study Design | Study Participants | Dietary Assessment Method | Dietary Patterns Assessed | Measure of Telomere | Outcomes |
|---|---|---|---|---|---|---|
| Boccardi, 2013 (Italy) | Cross-sectional | n = 217 elderly Caucasians (n = 115 men, n = 102 women); age range: 81–87 years, mean 78.0 ± 2.7 years | Dietary questionnaire | Mediterranean Diet Score (MDS) (Trichopoulou 2003) | Leukocyte telomere length and telomerase activity PBL/qPCR (Cawthon, 2002) | Greater adherence to MDS associated with longer LTL (p = 0.003) and higher telomerase activity (p = 0.013), and remained significant after adjustment. Every year increase in age LTL decreased by 0.072 Kb, 0.057 Kb, and 0.051 Kb in low, medium, and high adherence respectively (p = 0.001). |
| Crous-Bou, 2014 (USA) | Cross-sectional from the Nurses’ Health Study | n = 4676 females; age range: 42–70 years, mean 59 ± 6.6 years | Semi-quantitative FFQ | Alternative Mediterranean Diet Score (AMDS) (Trichopoulou 2003) | Leukocyte telomere length PBL/qPCR (Cawthon, 2002) | Greater adherence to the AMDS was associated with LTL z-score (p for trend = 0.02 (without adjustment); and p for trend = 0.004 after adjustment). No independent association with nut consumption (p for trend = 0.24). |
| Garcia-Calzon, 2016 (Spain) | Cross-sectional analysis of the PREDIMED-NAVARRA trial | n = 520 (n = 234 male, n = 286 female) at high risk of CVD; age range 60–80 years females, 55–80 years males; mean age 67 ± 6.0 years | FFQ and 14-item questionnaire to evaluate adherence to Mediterranean dietary pattern | Mediterranean diet adherence score (MedDiet) | Leukocyte telomere length PBL/qPCR (Cawthon, 2002) | Higher adherence to MedDiet was associated with greater age-adjusted z-score LTL) and a lower risk of having short telomeres in women, but not men. Nut intake was not associated with LTL. |
| Gu, 2015 (USA) | Cross-sectional | n = 1743, aged ≥65 years; n = 506 white, n = 536 African American, n = 679 Hispanic, n = 22 other | Semi-quantitative FFQ | Mediterranean Diet Score | Leukocyte telomere length PBL/qPCR (Cawthon, 2009) | No association overall between MDS and telomere length. There was a positive association among Whites, 1 unit increase in MDS corresponds to 48 bp increase in LTL, but not African Americans or Hispanics. No association between nuts and telomere length. |
| Leung, 2018 (USA) | Cross-sectional analysis of NHANES 1999–2002 | n = 4758 (n = 2208 males, n = 2550 females), age range 20–75 years, mean age 39.5 years | Single 24 h recall | Healthy Eating Index 2010 scores (HEI-2010); Alternative Healthy Eating Index 2010 scores (AHEI-2010); Mediterranean Diet scores (MDS), Dietary Approaches to Stop Hypertension (DASH) score | LTL from whole blood (Cawthon, 2002) | Comparison of the top and bottom quintiles showed higher scores for all diet quality indices were associated with longer telomere length in women, but not men. |
| Meinilä, 2019 (Finland) | Cross-sectional and prospective (mean follow up period 9.9 y for females and 9.7 y for males) | n = 1046 (n = 456 men, n = 590 women); mean age 61 years | Semi-quantitative FFQ | Baltic Sea diet score (BSDS); modified Mediterranean diet score (mMed); Dietary Inflammation index (DII) | Leukocyte telomere length PBL/qPCR (Cawthon, 2009) | Adherence to the any of the 3 dietary indices was not associated with LTL in the cross-sectional analysis. In the prospective analysis adherence to mMed was associated with slightly higher rates of shortening in women (largely driven by the fruit and nut food component)—this was not considered clinically important. |
| Milte, 2018 (Australia) | Cross-sectional | n = 679 (n = 330 men, n = 349), age range 55–65 years, mean age 62.7 years | FFQ | Dietary Guideline Index; Recommended Food Score; Mediterranean Diet Score (MDS) | LTL from whole blood (Cawthon, 2002) | There were no associations between any of the diet indices and LTL. |
| Ojeda-Rodriguez, 2019 (Spain) | Cross-sectional (SUN Project) | n = 886 (n = 645 males, n = 241 females), aged ≥55 years | Semi-quantitative FFQ | Prime Diet Quality Score (PDQS); Fat Quality Index (FQI); Alternative Healthy Eating Index 2010 scores (AHEI-2010); Mediterranean Diet Adherence Screener (MEDAS), Dietary Approaches to Stop Hypertension (DASH) | Salivary telomere length PBL/qPCR (Cawthon, 2009) | There were fewer participants with short telomeres in the top tertile for each diet quality index, specifically the PDQS, MEDAS and DASH in crude and adjusted models; and all indices for adjusted models. |
| Ventura Marra, 2019 (USA) | Cross-sectional | n = 96 (n = 41 men, n = 55 women), aged 45–60 years, with at least one risk factor for CVD | Three 24 h recalls for the HEI-2015 index and aMed; 24-item questionnaire for the DST | Healthy Eating Index 2015 (HEI-2015); alternative Mediterranean diet score (aMed); Dietary Screening Tool (DST) | LTL from whole blood (Cawthon, 2002) | There were no associations between the HEI-2015 or aMed and LTL. Those scoring “at risk” by the DST were more likely to have short LTL. |
| Chan, 2010 (Hong Kong) | Cross-sectional | n = 2006 (n = 976 men, n = 1030 females), aged ≥65 years | FFQ | Included food group nuts/legumes/seeds | LTL from whole blood (Cawthon, 2002) with modifications | No association between the nuts/legumes/seeds group and LTL. |
| Gong, 2017 (China) | Cross-sectional | n = 553 (n = 281 men, n = 272 women); mean age: 45.1 years men; 48.3 years women | FFQ | PCA was used to derive 4 dietary patterns: Vegetable-rich (higher in fruits, vegetables, whole grains, dairy products, nuts, eggs, tea); Macho; Traditional; High energy-density | Leukocyte telomere length (Zhao, 2009) | Only the Vegetable-rich dietary pattern was associated with longer TL in women, but not men. The longer length of 160 bp corresponded with a difference of 4 years of aging |
| Karimi, 2018 (Iran) | Cross-sectional | n = 300 men, aged 25–40 years | Semi-quantitative FFQ | PCA was used to derive 4 dietary patterns: healthy diet pattern, Western pattern, traditional pattern, vegetarian diet pattern. Nuts and seeds were examined as a food group | LTL from whole blood (Cawthon, 2002) | Nuts and seeds were negatively, but not statistically significantly, associated with LTL. |
| Lee, 2015 (Korea) | Prospective (10 y follow up) | n = 1958 (n = 1018 men, n = 940 women), age range 40–69 years baseline | Semi-quantitative FFQ | Factor analysis characterised a Prudent dietary pattern and a Western dietary pattern | LTL from whole blood (Cawthon, 2002) | The Prudent dietary pattern was positively associated with LTL. The Western diet was not associated with LTL. Nuts were one food component positively associated with LTL. |
| Nettleton, 2008 (USA) | Cross-sectional of the Multi-Ethnic Study of Atherosclerosis (MESA) study | n = 840 (n = 434 women, n = 406 men), n = 157 whites, n = 228 African Americans, n = 455 Hispanics), age range 45–84 years | Semi-quantitative FFQ | PCA was used to derive 2 dietary patterns: fats and processed meat; and whole grains and fruits (including nuts). Nuts or seeds were also analysed as a food group component. | LTL from whole blood (Cawthon, 2002) | No association between dietary pattern andLTL. The food group nuts or seeds was not associated with LTL. |
| Zhou, 2016 (China) | Cross-sectional | n = 556 (n = 213 males, n = 343 females), mean age early 50s | Semi-quantitative FFQ | Nuts and seeds food group was examined | Leukocyte telomere length PBL/qPCR (Cawthon 2009) | Intakes of nuts or seeds were highest among those in the upper tertile for telomere length. Intakes of nuts and seeds were positively associated with telomere length. |
Abbreviations: AHEI-2010, Alternative Healthy Eating Index; AMDS, Alternative Mediterranean Diet Score; bp, base pairs; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DII, DIetary Inflammatry Index; FQI, Fat Quality Index; LTL, leukocyte telomere length; MDS, Mediterranean Diet Score; MEDAS, Mediterranean Diet Adherence Screener; n, number; NHANES, National Health and Nutrition Examination Survey; PCA, principal component analysis; PDQS, Prime Diet Quality Score; PREDIMED, Prevención con Dieta Mediterránea trial; SUN, Seguimiento Universidad de Navarra.
Nine studies examined adherence to pre-determined diet quality indices which emphasised nut intake. These included the Mediterranean diet score, Dietary Approaches to Stop Hypertension (DASH) score, Health Eating Index 2010 score (HEI-2010), Alternative Health Eating Index 2010 score (AHEI-2010), and the Prime Diet Quality Score (PDQS).
The dietary pattern which has received the most attention in this area is the Mediterranean diet, which has been correlated with healthy ageing and longevity [48]. One of the key components of the Mediterranean dietary pattern is nut consumption. In addition, this pattern is characterised as being largely plant-based with high amounts of olive oil, fruits and vegetables, legumes, and wholegrains [49]. When study populations were analysed as a whole, three studies showed a positive association between the Mediterranean diet and telomere length [25][39][41], while two showed no association [44][46]. Two studies showed positive associations in women only [38][43], and one U.S. study showed a positive association among white, but not African American or Hispanic participants [42]. Meinilä et al. found no association in a cross-sectional analysis of their Finnish population, but found slight, although statistically significantly, higher rates of telomere shortening among women adhering to a Mediterranean dietary pattern in the 10-year follow-up prospective analysis. Interestingly, this was largely driven by the fruit and nut food group; however, this difference was small and not considered clinically important [47].
Two studies that showed a positive association with the Mediterranean dietary pattern also analysed nuts as a food group, but found no association between nuts and telomere length [25][38]. Trichopoulou et al. have previously suggested that components of the Mediterranean eating pattern may be additive; hence, the lack of association based on a single nut food group. Additionally, looking at individual foods may be more susceptible to residual confounding [50]. Therefore, the potential benefit of nuts on telomere length is likely to be mediated through better overall diet quality. Of note is the finding that studies on the Mediterranean eating pattern carried out in southern European countries tended to show positive associations with telomere length compared to those carried out in countries such as Australia [46], Finland [47], and the United States [44]. This may indicate higher overall adherence to such an eating pattern in southern Europe, which may be more likely to show positive associations.
A meta-analysis including eight of the aforementioned cross-sectional studies collectively assessed the association between adherence to the Mediterranean diet and telomere length maintenance [51]. In the fully adjusted model for all participants, there was a positive association between adherence to the Mediterranean diet and telomere length maintenance, but this association disappeared when males and females were separated. This may be due to reduced power, but of note, no significant associations were seen in any of the models for men.
Two of the aforementioned studies that investigated the Mediterranean diet also examined other nut-containing dietary patterns [39][43]. Ojeda-Rodriquez showed that, similar to their findings of the Mediterranean diet, greater adherence to the Prime Diet Quality Score (PDQS), the Alternative Healthy Eating Index 2010 score (AHEI-2010), and Dietary Approaches to Stop Hypertension (DASH) scores was associated with longer telomere length among the Seguimiento Univeridad de Navarra (SUN) cohort [39]. In contrast, Leung et al., using data from the 1999–2002 cycles of the National Health and Nutrition Examinations Survey (NHANES), showed significant trends for longer telomere lengths from the lowest to the highest quintiles for each diet score (the Healthy Eating Index 2010 score (HEI-2010), the Alternative Healthy Eating Index 2010 score (AHEI-2010), or the DASH diet score) among women, but not men. This reflects their findings in terms of adherence to the Mediterranean diet [43].
Three studies (two cross-sectional and one prospective) analysed FFQ data and derived dietary patterns using principal component analysis (PCA) or factor analysis [27][36][52]. One study conducted in South China found that a dietary pattern containing nuts was associated with longer telomeres among women, but not men [36]. In a prospective study conducted in South Korea, a prudent diet was positively associated with telomere length [27]. When analysing individual food items which contributed to the prudent dietary pattern, nuts were positively associated with telomere length.
One U.S. study, including data from 840 Black, White, and Hispanic adults taking part in the Multi-Ethnic Study of Atherosclerosis (MESA), used PCA and reported no association between consuming a nut-containing food pattern and telomere length [52]. They also failed to show an association when nut or seeds were considered alone.
A further three studies used FFQs to examine the association of nuts and seeds consumed as a food group and telomere length. One study conducted in China showed that intakes of nuts or seeds were highest among those in the upper tertile for telomere length, and intakes were positively associated with telomere length [37]. In contrast, Chan et al., reported no associations between nut consumption and telomere length in a cross-sectional study of 2006 Chinese males and females living in Hong Kong [40]. Here, nuts were combined with the food group of legumes, seeds and nuts. A further study among males in Iran showed that nuts and seed were negatively, albeit not statistically significantly, associated with telomere length [45]. It should be noted that participants in this study were younger (25–40 years) compared to most other studies in this area.
Collectively, these observational studies on dietary patterns containing nuts have produced mixed results, making it difficult to form conclusions. Of the fifteen studies, nine showed a positive association between the consumption of a nut-containing dietary pattern and telomere length in the population as a whole or in a sub-group, five showed no association, and one showed a negative association, albeit not clinically important. Some factors which make interpretation of these observational studies difficult include the inability to examine the independent effects of nuts, variation in the age of participants, and the use of different dietary assessment tools. There also appears to be some sex differences, with three studies showing positive associations with consumption of nut-containing dietary patterns and telomere length in females only [36][38][43]. The reason for these sex-specific associations cannot be speculated based on the observational nature of these studies, and future clinical trials are therefore needed to understand the potential underlying biological explanations. A further factor to consider when interpreting the results of observational studies is survivor bias. This is where people who live longer tend to be more resilient to chronic disease. Although these studies cannot infer causality, they do provide useful information on which to base hypotheses that can be explored in intervention studies.
To the best of our knowledge, only two intervention studies have specifically assessed the effects of nut consumption on telomere length [35][53]. Freiras-Simoes et al. examined the inclusion of 15% of dietary energy from walnuts (n = 80), compared to a control group (n = 69), who continued to consume their usual diet while abstaining from walnuts, on the maintenance of telomere length in a group aged 63–79 years [53]. This parallel study was conducted over two years. There was a significant increase in red blood cell alpha-linolenic acid in the walnut group compared to the control, indicating compliance to the interventions. There was a tendency (p = 0.079) for the control group to have greater reduction in telomere length over the two years compared to the walnut group. In addition, the change in the percentage of telomeres with lengths less than 3 kb at the end of two years was marginally statistically significantly lower in the walnut group. Taken together, these results suggest that consuming walnuts may reduce telomere attrition.
Canudas et al. investigated the effect of pistachio consumption on telomere length and gene expression related to telomere maintenance in 49 participants aged 25–65 years with pre-diabetes using a crossover design [35]. Participants consumed a diet supplemented with 57 g/d pistachios compared with an isocaloric control diet for four months each. Telomere length did not differ between the two treatments; however, genes associated with telomere length were significantly upregulated in the pistachio treatment compared with the control.
While the findings of these intervention studies are promising, they need to be confirmed in long-term, adequately powered, randomised intervention studies using different types and doses of nuts.
There are only two intervention studies which have assessed telomere length in response to interventions with dietary patterns that emphasise nut consumption.
Garcia-Calzón et al. performed several analyses on the association between telomere length and diet using a subgroup from the PREDIMED-NAVARRA trial involving 520 participants aged 55–80 years who were at high risk of CVD [38]. In this trial, participants were randomly assigned to one of two Mediterranean diets supplemented with either extra virgin olive oil or mixed nuts, or to a low-fat control diet. Intervention with the Mediterranean diets with nuts was associated with a higher risk of telomere shortening compared to the control, whereas, there were no differences between the Mediterranean diet with extra virgin olive oil and the control group [38]. When analysing the individual components of the Mediterranean diet including nuts and extra virgin olive oil, there was no association between these components and telomere length. This is in agreement with other observational studies [25][42]. The sample size may not have had sufficient power to identify small changes in telomere length by individual dietary components. The finding that the intervention with the Mediterranean diet with nuts had a detrimental effect on telomere length was unexpected; although, it should also be noted that the control group increased adherence to the Mediterranean diet over the intervention, which made the results difficult to interpret. The authors also suggest that lifetime exposure to a Mediterranean diet may be more meaningful in determining telomere length than exposure during a five-year intervention. Further analysis of this group showed that among those with the Pro12Ala polymorphism—a gene variant associated with lower CVD risk—greater adherence to the Mediterranean dietary pattern was associated with greater prevention of telomere shortening [54].
In a small crossover study, 20 participants aged over 65 years consumed three diets for four weeks each: a Mediterranean diet enriched in olive oil; a saturated fat-rich diet; and a low fat, high carbohydrate diet enriched in n-3 PUFA from walnuts [28]. The Mediterranean diet was associated with a lower percentage of telomere shortening compared to the other two interventions. It was proposed that the Mediterranean diet protected against oxidative stress, and thus prevented telomere shortening. A Mediterranean diet usually contains nuts; however, it is unclear to what extent nuts were included in the Mediterranean arm of this study.
Overall, research on the effects of nut consumption and telomere length is inconsistent. Some observational studies show greater telomere length among those consuming dietary patterns including nuts, while others do not. Many of these studies failed to show an association with nuts consumption per se. Several systematic reviews have shown that intensive lifestyle interventions delay telomere shortening [29]. Therefore, more comprehensive changes may be more effective than changing only one component of the diet, such as increasing nut consumption. This suggests that the synergistic effect of nutrients may be important. It is possible that nuts, as part of a healthy diet and lifestyle, may be one contributing factor telomere health, and may be one of the underlying mechanisms whereby regular nut consumption reduces the risk of age-related diseases. However, it is important to note that much evidence derives from observational studies, and hence the relationship between nut intake and telomere length is largely associative rather than causal. How telomere length translates into lifespan is not straightforward, so it is also not possible to propose that nut intake contributes to longevity in older adults.
According to the revised European Working Group on Sarcopenia in Older People 2 (EWGSOP2), sarcopenia is characterised by low levels of muscle strength and muscle quantity or quality, and severe sarcopenia is characterised by sarcopenia and low physical performance [55].
Ageing is associated with the loss of muscle mass and strength, although the rate of decline differs between individuals, suggesting that lifestyle factors such as diet and physical activity may be important determinants of muscle health [56][57]. Current evidence suggests that nutrients such as protein, beta-hydroxy-beta-methylbutyrate, vitamin D, antioxidant nutrients, and long-chain PUFAs, as well as physical activity, may ameliorate the risk of sarcopenia [57][58][59], through muscle protein synthesis or preventing muscle breakdown, which helps to preserve muscle mass and function. Nuts are rich sources of plant protein, unsaturated fatty acids, phytochemicals, vitamins and minerals; therefore, these nutrients may act synergistically for the prevention and management of sarcopenia in older adults.
This section will review observational studies that examined the association between nut consumption, either alone or as part of a dietary pattern, and sarcopenia and related factors in older adults. A total of seven observational studies have been identified (Table 2) [60][61][62][63][64][65][66]. To the best of our knowledge, no intervention studies have been specifically designed to determine the effect of nut consumption on sarcopenia.
Table 2. Studies on nuts and sarcopenia related factors.
| Author (Year) Study Location |
Study Design | Study Participants | Dietary Assessment Method | Nuts/Dietary Patterns Assessed | Measure of Functional or Related Outcomes | Results |
|---|---|---|---|---|---|---|
| Nut-specific studies | ||||||
| Arias-Fernández, 2019 (Spain) |
Prospective study: Seniors-ENRICA cohort Cohort was established in 2008–2010, with 7.2 years of follow-up |
3289 individuals aged ≥60 years | A validated computerised diet history was used to assess nut consumption in 2008–2010 and 2012. Average nut consumption at baseline (2008–2010) and in the first follow-up wave of data collection (2012) was calculated to represent cumulative intake over follow-up. | Diet history included 20 types of nuts, which were grouped as follows: almonds, hazelnuts, peanuts, chestnuts, walnuts, pine nuts, sunflower seeds, pistachios, sesame seeds, cashews, macadamia nuts, and other types of nuts. | Five domains were considered to characterise participants’ physical function: (1) Agility: Rosow and Breslau scale (self-reported) (n = 1502) (2) Mobility: Rosow and Breslau scale (self-reported) (n = 1502) (3) Overall physical function: physical component summary (PCS) score of the 12-Item Short-Form Health Survey SF-12 (self-reported) (n = 1665) (4) Grip strength: highest value in two consecutive measures on the dominant hand (objective measure of muscle strength) (n = 1256) (5) Gait speed: 3 m walking speed test (objective measure of physical performance) (n = 1233) |
In men, compared with no consumption, an intake of nuts ≥11.5 g/d (median) in nut consumers was associated with lower risk of self-reported impaired agility (fully-adjusted HR = 0.59, 95% CI: 0.39–0.90) and mobility (fully-adjusted HR = 0.50, 95% CI: 0.28–0.90). In women, compared with no consumption, the fully-adjusted HR (95% CI) of impaired self-reported overall physical function was 0.65 (0.48–0.87) for intake ≥11.5 g/d. No association was found between nut consumption and grip strength and gait speed. |
| Studies on dietary patterns that include nuts (as a food group) | ||||||
| Bradlee, 2018 (United States) | Prospective study: Framingham Offspring Study Began in 1972, with a median follow-up of 13.0 years |
5124 offspring were enrolled in 1972 For skeletal muscle mass outcomes, participants aged 40 years or older were included. For functional status outcomes, participants aged 50 years or older at the time of the dietary assessments were included; follow-up for functional status outcomes continued for up to 16 years. |
Diet records (six days) | Protein-source foods: Legumes, Soy, Nuts, Seeds | Skeletal muscle mass was estimated using BIA. Functional status was measured using standardised instruments: (1) Rosow–Breslau scale measures gross-mobility capacity (2) Nagi scale assesses self-reported functional limitations |
Higher intake of “legumes, soy, nuts and seeds” was associated with higher percent skeletal muscle mass over 9 years. In men, compared with consumption <0.25 serving/day of legumes, soy, nuts and seeds, those who consumed ≥1.25 serving/day had higher percent skeletal muscle mass (36.8% vs. 37.5%, p = 0.0197). In women, compared with consumption <0.25 serving/day of legumes, soy, nuts and seeds (27.3%), those who consumed 0.25 to <1.25 serving/day and ≥1.25 serving/day had higher percent skeletal muscle mass (28.2% and 28.1% respectively, both p ≤ 0.0156). In the multivariable Cox proportional hazards models, “legumes, soy, nuts and seeds” was not a predictor of limitation in two or more functional tasks from the Rosow–Breslau and Nagi scales (HR = 0.96, 95% CI: 0.72, 1.30). |
| Hai, 2017 (China) |
Cross-sectional study | 848 individuals aged ≥60 years who lived in the community for more than 12 months. Data from 834 participants were used for the analysis. |
A validated simplified FFQ was used. Frequency units: day, week, month or never. | Nine food categories based on the Chinese Food Guide Pagoda: (1) Grain or cereals (2) Vegetables (3) Fruit (4) Meat (pork, beef, poultry, and mutton) (5) Eggs (6) Fish and shrimp (7) Milk and milk products (8) Legumes (9) Nuts |
Sarcopenia, i.e., presence of low muscle mass, plus low muscle strength or low physical performance. Muscle mass was measured using BIA. Grip strength was measured using a dynamometer. Usual gait speed (m/s) on a 6 m course was used to measure physical performance and a slow walking speed was defined as a walking speed <0.8 m/s. |
In females, participants with sarcopenia had significantly lower frequency of nut consumption than those without sarcopenia (0.05 times vs. 0.81 times per week, p = 0.022). This was not found in male participants (p = 0.135). After adjusting for potential confounders, there was a significant association between prevalence of sarcopenia and frequency of nut consumption per week (OR = 0.724, 95% CI: 0.532, 0.985, p < 0.05). |
| Lim, 2020 (Korea) |
Cross-sectional study. 2008 to 2011 Korea National Health and Nutrition Examination Survey (KNHANES). |
3350 elderly over 65 years, 862 had sarcopenia. | 24 h dietary intake | Food intake analysis was based on the guideline of 15 food groups: (1) Cereals (2) Potato and starches (3) Sugars and sweeteners (4) Pulses (5) Nuts and seeds (6) Vegetables (7) Fungi and mushrooms (8) Fruits (9) Meat (10) Eggs (11) Fish and shellfish (12) Seaweeds (13) Milk (14) Oil and fat (15) Beverages |
Sarcopenia was defined as muscle mass excluding bones and fats of limbs measured by dual energy X-ray absorptiometry divided by weight in the form of percent is under the twice of standard deviation. | In males, the sarcopenia group had significantly lower intake of nuts and seeds than the non-sarcopenia group (5.2 g/day vs. 3.1 g/day, p = 0.002). This was not found in female participants (p = 0.258). Logistic regression analyses showed no significant association between prevalence of sarcopenia and tertiles of nut and seed intake in both males and females. |
| Studies on dietary patterns that include nuts (diet quality indices) | ||||||
| Ballesteros, 2020 (Spain) |
Prospective study: Seniors-ENRICA cohort Cohort was established in 2008–2010, with a median follow-up of 3.5 years |
3289 individuals aged ≥60 years 2071 included in the analysis |
A validated computer-assisted face-to-face dietary history. | Mediterranean Diet Adherence Screener (MEDAS) score was used to determine the adherence to the Mediterranean diet, with a higher score indicating greater adherence. | Risk of falling | There was an inverse dose-response relationship between the MEDAS score and the risk of falling in older adults (p for trend = 0.04). Compared with the people in the lowest tertile of the MEDAS score, those in the second tertile (OR = 0.93, 95% CI: 0.71–1.21) and highest tertile (OR = 0.72, 95% CI: 0.53–0.98) showed lower risk of falling after adjustment for potential confounders. |
| Schacht, 2019 (Denmark) |
Cross-sectional study | 184 Danish older individuals aged 65 years and above participated in the “Counteracting Age-related Loss of Skeletal Muscle Mass” (CALM) study. | 3 days weighed food diaries from Wednesday to Friday. Average daily consumption of different food products was calculated. | Dietary index characterised by higher intakes of whole grains, dairy products, fish, legumes, nuts, fruit, and vegetables. | Muscle function (1) 30s chair stands (2) 400 m gait speed (3) Handgrip strength (dynamometer DHD-1 [SH100]) (4) Knee extensor maximal voluntary contractions was measured using an isokinetic dynamometer |
Dietary index was associated with faster 400 m walking speed (p for trend = 0.021). No associations were found between dietary index and 30s chair stands, handgrip strength, knee extensor maximal voluntary contractions (all p for trend > 0.05). |
| Hashemi, 2015 (Iran) |
Cross-sectional study | 300 elderly men and women aged 55 years and older | Semi-quantitative Food Frequency Questionnaire, frequency of 117 common Iranian food items by standard serving size | Mediterranean pattern was defined as a dietary pattern with high factor loadings (>0.4) in food groups such as olives and olive oil, low and high carotenoid vegetables, tomatoes, whole grains, nuts, fish, fresh and dried fruits, and pickles. | Sarcopenia is defined as low appendicular muscle mass with either low muscle strength or low muscle performance. Muscle mass (DXA) was calculated as the ratio of total lean mass of legs and arms (ASM) to squared height. Muscle strength was measured using a handgrip dynamometer. Muscle performance was measured using a 4 m walk gait speed test. Low muscle performance was defined as gait speed <0.8 m/s. |
There was a significant association between Mediterranean dietary pattern and prevalence of low gait speed (p = 0.02). The percentage of participants with low gait speed (< 0.8 m/s) in the top tertile was 29.3%, second tertile was 47.5%, and lowest tertile was 43.9%. After adjusting for potential confounders, Mediterranean diet was associated with lower odds of having sarcopenia. Odds ratio (95% CI): T1: 1.00 T2: 0.84 (0.40–1.70) T3: 0.40 (0.17–0.97) P for trend: 0.04 |
Seven observational studies have been identified in the nuts and sarcopenia area (Table 2) [60][61][62][63][64][65][66]. One prospective study examined the association between nut consumption and physical function [60], three observational studies (one prospective study and two cross-sectional studies) reported nuts as a food group [62][63][65], and another three studies (one prospective study and two cross-sectional studies) examined the adherence to diet quality indices in which nuts was a key component [61][64][66]. Two studies were conducted in Spain, and one each in the United States, China, Korea, Denmark, and Iran (Table 2).
To date, only one prospective study has examined the association between nut consumption and physical function [60]. The Seniors-ENRICA cohort study was conducted in Spain in 3289 community-dwelling older adults aged ≥60 years [60]. A validated diet history was used to assess consumption of 20 types of nuts in this study. Physical function was ascertained by five domains, namely agility, mobility, overall physical function, grip strength, and gait speed, with the first three domains being self-reported and the last two domains being objective measures.
Participants were classified into three categories based on their nut consumption: non-consumers, <median (<11.5 g/d), and ≥median (≥11.5 g/d). In men, there was a dose-response relationship between nut consumption and impaired agility (p for trend = 0.01) and mobility (p for trend = 0.02), in which higher nut consumption was associated with lower risk of these two impairments. Compared with non-consumers, nut intake of ≥11.5 g/d was associated with a lower risk of impaired agility (HR = 0.59, 95% CI: 0.39, 0.90) and impaired mobility (HR = 0.50, 95% CI: 0.28, 0.90) in the fully adjusted models. In women, there was a dose-response relationship between nut consumption and impaired overall physical function (p for trend = 0.004). Compared with non-consumers, nut intake ≥11.5 g/d was associated with lower risk of impaired overall physical function (HR = 0.65, 95% CI: 0.48, 0.87). However, nut consumption was not associated with grip strength and gait speed. The authors reported that this could be due to the low sensitivity of method used to detect the differences.
Overall, this study revealed that nut consumption was associated with a lower risk of self-reported impaired agility and mobility in men, and lower risk of impaired overall physical function in women [60]. However, such associations were not observed in objective measures of grip strength and gait speed. Further investigation is warranted to confirm this finding.
Six observational studies have examined the association between dietary patterns that include nuts and sarcopenia. Three studies reported nuts as a food group [62][63][65], while the other three studies reported the adherence to diet quality indices in which nuts was a key component [61][64][66].
Three studies, consisting of one prospective study and two cross-sectional studies, examined nuts as part of a dietary pattern and their relationship with muscle mass and function [62] and sarcopenia [63][65]. The Framingham Offspring study was the first prospective study to investigate the association between a diet rich in protein-source foods (including nuts) and skeletal muscle mass and functional status among community-dwelling adults [62]. Results showed that compared to consumption <0.25 servings/day of “legumes, soy, nuts, seeds”, those who consumed ≥1.25 servings/day had a higher mean percent skeletal muscle mass in men (difference of 0.7%, p = 0.0197) and women (difference of 0.8%, p = 0.0156), although the difference in percent skeletal muscle mass was small.
Two cross-sectional studies in China and Korea examined the association between food groups (in which nuts was one of the food groups) and sarcopenia in older adults [63][65]. Hai et al. [63] reported that female participants with sarcopenia had a significantly lower frequency of nut consumption compared to those without sarcopenia (0.05 times per week vs. 0.81 times per week, p = 0.022). In addition, there was a 28% reduction in the prevalence of sarcopenia and frequency of nut consumption (OR = 0.724, 95% CI: 0.532, 0.985). In line with these findings, a cross-sectional study showed that male participants with sarcopenia had a significantly lower intake of nuts and seeds compared to those without sarcopenia (3.1 g per day vs. 5.2 g per day, p = 0.002) [65].
Overall, the evidence from observational studies where nuts were included as a food group showed an inverse association between nut consumption and sarcopenia [63][65]. In addition, a prospective study reported higher skeletal muscle mass among people who consumed more servings/day of “legumes, soy, nuts, seeds” [62]. The current, albeit limited, epidemiologic evidence suggests a protective effect of nut consumption on sarcopenia in older adults.
Three studies, including one prospective study and two cross-sectional studies, examined adherence to diet quality indices, such as the Mediterranean diet and Mobility diet, and the risk of sarcopenia and related factors [61][64][66]. The Seniors-ENRICA prospective study reported a dose-response relationship between the Mediterranean Diet Adherence Screener (MEDAS) score and risk of falling (p for trend = 0.04) [61]. Participants in the highest tertile of the MEDAS score had a 28% reduction in the risk of falling, in comparison with those in the lowest tertile of the MEDAS score. Similarly, Hashemi et al. [64] reported an inverse dose-response relationship between the Mediterranean pattern and sarcopenia (p for trend = 0.04). Participants in the highest tertile of the Mediterranean pattern had a 60% reduction in the odds of having sarcopenia, compared to those in the lowest tertile of the Mediterranean pattern. There was a significant association between a Mediterranean dietary pattern and prevalence of low gait speed (p = 0.02). The percentage of participants with low gait speed (<0.8 m/s) was lowest in the top tertile of the Mediterranean dietary pattern. In line with these findings, another cross-sectional study reported that dietary index characterised by higher intakes of whole grains, dairy products, fish, legumes, nuts, fruit, and vegetables was associated with faster 400 m walking speed (p for trend = 0.021) [66].
Overall, the evidence showed an association between dietary patterns with nuts and a lower risk of falling, sarcopenia, and low gait speed. Nuts were one of the many components in these diet quality indices, which makes it difficult to estimate the independent effect of nuts. It is likely that the combined effect of several food components within a diet will exert greater protective benefits than the individual effect of a single food. Nevertheless, these results are promising and warrant further investigation.
There is limited published research investigating the association between nut consumption and sarcopenia and its components. Results from the prospective studies and cross-sectional studies reported positive associations between nut consumption and physical function. It is important to note that no study to date has reported a detrimental effect on muscle function after nut consumption. Further research is required to draw definitive conclusions of the association between nut consumption and sarcopenia.