1000/1000
Hot
Most Recent

Hair loss (HL), also known as alopecia or baldness, is a common clinical disorder that affects millions of people worldwide and often causes a significant source of patient distress.
Hair loss (HL), also known as alopecia or baldness, is a common clinical disorder that affects millions of people worldwide and often causes a significant source of patient distress [1]. The Ebers papyrus from 1500 BCE [2] was the first known historical record of alopecia. Since then, numerous studies have been conducted to understand its main causes and pathophysiology better and to find possible treatment methods to stimulate the growth of HFs. The properties of HFs to regenerate and their cyclical growth pattern have become interesting in stem cell biology since HFs have a niche for mature stem cells—hair follicular stem cells (HFSCs). HFSCs are found in the bulge region, which resides in the attachment region of the arrector pili muscles and contains both epithelial and melanocyte stem cells [3].
The diagnosis of HL is accomplished with thorough physical examination and history-taking. Physical examination of patients presenting symptoms of HL should be conducted to evaluate the pattern of HL by examining the scalp and follicular openings with the help of a hand lens or dermatoscopy and, lastly, conducting a hair pull test. Generalized thinning of hair should not be mistaken for HL [4].
Alopecia, in general, can be divided into two groups—noncicatricial and cicatricial alopecia, as shown in Figure 3. The main difference between noncicatricial and cicatricial alopecia is that cicatricial alopecia is accompanied by scarring, in contrast to noncicatricial alopecia, where scarring is not present [5].
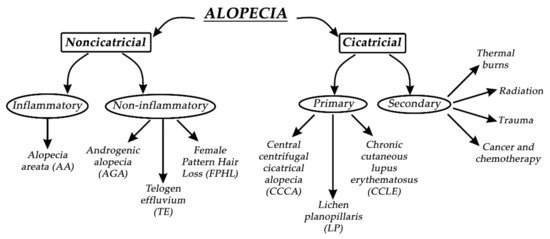
Figure 3. Types of alopecia.
In primary cicatricial alopecia (PCA), HFs are irreversibly destroyed and actively replaced by fibrous tissue [6]. The regeneration of HFs is prevented since the epithelial stem cells are destroyed in the outer root sheath’s bulge at the arrector pili muscle insertion [7]. Secondary cicatricial alopecia (SCA) is mostly caused by cancer and chemotherapy, trauma, radiation, and thermal burns [8]. PCA is further divided into three subtypes: chronic cutaneous lupus erythematosus (CCLE), lichen planopilaris (LP), and central centrifugal cicatricial alopecia (CCCA). Based on The North American Hair Research Society classification, PCA is further divided according to the nature of the predominant inflammatory infiltrate detected in scalp biopsy [9]. Since it is not rare, it represents approximately 7% of patients seen in specialist HL clinics, where one or more patches of permanent alopecia are commonly seen on the scalp. The skin around the active HFs is bald, shiny, and smooth, with closed pores, because of the complete loss of follicular openings [3][9]. The diagnosis of PCA is the most challenging, and the best opportunity to make a definitive diagnosis is to recognize the active inflammation at the site of the bald skin. It is worth mentioning that histological features in the affected sites of bald skin are not specific for PCA, and therefore, clinical examination is more reliable for diagnosis. If alopecia is not diagnosed early, it will result in irreversible HL, with no effective treatment to stimulate the growth of HFs [2]. Therefore, it is crucial to prevent further HL or transplant undamaged donor hair elsewhere on the scalp if the disease is inactive [10].
Noncicatricial alopecia can be divided into inflammatory or noninflammatory types of alopecia. Alopecia areata (AA) is a common psychologically distressing disease, which involves eyebrows, body hair, beard, eyelashes, and scalp; its etiology is closely related to autoimmune conditions such as autoimmune thyroid disease and type 1 diabetes. Due to HL on the scalp region, patients most commonly seek medical help. Patches of lost hair are sometimes light pink; this is caused by inflammation. The pathophysiological origins of AA are closely related to immune response, which specifically targets melanocytic antigens in HFs, and white hairs are commonly spared in patients with grey hair. If the regrowth of hair occurs, it is most commonly white [11]. AA is treated with intralesional corticosteroid injections into the deep dermis of affected regions, but unfortunately, acne and atrophy can result as side effects. Investigational treatments for AA are interleukin-2, interleukin 17, quercetin, JAK inhibitors, and many other active substances, with rather modest effects [12]. Pattern HL is the second most common condition, which independently affects both men and women. By the age of 50, nearly 50% of men develop androgenic alopecia (AGA), which is driven by genetic predisposition and the androgen insensitivity of HFs to androgens (specifically to dihydrotestosterone). Temporal and occipital regions of the scalp are commonly unaffected due to different responses to androgens in different scalp locations. Moreover, hair in AGA does not disappear but becomes short and miniaturized, which visually reflects the appearance of baldness. Female pattern hair loss (FPHL) is a socially distressing cause of HL in the female population, affecting up to 30% of women by the age of 50. It is similar to AGA but most commonly results in hair thinning rather than balding. Hair loss occurs at the crown, with the preservation of the frontal hairline [2][13]. Interestingly, women with FPHL have normal hormone levels. Minoxidil and finasteride are commonly used drugs for treating AGA. Topical minoxidil normalizes HF morphology by increasing the duration of anagen and affecting potassium channels. Finasteride competitively inhibits the enzyme 5α-reductase type II, which blocks the conversion of testosterone to dihydrotestosterone. It is crucial when treating FPHL to evaluate whether androgens are present or not so that the appropriate therapy can be selected [14]. Women are also more affected by telogen effluvium, which is characterized by a diffused hair loss on the scalp. Telogen effluvium (TE) starts with a trigger event 2–4 months before the onset of symptoms, which can be related to an endocrine pathophysiological event, psychological stress, drug-related event, or vitamin deficiency. Acute TE is a self-limited condition, and hair transplantation has no role in the treatment of TE [15]. A recent study has suggested that the stress hormone corticosterone—which is derived from the adrenal gland and is the rodent equivalent of cortisol in humans—regulates hair follicle stem cell (HFSC) quiescence and hair growth in mice. In the absence of systemic corticosterone, HFSCs enter substantially more rounds of the regeneration cycle throughout life. During periods of chronic stress, increased corticosterone levels prolong HFSC quiescence and maintain hair follicles in an extended resting phase [16].
Since the middle of the twentieth century, there has been a strong interest in developing in vitro skin models. From the culturing of 3D human skin biopsies ex vivo to the implementation of 3D printing, there has been a progressive scientific advancement to design skin models in vitro that can mimic human skin morphology and physiology almost entirely [17][18]. Although there have been advancements in bioengineering skin equivalents (SEs), they still do not possess the full complexity of mammalian skin. The complexity of the hypodermis, vasculature, and, especially, hair follicles (HFs) are still not fully understood and, hence, cannot be recapitulated in full detail in the laboratory environment. Current models for HF research are hair follicle organ cultures (HFOCs), monolayer cultures of specific cell types from HFs, 3D coculture systems, human skin transplantation assays, and in vivo reconstitution in immunocompromised mice. Due to legal restrictions in the use of animals in the cosmetic industry (including the 3R principle), there is an increasing need for innovation [17].
Despite all the work done, there is still a need for the development of human HF in vitro cultures to make them a suitable platform for testing various hair-growth-inducing substances and/or transplantation. For such models, the main goal lies in their successful utilization in experiments, aiming to provide previously inaccessible insights into human HF biology and pathology. Furthermore, such models could serve as tools to elucidate our knowledge of signaling within the DP as it relates to induction, maintenance, or even inhibition of hair growth [19].
Comparing 2D cultured dermal papilla cells (DPCs) with intact DPCs and 3D cultures has revealed important differences in morphology, biosynthetic activities, and DPC behavior [20][21]. The problem with monolayer 2D hair follicle models is that they fail to replicate several key features of the HF microenvironment [21]. One of the more crucial drawbacks of 2D cultures is the loss of primary inductive potency of dermal papilla cells (DPCs), which can be seen after a certain period. It predominantly correlates with a loss of the extracellular matrix protein versican and the enzyme alkaline phosphatase at higher passage numbers in the DP [22]. Versican expression in the DP is the highest in the anagen stage, decreased in the catagen stage, and apparently nondetectable in telogen, indicating its importance in the process of maintaining normal HF growth. Patients affected by male pattern baldness were found to have little-to-no versican expression in the DP [23]. It has been proven many times that simple 2D cultures do not show the expression of markers associated with HF inductivity, whereas spheroid-like 3D cultures have the ability to restore the expression of the markers, confirmed by methods such as RT-PCR and immunofluorescence. Miao et al. have examined protein expression in response to DPC cultures on Matrigel-coated plates, representing 3D-like in vitro HF models. Immunoblotting studies showed that adherent DPCs cultured on plastic (2D-style HF model) barely expressed NCAM, versican, and a-SMA proteins, while DP spheroids formed on Matrigel expressed all of them [20][24][25] β-catenin expression was there as well, which is important because of the known fact that the upregulation of canonical WNT–β-catenin signaling is essential for HF growth [26][27][28].
Since 2D models do not offer the characteristics of real hair follicles, most of the studies have put their efforts into developing 3D-like structures that resemble in vivo HFs the most in structure, signaling pathways, and cycling. There are a lot of similarities between the different models, most of them trying to establish and maintain an epidermal–mesenchymal connection to simulate its in vivo counterpart [28]. It seems that one of the simplest and most effective ways to assure this kind of formation is by seeding DPCs into microwells to allow a physiological arrangement of cells in a hair follicle [29]. H.E. Abaci, with his team, used a method of seeding DPCs into microwells, crafted in a way to allow a physiological arrangement of cells in a hair follicle.
In order to create microwells of proper dimensions, a mold was crafted with the help of a 3D printing machine. The base of the model in Figure 4, on which the HF-like extensions of the mold are printed, usually consists of one of the molecules building the extracellular matrix (ECM) in vivo, often chosen to be type-1 collagen, either on its own or combined with fibroblasts [28]. However, more appropriate molecules, with fewer limitations, are being researched [28]. Abaci et al. concluded that overexpressing the MR gene Lef-1 in combination with spontaneous DPC spheroid formation in the HSCs showed a significant increase (from 19% to 70%) in the success rate of ex vivo HF formation compared to empty-vector-transfected DPCs. However, appropriate selection of growth factors, small molecules, and proliferation enhancers needs to be addressed in future studies [26].
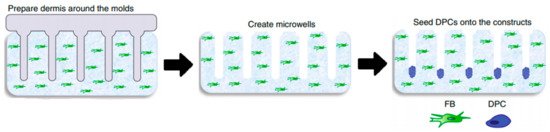
Figure 4. Molds were used to create an array of microwells from the collagen gel containing dermal fibroblasts, in which DPCs were allowed to form spontaneous aggregates. DPCs—dermal papilla cells; FB—fibroblasts. Reproduced with permission from [28].
The process of forming the perfect HF model that closely resembles the in-vivo-formed HF in all its important characteristics presents a challenge for research teams, presenting them with many problems. Methods using 3D printing technology, particularly 3D printed molds, have shown the most promising results but are still limited by resolution in the spatial control of cells. Adding a stem cell niche, such as the bulge region, is crucial in future research. Using 3D-bioprinters that would operate at single-cell resolution could mean the ability to add stem cells and melanocytes to the HF, allowing in-vitro-formed HFs to be capable of cycling and producing pigment [23].
Current models also have lots of limitations regarding the materials used, such as collagen-based models, for which the main problem lies in an extensive contraction that may happen after transplantation due to a number of proteinases active in vivo [23]. Another example is Matrigel, the origin of which (it is derived from murine sarcoma cells) causes doubts regarding its adequacy for the development of human-cell-based HF models, despite its reportedly successful use in similar models [29]. The materials mentioned above are two of the most common in vitro models now, but there is an increasing number of ongoing research studies focused on the development of a more favorable model in terms of achieving an optimal environment for human HF growth. One of the newer materials, first used by Gupta et al. in 2018, is cytocompatible silk fibroin protein. Furthermore, a gelatin-conjugated hydrogel has been developed to overcome some of the mentioned drawbacks. The hydrogel was shown to support long-term cell viability in a number of primary and stem cell populations, providing an optimal 3D microenvironment for the immobilization of growth factors, supporting the remodeling of the pericellular matrix and triggering the activation of WNT–β-catenin signaling [22]. Recently a human-derived ECM hydrogel from placenta cells was used to grow HFs in 3D culture, managing to successfully restore the hair-inductive capacity of high-passaged DPCs (shown in Figure 5) [26].
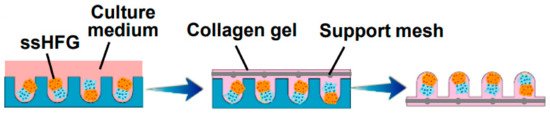
Figure 5. Self-sorted HF germs (ssHFGs) were grown in culture medium and later embedded in collagen gel to enable their transplantation. Reproduced with permission from [26].
When considering the choice of material used for the base (ECM) of 3D spheroid cultures, it is also important for the surface to be designed in the right way, allowing DPCs to form spheroids. For instance, DPCs cannot form spheroids on the surface of plates pre-coated with a thin layer of Matrigel (2D), but with a thicker coating (3D), the formation of spheroid DPCs is successful. However, it is important to note that some materials do not provide a suitable environment for the formation of spheroids in 3D culture, such as hyaluronan, even though it is a major part of the ECM [30]. Another important feature that should not be disregarded is to assure sufficient oxygen supply, which is critical for proper cell growth, especially when 3D aggregates are prepared within a condensed space with very limited interspaces between them [31].
For purposes of culturing HFs in vitro, researchers have experimented with different cell types that would mimic the actual morphology and physiological environment in human skin tissue. DPCs are a population of mesenchymal cells in the human skin, regulating HF growth and serving as a reservoir of multipotent stem cells [32]. In the 1960s, transplanted DPCs from adult HFs demonstrated a regenerative capacity to induce the growth of new HFs [21]. There is a demand to expand human DPCs in vitro without any impact on their in situ properties. Ohyama et al. were the first to elucidate the molecular structure of noncultured human DPCs. In total, 118 human DP signature genes were identified. Bioinformatics analysis of this DP gene list revealed that WNT, BMP, and FGF signaling pathways were upregulated in intact DPs. The addition of 6-bromoindirubin-39-oxime, recombinant BMP2, and basic FGF to stimulate these respective signaling pathways resulted in the maintained expression of in situ DP signature genes in primarily cultured human DPCs. It was concluded that DPC exposure to the stimulants mentioned above restored DP biomarker expression, which is normally reduced in cultured human DPCs [33]. Havlickova et al. introduced the minimal criteria when designing a human folliculoid in vitro system, supporting the claim that it sufficiently imitates the in vitro situation. One of their claims is that outer root skin keratinocytes (ORSKs) should be physically interacting under 3D conditions and that basement membrane components should be included in the extracellular matrix because the mesenchyme and HF epithelium interact via a basement membrane. Folliculoid 3D systems should be continuously submerged in the culture to mimic the natural environment of human HFs, and the interaction of the HF epithelium and mesenchyme should be supported by fibroblast-contracted collagen type I gel. Furthermore, epithelial HFCs should form concentric cell aggregates to mimic the epithelial tissue compartments in the hair matrix of the HF in vivo. Epithelial HF cells should possess the ability to proliferate HF/ORSK-type keratinization and express a high level of glycogen expression and a low apoptosis level. Minimal proliferation, minimal apoptosis, specific secretory activities, and strong expressions of hepatocyte growth factor (HGF), neural cell adhesion molecule NCAM, and, especially, versican are essential to obtain appropriate interactions between HF mesenchymal cells in such 3D systems [20][23][26][27][28][33][34][35]. Versican is a large chondroitin sulfate proteoglycan molecule, an important initiator of hair regeneration and hair growth maintenance and an inductor of hair morphogenesis. Versican immunoreactivity in DPC is lost when HFs are affected by male pattern baldness [36][37]. When DPCs are removed from the HF microenvironment in human tissue, they lose their ability to induce hair growth. In 2013, DPCs were first successfully grown in three-dimensional papilla spheroids using hanging drop culture systems between separated epidermis and dermis [38]. Since then, further research has been conducted to prove the hypothesis that the transcriptional signature can be partially restored by the growth of DPCs in 3D spheroid cultures [39]. Nonfollicular cell populations have also gained more interest recently due to the scarcity of donor hair. Multipotent dermal progenitors (SKPs) are similar to DPC and have proven clinical relevance for stem cell replacement applications [30]. Unfortunately, their inductive hair potential in the human species remains a mystery [38]. Although human-induced pluripotent stem cells (hiPSCs) also express the ability to generate DPCs and hair follicle stem cells (HFSCs), strong safety standards should be considered [32].
Once more, it is worth mentioning that DPCs quickly lose their ability to induce HFs after they are cultured in a culture medium. Furthermore, it is necessary to activate the WNT and BMP signaling pathways, as well as grow DPCs in 3D aggregates and, finally, culture them with keratinocytes to mimic their native in vivo microenvironment [22]. Different 3D models of HFs require scaffolds made mostly from biomaterials. Biomaterials can be implemented as a matrix that supports the encapsulation of dissociated cells or as a supportive scaffold with known rheological properties for designing 3D cell cultures [37]. The organotypic folliculoid 3D system was prepared from collagen I mixed with human dermal fibroblasts (HDFs) and layered, first, with Matrigel and DPCs and, second, with ORSK on the top. When the cell culture was grown in low calcium and serum-free conditions, it represented optimal growth [35]. In another study, DPCs were also cultured in Matrigel medium, which mimics the niche of DPCs [28]. DPCs were cocultured with human extracellular matrix (ECM) proteins such as collagen IV, fibronectin, and laminin [27]. In another study, DPCs and epithelial cells were cultured in collagen–chitosan scaffolds to stimulate epithelial–mesenchymal cellular interactions [40]. New ECM-like compounds are continuously being tested. In a recent study by Tan et al., the authors indicated some interesting outcomes. Using gelatin methacrylate (GelMA)-coated microwells for HF morphogenesis resulted in the production of heterotypic aggregates over prolonged culture duration, without exfoliation of keratinocytes [41]. 3D-printing technology was also implemented, where plastic molds were microfabricated and used to create an array of microwells on a type I collagen gel containing dermal fibroblasts. DPCs were seeded over microgels, which led to spontaneous aggregate formation. With adjustments to the diameter of the microwells, they controlled the size of the DPC aggregates [26]. In 2019, a systematic approach was introduced to produce 3D core-shell heterotypic spheroids, implementing DPCs, keratinocytes, and HDFs into microarray hydrogels fabricated from poly(ethylene glycol) diacrylate (PEGDA) with the application of soft photolithography [23]. Another study concluded that when DPCs and keratinocytes are compartmented in a core-shell structure and both seeded on an ethylene vinyl alcohol (EVAL) surface, they express the ability to grow into multicellular spheroids [42]. Kageyama et al. presented a method to grow epidermal and mouse/human mesenchymal cells in microwells of a custom-designed array plate, fabricated using gas-permeable PDMS. Spontaneous hair follicle germ (HFG) formation in vitro was observed, which presented a suitable method for large-scale production of HFG cells [43]. A more sophisticated HF organoid model was developed by encapsulating the DP spheroid with HF keratinocytes and HF stem cells in a silk–gelatine hydrogel. A hypoxia-induced medium enhanced DP-specific gene expression and cellular proliferation [44].
Since 2004 (the restriction was amended in 2009), animal testing in the European Union is prohibited for cosmetic ingredients and products. In addition, cosmetic products containing ingredients tested on animals were forbidden in the year 2009. Since then, many in vitro models to study cosmetic ingredients have become available, serving as a valuable and effective tool for testing the absorption and permeability of cosmetic ingredients. For instance, EpiSkinTM, developed by L’Oreal, was validated and recognized as a screening and replacement model. Histological stained images of T-Skin™ with all of its components is presented on Figure 6.
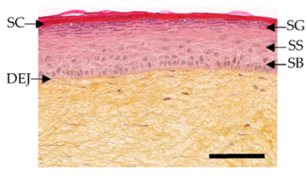
Figure 6. Histological stained images of T-Skin™ with stratum corneum (SC), stratum granulosum (SG), stratum spinosum (SS), stratum basale (SB) and dermo-epidermal junction (DEJ). Reproduced with permission from.
This model represents the skin's epidermis layers, mimicking the native tissue epidermal layers: stratum basale, stratum spinosum, stratum granulosum, and stratum corneum. It consists of a dermal substrate based on type I bovine collagen matrix, which represents the dermis. A stratified differentiated epidermis is laid upon after 13 days in culture. In vitro toxicology test for dermal corrosivity, skin irritation, and phototoxicity could also be achieved with EpidermTM, SkinEthicsTM, and epiCS®. Predicting the possible skin sensitization, also known as contact allergic dermatitis (ACD), could be tested with Direct Peptide Reactivity Assay (DPRA), KeratinoSens™. Systemic toxicity, carcinogenicity, genotoxicity, reproductive toxicity, and endocrine toxicity should also be tested in vitro accordingly.
Hair growth stimulating active compounds have been tested both in vivo and in vitro. In vivo tests provide a better understanding of pathophysiological processes involved in alopecia. Still, due to legal restrictions, amendments to the European Union's Cosmetics Directive phased animals' use in testing for any acute toxic effects of beauty products, and toiletries. There are different arguments regarding the inappropriateness of the animal models besides the ethical ones. For example, human HFs growth phases differ in comparison to animals. Secondly, human HF growth does not occur synchronized. Therefore, in vitro human HF models to study hair growth are essential to screen for active compounds and test their efficacy for treating alopecia or simply improving the rate of hair growth. On the other hand, there is scarceness of donated HFs, mainly due to invasive extraction methods and a limited number of HFs.
Since DPCs regulate HF's growth and cycling, they are commonly used to study hair growth and regeneration. Because of their short life span in vitro, Kwack et al. developed an immortalized human DPC line, SV40ThTERT-DPC, by introducing the human telomerase reverse transcriptase (hTERT) gene into the transformed cell line, simian virus 40 large T DPC (SV40T-DPC). Following the first study, they co-transfected the SV40T antigen (SV40T-Ag) and hTERT into DPC from male scalp HFs with AA and established five immortalized DPC lines. Primary and immortalized DPCs are already commercially available. Since HF growth is intertwined with the activity of numerous growth factors (VEGF, IGF, HGF, KGF, TGF-β2) and signaling pathways (ALP, WNT/b catenin pathway, Akt and MAPK, JNK, ERK), the evaluation of DPC proliferation is challenging. A comprehensive review article was published in 2018. Numerous active compounds for HF growth tested on DPC were evaluated, and their efficacy was later studied in vivo and ex vivo. Active compounds that stimulate DPC in vitro are minoxidil, numerous herbal extracts, plant actives, natural products, growth factors, cytokines, platelet-rich plasma, placental extract, stem cells, conditioned medium, peptide/proteins, squarticles, mimetics etc. Effects of light, electromagnetic field, and electrical stimulation also induce HF proliferation and stimulate DPC in vitro. Testosterone and DHT exerted inhibitory effects on DPC. The assessment of the stemness and senescence properties of DPC were also taken into consideration.
Although DPCs present an established method for assessing hair growth, they do not resemble HF morphology's wholeness. Existing assays with DPCs should be co-cultured with other HF cells, such as keratinocytes, fibroblasts to understand better the mechanisms of action of selected active compounds, which might possess the ability to induce growth of HFs.
Gupta et al. tested three-dimensional DP spheroids with and without a silk-gelatin microenvironment and used them as a screening assay by using a standard drug for AA – minoxidil. DP spheroids were able to express DP-specific gene, which resulted in enhanced ECM production compared to monolayer culture. Furthermore, they established an in vitro 3D organoid model where they encapsulated DP spheroids in silk-gelatin and combined them with keratinocytes and stem cells. Elevated HF markers with epithelial-mesenchymal crosstalk were established, which provides new insights into understanding cell-cell interactions and mechanisms responsible for HF cycling in vivo. Their 3D HF assay could be used to screen and evaluate different molecules that might represent inductive HF growth properties.
Human HFs can be switched from anagen to catagen in vitro but modeling the human hair growth cycle in vitro is impossible. Therefore, the best model to study HF cycling is the murine model. Screening models for the hair growth assessment are in vivo C57BL/6 and C3H mice model. Ex vivo human and mouse HF cultures are also commonly used to induce the anagen growth phase of HF and, secondly, to achieve hair shaft elongation. The HF research has been conducted so far on mice, rats, hamsters, rabbits, and sheep in laboratory conditions. The widely used de novo model for testing hair growth is the silicone chamber assay, where cells are implanted inside grafting chambers onto the backs of nude mice. Epidermal and dermal cells from a new-born mouse serve as a positive control. Three weeks after grafting, an area of visible hair can be seen at the site of implantation. When using cells from new-born mice to serve as a positive control, an area of visible hair develops in each chamber about 3 weeks after grafting. Either one or several cell components can then be replaced with candidate cells. Since silicone chamber assay requires surgical procedure with the help of a special apparatus and since it is labor-intensive, it is not efficient in high throughput screening procedures, but rather used in testing candidate molecules and cells. Furthermore, it could be used for evaluating the effects of specific gene products and their hair inductive abilities in vivo.
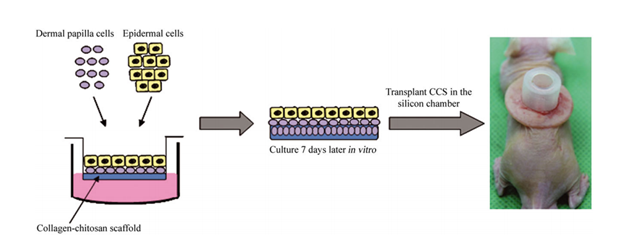
Figure 7. Schematic representation of inoculated DPCs and epidermal cells being transplanted in the silicon chamber on the back of the nude mouse. Reproduced with permission from.
Another assay method was proposed by Zheng et al., where epithelial and mesenchymal cells were injected at certain rations into mice’s hypodermis. K15 positive adult epidermal cells from transgenic mice were isolated, and their ability to generate hair was further evaluated by combining them with neonatal dermal cells in the patch assay. Furthermore, genetic analysis of HF stem cells exposed several known and unknown receptors and signaling pathways, which are important for maintaining stem cell phenotype. Such research targets could provide potential targets for treating hair loss and other disorders related to hair and skin. Figure 7 shows a similar approach of inoculated DPCs and epidermal cells being transplanted in the silicon chamber on the back of the nude mouse. As with other types of assays, the patch assay also has some limitations such as difficult visual interpretation of hair growth, which requires surgical biopsy and the loss of macro-environment since the orientation of HFs is random. The creation of HF in vitro was recreated in tissue culture, and the proposed method was capable of producing a well-defined hair shaft and revealed similarities in terms of their in vivo counterparts. The assembly of follicular keratinocytes, melanocytes, and fibroblasts was also studied electron microscopy and molecular analysis. De novo created human microfollicles were implemented in existing chip-based human skin equivalents for substance testing. Functional neopapillae or DP condensates needed more than 48 hours to form, and after adding keratinocytes and melanocytes, the microorganoids started to self-organize by generating sheath formations, polar segments, and finally, a hair shaft – like fibre.
The ideal model system for a precise understanding of the biochemical function of molecules should, therefore, be able to induce de novo HF or be able to reconstruct them from their component cells. Further improvements in designing HFs in vitro might establish a gold-standard screening assay and provide an opportunity for creating improved implants for treating wound conditions, transplanting hair in patients with alopecia, etc.
Hair loss (HL) in response to various factors and pathological mechanisms such as age, genetics, hormonal imbalances, medical procedures, and trauma is a common disorder affecting both men in about 50% and women in approximately 25%, by the age of 50 worldwide. Consequently, there is a huge demand for effective treatment. Currently, the most obvious choices to cure different types of alopecia are pharmacological or surgical treatments. Still, they are far from being the ultimate solution since they all lack in both efficacy and applicability. Pharmaceutical treatment, being useful only in the early stages of alopecia, brings many negative side effects, especially long term, making it anything but ideal. Treatment with Minoxidil (Rogaine) can cause scalp irritation and unwanted hair growth on the face and hands' skin. Finasteride (Propecia), on the other side, can cause diminished sex drive and sexual function and an increased risk of prostate cancer. On the other hand, surgical treatment has proved itself useful in most cases of hair loss, but in addition to being an invasive method, it has plenty of drawbacks. The two most reliable surgical options are autologous single follicles or follicular unit transplantation. Still, the limited number of donor follicles is a big disadvantage. It often occurs that there is not enough available HFs to cover the patient's alopecic regions, limiting the number of patients suitable for this kind of treatment. Scientists are still trying to develop methods that would be more clinically applicable in terms of invasiveness, cost, technical complexity, and reproducibility and are constantly testing new innovative solutions.
In the case of reconstructive medicine where larger defects are present together with full-thickness skin loss (not just HFs by conditions such as androgenic alopecia and alopecia areata), the use of human skin constructs (HSCs), also known as dermal-epidermal composites (DEC), is becoming a new promising approach. They are usually comprised of dermal fibroblasts embedded in a matrix such as collagen and overlaid with keratinocytes. The most prominent problem with HSCs was achieving a full thickness of skin, meaning the presence of subcutis alongside with formation of complete appendages, also involving mature shaft producing hair follicles associated with sebaceous glands. An important fact that was discovered is that the successful appendage formation correlates with the donor cells' age. Wu et al. discovered that HSCs with hairs formed from foreskin epidermal (FK Epi) together with fetal dermal (fDer) cells, showed that 15 out of 18 constructs produced hair. On the other hand, HSCs with no hairs formed from adult epidermal (Adult Epi) with adult dermal (Adult Der) cells showed that 0 out of 9 constructs produced hair. The results were observed 3 months after implanting HSCs on immunodeficient mice. Through the evolution of 3D bioprinting and robotic harvesting methods, it is expected that we will be able to produce HF rich HSCs resembling skin with all its components.
Hair regenerative medicine seems to be the most promising approach for the future. Nevertheless, it is held back by some limitations, particularly problems regarding the development of fully functional transplantable hair follicles of human origin grown in vitro. The goal for the cell-based regenerative medicine is to be autologous, avoiding tissue immune rejection and, at the same time restoring epithelial‐mesenchymal interactions, imitating the embryonic process of hair follicle neogenesis. During the anagen phase, the hair follicle was found to be the site of relative immune privilege, expressing a low amount of the major histocompatibility (MHC) class Ia antigens and locally producing potent immunosuppressive agents such α-MSH and TGF-β1. The latter could be an indication that the use of allogeneic cells could also be a suitable option for HF regeneration. Recently, it was shown that DPCs are not the only ones being able to induce hair follicle formation. Namely, multipotent skin-derived precursors (SKPs) have a similar ability, while exhibiting long term proliferation potential, when being cultured in spheroids. Current studies were executed only in mice, but SKPs have been able to induce de novo hair genesis, showing potential for future application of SKPs in hair follicle regeneration and bioengineering. Nevertheless, due to potential epigenetic instability, SKP cells may lose their inductive potential after culture expansion. Ling Guo et al. discovered that supplementation of Trichostatin A (TSA) upon culture expansion of SKPs caused an important increase in their hair induction ability by upregulating the level of histone H3 acetylation in K9 and K14, as well as elevating BMP signaling activity [85]. Also, other cellular sources with the ability to differentiate into hair follicles are being tested to determine their applicability for the formation of in vitro hair follicles, appropriate for hair transplants.
As of now, there have not been any reported cases of in vitro hair follicle transplantation into the human scalp, but plenty of studies researching the integration of human cell hair follicles into the back or scalp of nude mice. Promising results were noticed in studies testing culture array chips that ensure dense and spatially arranged manner of hair follicles replicating the density of the HFs found on the human head. Kageyama et al. developed a method for large-scale preparation of HFs. After the formation of HFs, they were poured with mesh reinforced collagen to ensure successful removal from the chip. Still, upon transplantation, there was severe contraction of collagen caused by enzyme activity, resulting in spaces between hairs shrinking to a tenth of the original one, making collagen base insufficient option for in vivo transplantation. Even with its drawbacks, the mentioned method could still be a viable option for producing large amounts of transplantable HFs in the future if collagen is replaced with more optimal material. The study incorporated a mixture of human and murine cells in the experiment, preventing properly assessing this method's feasibility in human HF transplantations, still leaving some room to further experimentation with human origin trichogenic cells.
When extracting only DPCs and expanding them in culture, the problem is losing their inductive potential and variable quality of the hair shaft. To preserve their ability of induction, specific factors can be added, such as WNT-CM [50], keratinocyte-conditioned medium basic FGF, Lef-1 overexpression or growing them in 3D cultures. The perfect transplantation method for DPCs has yet to be developed, despite all the efforts in this field. Aoi N. et al. tested 5 different transplantation methods on nude mice, using either DPCs or fully differentiated dermal papilla tissue (DPT). One method, particularly the hemi-vascularized sandwich (HSV) method, where the epidermis with the upper part of the dermis was detached, showed promise. The upper dermal fragment was discarded, and the DPT or DPCs were sandwiched between the remaining part of the dermis and epidermis, which was sutured at the end. Although the epidermis was fixated, the dislocation of DPCs was present to some degree. This method displayed high hair regeneration efficiency compared to the other 4 methods studied, mainly due to direct contact of DPCs with the basal layer and sufficient vascularization of the recipient bed. It has further shown the great importance of oxygenation in the induction of hair formation by DPCs transplantation.