
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carole Henique | + 1416 word(s) | 1416 | 2021-04-25 05:21:09 | | | |
| 2 | Camila Xu | Meta information modification | 1416 | 2021-05-05 11:40:00 | | |
Video Upload Options
VEGF-A (also called VEGF) is a member of the mammalian platelet-derived growth factor (PDGF) supergene family which also includes VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF).
1. VEGF, Pro-Angiogenic Factor, Renal Expression
The most important molecule that promotes angiogenesis and increases vascular permeability is VEGF-A (also called VEGF). It is a member of the mammalian platelet-derived growth factor (PDGF) supergene family which also includes VEGF-B, VEGF-C, VEGF-D and placental growth factor (PlGF). VEGF signals mainly through the VEGF receptor 2 (VEGFR2) and also binds VEGFR1, both members of the VEGFR tyrosine kinase family (VEGFR1, VEGFR2, and VEGFR3, encoded by the genes FLT1 (Fms Related Receptor Tyrosine Kinase 1), KDR (Kinase Insert Domain Receptor), and FLT4 (Fms Related Receptor Tyrosine Kinase 4), respectively), which is expressed at elevated levels in endothelial cells [1][2]. VEGFR1 has a substantially higher affinity for VEGF than for VEGFR2 but mediates a much lower pro-angiogenic activity [3]. VEGFR3 is mainly restricted to lymphatic endothelial cells.
Receptor tyrosine kinases (RTKs) have a similar molecular structure, with a ligand-binding site in the extracellular domain, a transmembrane region, and a cytoplasmic region that contains the protein tyrosine kinase (TK) domain with an ATP-binding site. Growth factors bind their specific extracellular domain and activate RTKs by inducing receptor dimerization, which, in turn, activates their autophosphorylation, thus initiating a cascade of downstream signaling events [4]. They are key regulators of critical cellular processes such as proliferation and differentiation, cell survival, metabolism, and migration.
Several studies have documented the importance of VEGF signaling in maintaining glomerular integrity [5][6][7]. In the glomerulus, VEGF is expressed and secreted by podocytes, which are highly differentiated visceral epithelial cells with foot processes, located on the urinary side of the glomerular basement membrane. VEGFRs are expressed on the surface of both endothelial cells and podocytes, even if the latter remains controversial [8][9][10][11][12]. Indeed, the autocrine effect of VEGF on the podocyte has been discussed. Bertuccio and Veron et al. have established that VEGFR2 is expressed in adult mouse podocytes and glomerular endothelial cells [10][13]. Müller-Deil et al. have identified VEGFRs in murine and human podocyte cell cultures [11], while Wang et al. have shown the expression and distribution of VEGFR2 both in endothelial cells and in podocytes by immune electron microscopy and immunofluorescence in human patients [9]. However, when Eremina et al. induced a podocyte-specific deletion of VEGFR2 in mice, they did not observe any effect on either glomerular development or function. They failed to detect any expression of VEGFR2 in podocytes [8].
This configuration allows a VEGF-mediated epithelial–endothelial crosstalk and contributes to the functional glomerular filtration barrier through survival, proliferation, and/or differentiation of the adjacent fenestrated glomerular capillary endothelial cells [14]. VEGF is known to exert bidirectional effects on podocytes, depending on its expression level. In adult mice, chronic VEGF knockout induced thrombotic microangiopathy (TMA) [6]. The best documented example for a pathological role of VEGF inhibition in the kidney is pre-eclampsia. Pre-eclampsia is a hypertensive disorder peculiar to pregnancy (4–5% of pregnant women). This systemic syndrome appears to originate in the placenta and is characterized by widespread maternal endothelial dysfunction, the presence of new-onset hypertension, and proteinuria or other end-organ damage occurring after 20 weeks of gestation [15]. The pathogenesis of pre-eclampsia relies on placental ischemia, abnormal spiral artery remodeling, and oxidative stress, leading to increased systemic levels of the circulating soluble form of VEGFR1 (also known as sFlt1, soluble Fms-like tyrosine kinase-1), an antagonist of VEGF, and PlGF [16][17]. Indeed, excess levels of the anti-angiogenic factor sFlt1 are associated with decreased circulating levels of VEGF and PlGF, resulting in maternal endothelial dysfunction, glomerular endotheliosis, and proteinuria, which may progress to thrombotic microangiopathy [15][18][19]. By contrast, podocyte VEGF overexpression induces collapsing glomerulopathy [10][14]. Renal expression of VEGF and its receptors is upregulated in patients with diabetic nephropathy, which induces new vessel formation in the kidney, stimulates renal hypertrophy, and causes proteinuria in experimental models [20][21]. An in vitro study showed that VEGF promotes podocyte survival through an autocrine pathway involving VEGFR2, inducing podocin upregulation and its association with CD2AP (CD2-associated protein), an adaptor molecule regulating podocyte actin polymerization [22]. Moreover, VEGFR2 interacts with nephrin, an adhesion protein and key regulator of podocyte survival via Akt signaling. Indeed, VEGFR2 is rapidly phosphorylated in response to VEGF and recruits the Src kinase Fyn, which binds to nephrin and initiates a cascade of phosphorylation, leading to actin cytoskeleton polymerization and actin stress fiber formation [13][23].
2. Anti-Angiogenic Drugs
Solid tumor growth and metastasis spreading depend on angiogenesis. Various signals may trigger the angiogenic switch, for example, metabolic stress (hypoxia, low pH, or hypoglycemia), mechanical stress, immune/inflammatory response, and genetic mutations [24][25][26]. VEGF secreted by tumor cells stimulates endothelial cell proliferation and survival, leading to the establishment of new blood vessels [3]. Indeed, hypoxia regulates angiogenesis at every step of this process through multiple pathways, including VEGF. The master oxygen homeostasis regulators of this process are the hypoxia-inducing factors, HIFs. The founding member of this family is HIF-1α [27].
In 1971, Folkman introduced anti-angiogenesis as a new anti-cancer strategy [28]. However, only in 2004, the US Food and Drug Administration (FDA) approved bevacizumab, a humanized anti-VEGF monoclonal antibody, for metastatic colorectal cancer treatment [29]. To date, several anti-angiogenic therapies have been developed and approved to treat cancers and other activated VEGFR-related diseases.
2.1. Anti-VEGF mAb and Tyrosine Kinase Inhibitors
These drugs can be classified into two categories: small-molecule inhibitors that target the ATP-binding site of RTK intracellular domain (tyrosine kinase inhibitor, TKI), and monoclonal antibodies (mAbs) that either interfere with the RTK extracellular domain or target the VEGF ligand (anti-VEGF) [1][30] (Figure 1).
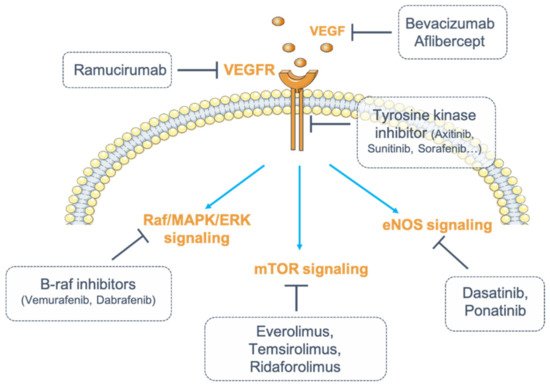
Figure 1. Inhibition of vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor (VEGFR) signaling. Numerous strategies exist to inhibit VEGF/VEGFR signaling. VEGF can be blocked by monoclonal antibodies (mAbs) (bevacizumab) or by fusion proteins (aflibercept). Its receptor, VEGFR, can be targeted by fully humanized monoclonal antibodies (ramucirumab). The receptor can also be targeted for its intracellular tyrosine kinase activity (tyrosine kinase inhibitors, TKIs). Finally, one strategy consists of inhibiting the downstream signaling pathways of VEGFR by targeting either the Raf (Rapidly Accelerated Fibrosarcoma)/mitogen-activated protein kinase (MAPK)/ERK (Extracellular signal-Regulated Kinase) pathway with B-Raf inhibitors (dabrafenib, vemurafenib), or the endothelial nitric oxide synthase (eNOS) pathway (dasatinib, ponatinib) or the mammalian target of rapamycin (mTOR) pathway (everolimus, temsirolimus, ridaforolimus).
VEGF inhibitors through antibody binding include bevacizumab, ranibizumab, aflibercept, and ramucirumab. Bevacizumab and ranibizumab are monoclonal antibodies (mAbs), and aflibercept is a recombinant fusion protein that acts as a soluble decoy receptor or VEGF trap. Ramucirumab is a fully humanized mAb that specifically inhibits VEGFR2. Among these angiogenesis inhibitors, some are used either alone or in combination with chemotherapy, while most TKIs are multi-kinase inhibitors targeting VEGFRs and other RTKs simultaneously [31] (Table 1).
Table 1. Incidence of renal manifestations and electrolytic disorders under anti-angiogenic targeted therapies.
| Drugs | Molecular Targets | Tumor Targets | Adverse Events (Incidence) | Electrolytic Disorders | References |
|---|---|---|---|---|---|
| Monoclonal antibodies | |||||
| Bevacizumab | VEGF | CRC, NSCLC, RCC, GBM, epithelial ovarian cancer, primary peritoneal cancer, cervical cancer, fallopian cancer, glioblastoma, ocular diseases | HTN (23–41%), Proteinuria (2–32%) | Hypophosphatemia, Hyponatremia | [32][33][34][35][36][37][38][39][40][41][42] |
| Ranibizumab | VEGF | Ocular diseases | HTN, Proteinuria | - | [32][33] |
| Ramucirumab | VEGFR2 | CRC, NSCLC, GC | HTN, Proteinuria | - | [32][33] |
| Recombinant fusion protein | |||||
| Aflibercept | VEGF | CRC, ocular diseases | HTN, Proteinuria | - | [32][33] |
| Multitargeted TKI | |||||
| Sorafenib | VEGFRs, PDGFRs, RAF, c-Kit, FLT3, Ret | RCC, HCC, DTC | HTN (17–55%), Proteinuria (10%) | Hypophosphataemia (16–85%), Hyponatremia (39%) | [43][44][45][46][47][48][49][50][51][52] |
| Sunitinib | VEGFRs, PDGFRs, FLT3, CSF1R, Ret | RCC, GIST, pNETs | HTN (22–60%), Proteinuria (10–65%) | Hypophosphatemia, Hyponatremia | [53][54][55][56][57][58][59][60] |
| Pazopanib | VEGFRs, PDGFRs, FGFR1, c-Kit | RCC, STS | HTN (40–52%), Proteinuria (13.5–18%) | Hypophosphatemia (34%), Hypocalcemia (33%), Hyponatremia (31%), Hypomagnesemia (11%) | [43][44][61][62][63][64][65][66][67] |
| Vandetanib | VEGFRs, EGFR, Ret | MTC | HTN (23.5–84%), Proteinuria (5.6–26%) | Hypomagnesemia (10–40%)Hypocalcemia (4–29%)Hypokaliemia (4–17%) | [68][44][69][70][71][72][73][74] |
| Axitinib | VEGFRs, PDGFRs, c-Kit | RCC | HTN (40–64%), Proteinuria (4.6–23%) | Hyponatremia, Hypophosphatemia (13%), Hypocalcemia (39%) | [68][43][44][75][76][77][78] |
| Regorafenib | VEGFRs, PDGFRs, FGFRs, Tie2, c-Kit, Ret, RAF | GIST, CRC, HCC | HTN (13–59%), Proteinuria (7–9.4%) | Hypophosphataemia (5–18%) | [68][43][44][49][79][80][81] |
| Cabozantib | VEGFRs, c-Met, AXL, c-Kit, FLT3, Ret | MTC, RCC | HTN (7–16), Proteinuria (6%) | Hypophosphatemia (4–8%) | [68][82][83][84][85][86] |
| Nintedanib | VEGFRs, PDGFRs, FGFRs, SRC | IPF, NSCLC | HTN, Proteinuria | - | [87] |
| Lenvatinib | VEGFRs, FGFRs, PDGFRa, Ret, c-Kit | DTC, RCC, HCC | HTN (45–100%), Proteinuria (26.9–100%) | Hypophosphatemia (45%) | [68][49][88] |
| Dasatinib | BCR-ABL, SRC, LCK, YES, FYN, c-Kit, VEGFR, PDGFR | CML, Ph+ ALL | Proteinuria | Hyponatremia | [89][90] |
| Ponatinib | VEGFRs, BCR-ABL, FLT3, Ret, c-Kit, FGFRs, PDGFR | CML, Ph+ ALL | HTN (9–32%) | - | [91][92][93] |
CML: chronic myeloid leukemia; CRC: colorectal cancer; CSF1R: colony stimulating factor 1 receptor; DTC: differentiated thyroid cancer; FGFR: fibroblast growth factor receptor; FLT3: fms like tyrosine kinase 3; GBM: glioblastoma multiforme; GC: gastric cancer (or gastroesophageal junction adenocarcinoma); GIST: gastrointestinal stromal tumor; HCC: hepatocellular carcinoma; HTN: hypertension; IPF: idiopathic pulmonary fibrosis; LCK: lymphocyte-specific protein tyrosine kinase; MTC: medullary thyroid cancer; NSCLC: non-small cell lung cancer; PDGFR: platelet-derived growth factor; Ph+ ALL: Philadelphia-chromosome-positive acute lymphoblastic leukemia; pNETs: progressive pancreatic neuroendocrine tumors; RAF: rapidly accelerated fibrosarcoma; RCC: renal cell carcinoma; STS: soft tissue sarcoma; VEGF: vascular endothelial growth factor; VEGFR: vascular endothelial growth factor receptor.
References
- Ivy, S.P.; Wick, J.Y.; Kaufman, B.M. An Overview of Small-Molecule Inhibitors of VEGFR Signaling. Nat. Rev. Clin. Oncol. 2009, 6, 569–579.
- Adams, R.H.; Alitalo, K. Molecular Regulation of Angiogenesis and Lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478.
- Kerbel, R.S. Tumor Angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049.
- Lemmon, M.A.; Schlessinger, J. Cell Signaling by Receptor Tyrosine Kinases. Cell 2010, 141, 1117–1134.
- Tanabe, K.; Wada, J.; Sato, Y. Targeting Angiogenesis and Lymphangiogenesis in Kidney Disease. Nat. Rev. Nephrol. 2020, 16, 289–303.
- Eremina, V.; Jefferson, J.A.; Kowalewska, J.; Hochster, H.; Haas, M.; Weisstuch, J.; Richardson, C.; Kopp, J.B.; Kabir, M.G.; Backx, P.H.; et al. VEGF Inhibition and Renal Thrombotic Microangiopathy. N. Engl. J. Med. 2008, 358, 1129–1136.
- Sivaskandarajah, G.A.; Jeansson, M.; Maezawa, Y.; Eremina, V.; Baelde, H.J.; Quaggin, S.E. Vegfa Protects the Glomerular Microvasculature in Diabetes. Diabetes 2012, 61, 2958–2966.
- Sison, K.; Eremina, V.; Baelde, H.; Min, W.; Hirashima, M.; Fantus, I.G.; Quaggin, S.E. Glomerular Structure and Function Require Paracrine, Not Autocrine, VEGF-VEGFR-2 Signaling. J. Am. Soc. Nephrol. JASN 2010, 21, 1691–1701.
- Wang, H.; Yue, Z.; Wu, J.; Liu, T.; Mo, Y.; Jiang, X.; Sun, L. The Accumulation of VEGFA in the Glomerular Basement Membrane and Its Relationship with Podocyte Injury and Proteinuria in Alport Syndrome. PLoS ONE 2015, 10, e0135648.
- Veron, D.; Reidy, K.J.; Bertuccio, C.; Teichman, J.; Villegas, G.; Jimenez, J.; Shen, W.; Kopp, J.B.; Thomas, D.B.; Tufro, A. Overexpression of VEGF-A in Podocytes of Adult Mice Causes Glomerular Disease. Kidney Int. 2010, 77, 989–999.
- Müller-Deile, J.; Worthmann, K.; Saleem, M.; Tossidou, I.; Haller, H.; Schiffer, M. The Balance of Autocrine VEGF-A and VEGF-C Determines Podocyte Survival. Am. J. Physiol. Renal Physiol. 2009, 297, F1656–F1667.
- Ku, C.-H.; White, K.E.; Dei Cas, A.; Hayward, A.; Webster, Z.; Bilous, R.; Marshall, S.; Viberti, G.; Gnudi, L. Inducible Overexpression of SFlt-1 in Podocytes Ameliorates Glomerulopathy in Diabetic Mice. Diabetes 2008, 57, 2824–2833.
- Bertuccio, C.; Veron, D.; Aggarwal, P.K.; Holzman, L.; Tufro, A. Vascular Endothelial Growth Factor Receptor 2 Direct Interaction with Nephrin Links VEGF-A Signals to Actin in Kidney Podocytes. J. Biol. Chem. 2011, 286, 39933–39944.
- Eremina, V.; Sood, M.; Haigh, J.; Nagy, A.; Lajoie, G.; Ferrara, N.; Gerber, H.-P.; Kikkawa, Y.; Miner, J.H.; Quaggin, S.E. Glomerular-Specific Alterations of VEGF-A Expression Lead to Distinct Congenital and Acquired Renal Diseases. J. Clin. Invest. 2003, 111, 707–716.
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-Eclampsia: Pathogenesis, Novel Diagnostics and Therapies. Nat. Rev. Nephrol. 2019, 15, 275–289.
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess Placental Soluble Fms-like Tyrosine Kinase 1 (SFlt1) May Contribute to Endothelial Dysfunction, Hypertension, and Proteinuria in Preeclampsia. J. Clin. Invest. 2003, 111, 649–658.
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the SFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22.
- Henao, D.E.; Saleem, M.A.; Cadavid, A.P. Glomerular Disturbances in Preeclampsia: Disruption between Glomerular Endothelium and Podocyte Symbiosis. Hypertens. Pregnancy 2010, 29, 10–20.
- Henao, D.E.; Saleem, M.A. Proteinuria in Preeclampsia from a Podocyte Injury Perspective. Curr. Hypertens. Rep. 2013, 15, 600–605.
- Nakagawa, T.; Tanabe, K.; Croker, B.P.; Johnson, R.J.; Grant, M.B.; Kosugi, T.; Li, Q. Endothelial Dysfunction as a Potential Contributor in Diabetic Nephropathy. Nat. Rev. Nephrol. 2011, 7, 36–44.
- Schrijvers, B.F.; Flyvbjerg, A.; De Vriese, A.S. The Role of Vascular Endothelial Growth Factor (VEGF) in Renal Pathophysiology. Kidney Int. 2004, 65, 2003–2017.
- Guan, F.; Villegas, G.; Teichman, J.; Mundel, P.; Tufro, A. Autocrine VEGF-A System in Podocytes Regulates Podocin and Its Interaction with CD2AP. Am. J. Physiol. Renal Physiol. 2006, 291, F422–F428.
- Ollero, M.; Sahali, D. Inhibition of the VEGF Signalling Pathway and Glomerular Disorders. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2015, 30, 1449–1455.
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257.
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674.
- Ronca, R.; Benkheil, M.; Mitola, S.; Struyf, S.; Liekens, S. Tumor Angiogenesis Revisited: Regulators and Clinical Implications. Med. Res. Rev. 2017, 37, 1231–1274.
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-Inducible Factor 1 Is a Basic-Helix-Loop-Helix-PAS Heterodimer Regulated by Cellular O2 Tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514.
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186.
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N. Engl. J. Med. 2004, 350, 2335–2342.
- Estrada, C.C.; Maldonado, A.; Mallipattu, S.K. Therapeutic Inhibition of VEGF Signaling and Associated Nephrotoxicities. J. Am. Soc. Nephrol. JASN 2019, 30, 187–200.
- Qin, S.; Li, A.; Yi, M.; Yu, S.; Zhang, M.; Wu, K. Recent Advances on Anti-Angiogenesis Receptor Tyrosine Kinase Inhibitors in Cancer Therapy. J. Hematol. Oncol. 2019, 12, 27.
- Hanna, R.M.; Barsoum, M.; Arman, F.; Selamet, U.; Hasnain, H.; Kurtz, I. Nephrotoxicity Induced by Intravitreal Vascular Endothelial Growth Factor Inhibitors: Emerging Evidence. Kidney Int. 2019, 96, 572–580.
- Shye, M.; Hanna, R.M.; Patel, S.S.; Tram-Tran, N.; Hou, J.; Mccannel, C.; Khalid, M.; Hanna, M.; Abdelnour, L.; Kurtz, I. Worsening Proteinuria and Renal Function after Intravitreal Vascular Endothelial Growth Factor Blockade for Diabetic Proliferative Retinopathy. Clin. Kidney J. 2020, 13, 969–980.
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for Advanced Cervical Cancer: Final Overall Survival and Adverse Event Analysis of a Randomised, Controlled, Open-Label, Phase 3 Trial (Gynecologic Oncology Group 240). Lancet 2017, 390, 1654–1663.
- Gridelli, C.; de Castro Carpeno, J.; Dingemans, A.-M.C.; Griesinger, F.; Grossi, F.; Langer, C.; Ohe, Y.; Syrigos, K.; Thatcher, N.; Das-Gupta, A.; et al. Safety and Efficacy of Bevacizumab Plus Standard-of-Care Treatment Beyond Disease Progression in Patients With Advanced Non-Small Cell Lung Cancer: The AvaALL Randomized Clinical Trial. JAMA Oncol. 2018, 4, e183486.
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.-G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and Paclitaxel-Carboplatin Chemotherapy and Secondary Cytoreduction in Recurrent, Platinum-Sensitive Ovarian Cancer (NRG Oncology/Gynecologic Oncology Group Study GOG-0213): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2017, 18, 779–791.
- Saito, H.; Fukuhara, T.; Furuya, N.; Watanabe, K.; Sugawara, S.; Iwasawa, S.; Tsunezuka, Y.; Yamaguchi, O.; Okada, M.; Yoshimori, K.; et al. Erlotinib plus Bevacizumab versus Erlotinib Alone in Patients with EGFR-Positive Advanced Non-Squamous Non-Small-Cell Lung Cancer (NEJ026): Interim Analysis of an Open-Label, Randomised, Multicentre, Phase 3 Trial. Lancet Oncol. 2019, 20, 625–635.
- Carvalho, B.; Lopes, R.G.; Linhares, P.; Costa, A.; Caeiro, C.; Fernandes, A.C.; Tavares, N.; Osório, L.; Vaz, R. Hypertension and Proteinuria as Clinical Biomarkers of Response to Bevacizumab in Glioblastoma Patients. J. Neurooncol. 2020, 147, 109–116.
- Wu, S.; Kim, C.; Baer, L.; Zhu, X. Bevacizumab Increases Risk for Severe Proteinuria in Cancer Patients. J. Am. Soc. Nephrol. JASN 2010, 21, 1381–1389.
- Zhao, T.; Wang, X.; Xu, T.; Xu, X.; Liu, Z. Bevacizumab Significantly Increases the Risks of Hypertension and Proteinuria in Cancer Patients: A Systematic Review and Comprehensive Meta-Analysis. Oncotarget 2017, 8, 51492–51506.
- Al-Samkari, H.; Kasthuri, R.S.; Parambil, J.G.; Albitar, H.A.; Almodallal, Y.A.; Vázquez, C.; Serra, M.M.; Dupuis-Girod, S.; Wilsen, C.B.; McWilliams, J.P.; et al. An International, Multicenter Study of Intravenous Bevacizumab for Bleeding in Hereditary Hemorrhagic Telangiectasia: The InHIBIT-Bleed Study. Haematologica 2020.
- Kreisl, T.N.; Zhang, W.; Odia, Y.; Shih, J.H.; Butman, J.A.; Hammoud, D.; Iwamoto, F.M.; Sul, J.; Fine, H.A. A Phase II Trial of Single-Agent Bevacizumab in Patients with Recurrent Anaplastic Glioma. Neuro Oncol. 2011, 13, 1143–1150.
- Agarwal, M.; Thareja, N.; Benjamin, M.; Akhondi, A.; Mitchell, G.D. Tyrosine Kinase Inhibitor-Induced Hypertension. Curr. Oncol. Rep. 2018, 20, 65.
- Zhang, Z.-F.; Wang, T.; Liu, L.-H.; Guo, H.-Q. Risks of Proteinuria Associated with Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e90135.
- Bellini, E.; Pia, A.; Brizzi, M.P.; Tampellini, M.; Torta, M.; Terzolo, M.; Dogliotti, L.; Berruti, A. Sorafenib May Induce Hypophosphatemia through a Fibroblast Growth Factor-23 (FGF23)-Independent Mechanism. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 988–990.
- Escudier, B.; Eisen, T.; Stadler, W.M.; Szczylik, C.; Oudard, S.; Siebels, M.; Negrier, S.; Chevreau, C.; Solska, E.; Desai, A.A.; et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N. Engl. J. Med. 2007, 356, 125–134.
- Lalami, Y.; Garcia, C.; Flamen, P.; Ameye, L.; Paesmans, M.; Awada, A. Phase II Trial Evaluating the Efficacy of Sorafenib (BAY 43-9006) and Correlating Early Fluorodeoxyglucose Positron Emission Tomography–CT Response to Outcome in Patients with Recurrent and/or Metastatic Head and Neck Cancer. Head Neck 2016, 38, 347–354.
- Kelley, R.K.; Nimeiri, H.S.; Munster, P.N.; Vergo, M.T.; Huang, Y.; Li, C.-M.; Hwang, J.; Mulcahy, M.F.; Yeh, B.M.; Kuhn, P.; et al. Temsirolimus Combined with Sorafenib in Hepatocellular Carcinoma: A Phase I Dose-Finding Trial with Pharmacokinetic and Biomarker Correlates. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 1900–1907.
- Terashima, T.; Yamashita, T.; Takata, N.; Takeda, Y.; Kido, H.; Iida, N.; Kitahara, M.; Shimakami, T.; Takatori, H.; Arai, K.; et al. Safety and Efficacy of Sorafenib Followed by Regorafenib or Lenvatinib in Patients with Hepatocellular Carcinoma. Hepatol. Res. Off. J. Jpn. Soc. Hepatol. 2020.
- Grignani, G.; Palmerini, E.; Ferraresi, V.; D’Ambrosio, L.; Bertulli, R.; Asaftei, S.D.; Tamburini, A.; Pignochino, Y.; Sangiolo, D.; Marchesi, E.; et al. Sorafenib and Everolimus for Patients with Unresectable High-Grade Osteosarcoma Progressing after Standard Treatment: A Non-Randomised Phase 2 Clinical Trial. Lancet Oncol. 2015, 16, 98–107.
- Gounder, M.M.; Mahoney, M.R.; Van Tine, B.A.; Ravi, V.; Attia, S.; Deshpande, H.A.; Gupta, A.A.; Milhem, M.M.; Conry, R.M.; Movva, S.; et al. Sorafenib for Advanced and Refractory Desmoid Tumors. N. Engl. J. Med. 2018, 379, 2417–2428.
- Ueda, T.; Uemura, H.; Tomita, Y.; Tsukamoto, T.; Kanayama, H.; Shinohara, N.; Tarazi, J.; Chen, C.; Kim, S.; Ozono, S.; et al. Efficacy and Safety of Axitinib versus Sorafenib in Metastatic Renal Cell Carcinoma: Subgroup Analysis of Japanese Patients from the Global Randomized Phase 3 AXIS Trial. Jpn. J. Clin. Oncol. 2013, 43, 616–628.
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115.
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Arén Frontera, O.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus Ipilimumab versus Sunitinib in First-Line Treatment for Advanced Renal Cell Carcinoma: Extended Follow-up of Efficacy and Safety Results from a Randomised, Controlled, Phase 3 Trial. Lancet Oncol. 2019, 20, 1370–1385.
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127.
- Fujita, T.; Hirayama, T.; Ishii, D.; Matsumoto, K.; Yoshida, K.; Iwamura, M. Efficacy and Safety of Sunitinib in Elderly Patients with Advanced Renal Cell Carcinoma. Mol. Clin. Oncol. 2018, 9, 394–398.
- Jin, H.; Zhang, J.; Shen, K.; Hao, J.; Feng, Y.; Yuan, C.; Zhu, Y.; Ma, X. Efficacy and Safety of Perioperative Appliance of Sunitinib in Patients with Metastatic or Advanced Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Medicine 2019, 98, e15424.
- Zhu, X.; Stergiopoulos, K.; Wu, S. Risk of Hypertension and Renal Dysfunction with an Angiogenesis Inhibitor Sunitinib: Systematic Review and Meta-Analysis. Acta Oncol. 2009, 48, 9–17.
- Ravaud, A.; Motzer, R.J.; Pandha, H.S.; George, D.J.; Pantuck, A.J.; Patel, A.; Chang, Y.-H.; Escudier, B.; Donskov, F.; Magheli, A.; et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N. Engl. J. Med. 2016, 375, 2246–2254.
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus Bevacizumab versus Sunitinib in Patients with Previously Untreated Metastatic Renal Cell Carcinoma (IMmotion151): A Multicentre, Open-Label, Phase 3, Randomised Controlled Trial. Lancet 2019, 393, 2404–2415.
- Miyamoto, S.; Kakutani, S.; Sato, Y.; Hanashi, A.; Kinoshita, Y.; Ishikawa, A. Drug Review: Pazopanib. Jpn. J. Clin. Oncol. 2018, 48, 503–513.
- Toulmonde, M.; Pulido, M.; Ray-Coquard, I.; Andre, T.; Isambert, N.; Chevreau, C.; Penel, N.; Bompas, E.; Saada, E.; Bertucci, F.; et al. Pazopanib or Methotrexate-Vinblastine Combination Chemotherapy in Adult Patients with Progressive Desmoid Tumours (DESMOPAZ): A Non-Comparative, Randomised, Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2019, 20, 1263–1272.
- Motzer, R.J.; Haas, N.B.; Donskov, F.; Gross-Goupil, M.; Varlamov, S.; Kopyltsov, E.; Lee, J.L.; Melichar, B.; Rini, B.I.; Choueiri, T.K.; et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo after Nephrectomy in Patients with Localized or Locally Advanced Renal Cell Carcinoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3916–3923.
- Motzer, R.J.; Hutson, T.E.; Cella, D.; Reeves, J.; Hawkins, R.; Guo, J.; Nathan, P.; Staehler, M.; de Souza, P.; Merchan, J.R.; et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2013, 369, 722–731.
- Van der Graaf, W.T.A.; Blay, J.-Y.; Chawla, S.P.; Kim, D.-W.; Bui-Nguyen, B.; Casali, P.G.; Schöffski, P.; Aglietta, M.; Staddon, A.P.; Beppu, Y.; et al. Pazopanib for Metastatic Soft-Tissue Sarcoma (PALETTE): A Randomised, Double-Blind, Placebo-Controlled Phase 3 Trial. Lancet 2012, 379, 1879–1886.
- Sternberg, C.N.; Davis, I.D.; Mardiak, J.; Szczylik, C.; Lee, E.; Wagstaff, J.; Barrios, C.H.; Salman, P.; Gladkov, O.A.; Kavina, A.; et al. Pazopanib in Locally Advanced or Metastatic Renal Cell Carcinoma: Results of a Randomized Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 1061–1068.
- Berardi, R.; Santoni, M.; Rinaldi, S.; Nunzi, E.; Smerilli, A.; Caramanti, M.; Morgese, F.; Torniai, M.; Savini, A.; Fiordoliva, I.; et al. Risk of Hyponatraemia in Cancer Patients Treated with Targeted Therapies: A Systematic Review and Meta-Analysis of Clinical Trials. PLoS ONE 2016, 11, e0152079.
- Zhang, W.; Feng, L.-J.; Teng, F.; Li, Y.-H.; Zhang, X.; Ran, Y.-G. Incidence and Risk of Proteinuria Associated with Newly Approved Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors in Cancer Patients: An up-to-Date Meta-Analysis of Randomized Controlled Trials. Expert Rev. Clin. Pharmacol. 2020, 13, 311–320.
- Leboulleux, S.; Bastholt, L.; Krause, T.; de la Fouchardiere, C.; Tennvall, J.; Awada, A.; Gómez, J.M.; Bonichon, F.; Leenhardt, L.; Soufflet, C.; et al. Vandetanib in Locally Advanced or Metastatic Differentiated Thyroid Cancer: A Randomised, Double-Blind, Phase 2 Trial. Lancet Oncol. 2012, 13, 897–905.
- Wells, S.A.; Robinson, B.G.; Gagel, R.F.; Dralle, H.; Fagin, J.A.; Santoro, M.; Baudin, E.; Elisei, R.; Jarzab, B.; Vasselli, J.R.; et al. Vandetanib in Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer: A Randomized, Double-Blind Phase III Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 134–141.
- Yoh, K.; Seto, T.; Satouchi, M.; Nishio, M.; Yamamoto, N.; Murakami, H.; Nogami, N.; Matsumoto, S.; Kohno, T.; Tsuta, K.; et al. Vandetanib in Patients with Previously Treated RET-Rearranged Advanced Non-Small-Cell Lung Cancer (LURET): An Open-Label, Multicentre Phase 2 Trial. Lancet Respir. Med. 2017, 5, 42–50.
- Hu, M.I.; Elisei, R.; Dedecjus, M.; Popovtzer, A.; Druce, M.; Kapiteijn, E.; Pacini, F.; Locati, L.; Krajewska, J.; Weiss, R.; et al. Safety and Efficacy of Two Starting Doses of Vandetanib in Advanced Medullary Thyroid Cancer. Endocr. Relat. Cancer 2019, 26, 241–250.
- Lee, J.S.; Hirsh, V.; Park, K.; Qin, S.; Blajman, C.R.; Perng, R.-P.; Chen, Y.-M.; Emerson, L.; Langmuir, P.; Manegold, C. Vandetanib Versus Placebo in Patients with Advanced Non-Small-Cell Lung Cancer after Prior Therapy with an Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor: A Randomized, Double-Blind Phase III Trial (ZEPHYR). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1114–1121.
- Thornton, K.; Kim, G.; Maher, V.E.; Chattopadhyay, S.; Tang, S.; Moon, Y.J.; Song, P.; Marathe, A.; Balakrishnan, S.; Zhu, H.; et al. Vandetanib for the Treatment of Symptomatic or Progressive Medullary Thyroid Cancer in Patients with Unresectable Locally Advanced or Metastatic Disease: U.S. Food and Drug Administration Drug Approval Summary. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 3722–3730.
- Rini, B.I.; Escudier, B.; Tomczak, P.; Kaprin, A.; Szczylik, C.; Hutson, T.E.; Michaelson, M.D.; Gorbunova, V.A.; Gore, M.E.; Rusakov, I.G.; et al. Comparative Effectiveness of Axitinib versus Sorafenib in Advanced Renal Cell Carcinoma (AXIS): A Randomised Phase 3 Trial. Lancet 2011, 378, 1931–1939.
- Gross-Goupil, M.; Kwon, T.G.; Eto, M.; Ye, D.; Miyake, H.; Seo, S.I.; Byun, S.-S.; Lee, J.L.; Master, V.; Jin, J.; et al. Axitinib versus Placebo as an Adjuvant Treatment of Renal Cell Carcinoma: Results from the Phase III, Randomized ATLAS Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 2371–2378.
- Motzer, R.J.; Escudier, B.; Tomczak, P.; Hutson, T.E.; Michaelson, M.D.; Negrier, S.; Oudard, S.; Gore, M.E.; Tarazi, J.; Hariharan, S.; et al. Axitinib versus Sorafenib as Second-Line Treatment for Advanced Renal Cell Carcinoma: Overall Survival Analysis and Updated Results from a Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 552–562.
- Hutson, T.E.; Lesovoy, V.; Al-Shukri, S.; Stus, V.P.; Lipatov, O.N.; Bair, A.H.; Rosbrook, B.; Chen, C.; Kim, S.; Vogelzang, N.J. Axitinib versus Sorafenib as First-Line Therapy in Patients with Metastatic Renal-Cell Carcinoma: A Randomised Open-Label Phase 3 Trial. Lancet Oncol. 2013, 14, 1287–1294.
- Van Cutsem, E.; Martinelli, E.; Cascinu, S.; Sobrero, A.; Banzi, M.; Seitz, J.-F.; Barone, C.; Ychou, M.; Peeters, M.; Brenner, B.; et al. Regorafenib for Patients with Metastatic Colorectal Cancer Who Progressed After Standard Therapy: Results of the Large, Single-Arm, Open-Label Phase IIIb CONSIGN Study. Oncologist 2019, 24, 185–192.
- Xu, J.; Xu, R.-H.; Qin, S.; Pan, H.; Bai, Y.; Chi, Y.; Wang, L.; Bi, F.; Cheng, Y.; Liu, T.; et al. Regorafenib in Chinese Patients with Metastatic Colorectal Cancer: Subgroup Analysis of the Phase 3 CONCUR Trial. J. Gastroenterol. Hepatol. 2020, 35, 1307–1316.
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 303–312.
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N. Engl. J. Med. 2018, 379, 54–63.
- Apolo, A.B.; Nadal, R.; Tomita, Y.; Davarpanah, N.N.; Cordes, L.M.; Steinberg, S.M.; Cao, L.; Parnes, H.L.; Costello, R.; Merino, M.J.; et al. Cabozantinib in Patients with Platinum-Refractory Metastatic Urothelial Carcinoma: An Open-Label, Single-Centre, Phase 2 Trial. Lancet Oncol. 2020, 21, 1099–1109.
- Drilon, A.; Rekhtman, N.; Arcila, M.; Wang, L.; Ni, A.; Albano, M.; Van Voorthuysen, M.; Somwar, R.; Smith, R.S.; Montecalvo, J.; et al. Cabozantinib in Patients with Advanced RET-Rearranged Non-Small-Cell Lung Cancer: An Open-Label, Single-Centre, Phase 2, Single-Arm Trial. Lancet Oncol. 2016, 17, 1653–1660.
- Italiano, A.; Mir, O.; Mathoulin-Pelissier, S.; Penel, N.; Piperno-Neumann, S.; Bompas, E.; Chevreau, C.; Duffaud, F.; Entz-Werlé, N.; Saada, E.; et al. Cabozantinib in Patients with Advanced Ewing Sarcoma or Osteosarcoma (CABONE): A Multicentre, Single-Arm, Phase 2 Trial. Lancet Oncol. 2020, 21, 446–455.
- Xu, J.; Higgins, M.J.; Tolaney, S.M.; Come, S.E.; Smith, M.R.; Fornier, M.; Mahmood, U.; Baselga, J.; Yeap, B.Y.; Chabner, B.A.; et al. A Phase II Trial of Cabozantinib in Hormone Receptor-Positive Breast Cancer with Bone Metastases. Oncologist 2020, 25, 652–660.
- Okamoto, I.; Miyazaki, M.; Takeda, M.; Terashima, M.; Azuma, K.; Hayashi, H.; Kaneda, H.; Kurata, T.; Tsurutani, J.; Seto, T.; et al. Tolerability of Nintedanib (BIBF 1120) in Combination with Docetaxel: A Phase 1 Study in Japanese Patients with Previously Treated Non-Small-Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 346–352.
- Yamazaki, H.; Iwasaki, H.; Takasaki, H.; Suganuma, N.; Sakai, R.; Masudo, K.; Nakayama, H.; Rino, Y.; Masuda, M. Efficacy and Tolerability of Initial Low-Dose Lenvatinib to Treat Differentiated Thyroid Cancer. Medicine 2019, 98, e14774.
- Hill, J.; Shields, J.; Passero, V. Tyrosine Kinase Inhibitor-Associated Syndrome of Inappropriate Secretion of Anti-Diuretic Hormone. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2016, 22, 729–732.
- Demetri, G.D.; Lo Russo, P.; MacPherson, I.R.J.; Wang, D.; Morgan, J.A.; Brunton, V.G.; Paliwal, P.; Agrawal, S.; Voi, M.; Evans, T.R.J. Phase I Dose-Escalation and Pharmacokinetic Study of Dasatinib in Patients with Advanced Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 6232–6240.
- Lipton, J.H.; Chuah, C.; Guerci-Bresler, A.; Rosti, G.; Simpson, D.; Assouline, S.; Etienne, G.; Nicolini, F.E.; le Coutre, P.; Clark, R.E.; et al. Ponatinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukaemia: An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2016, 17, 612–621.
- Cortes, J.E.; Kim, D.-W.; Pinilla-Ibarz, J.; le Coutre, P.D.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. Ponatinib Efficacy and Safety in Philadelphia Chromosome-Positive Leukemia: Final 5-Year Results of the Phase 2 PACE Trial. Blood 2018, 132, 393–404.
- Cortes, J.E.; Kim, D.-W.; Pinilla-Ibarz, J.; le Coutre, P.; Paquette, R.; Chuah, C.; Nicolini, F.E.; Apperley, J.F.; Khoury, H.J.; Talpaz, M.; et al. A Phase 2 Trial of Ponatinib in Philadelphia Chromosome-Positive Leukemias. N. Engl. J. Med. 2013, 369, 1783–1796.




