Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Chang Gue Son | + 1711 word(s) | 1711 | 2021-09-07 10:11:41 | | | |
| 2 | Enzi Gong | Meta information modification | 1711 | 2021-09-10 07:49:39 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Son, C.G. Korean Red Ginseng Ameliorates Fatigue. Encyclopedia. Available online: https://encyclopedia.pub/entry/14054 (accessed on 08 February 2026).
Son CG. Korean Red Ginseng Ameliorates Fatigue. Encyclopedia. Available at: https://encyclopedia.pub/entry/14054. Accessed February 08, 2026.
Son, Chang Gue. "Korean Red Ginseng Ameliorates Fatigue" Encyclopedia, https://encyclopedia.pub/entry/14054 (accessed February 08, 2026).
Son, C.G. (2021, September 10). Korean Red Ginseng Ameliorates Fatigue. In Encyclopedia. https://encyclopedia.pub/entry/14054
Son, Chang Gue. "Korean Red Ginseng Ameliorates Fatigue." Encyclopedia. Web. 10 September, 2021.
Copy Citation
Fatigue is both a physiological defense response and a disease-associated symptom; therefore, it is a common complaint in both the general population and patients with various disorders. Fatigue can be generally classified according to duration as acute (≤1 month), prolonged (1< and ≤6 months), and chronic, lasting over 6 months. Chronic fatigue is the main fatigue-related issue in the clinic, and its prevalence is approximately 10% in the general population. In particular, medically unexplained chronic fatigue, such as chronic fatigue syndrome (CFS), has a more serious impact on health-related quality of life than brain stroke, angina pectoris, or schizophrenia.
central fatigue
chronic fatigue
corticosterone
Korean red ginseng
serotonin
1. Overview
Fatigue is both a physiological defense response and a disease-associated symptom; therefore, it is a common complaint in both the general population and patients with various disorders [1]. Fatigue can be generally classified according to duration as acute (≤1 month), prolonged (1< and ≤6 months), and chronic, lasting over 6 months [2]. Chronic fatigue is the main fatigue-related issue in the clinic, and its prevalence is approximately 10% in the general population [3]. In particular, medically unexplained chronic fatigue, such as chronic fatigue syndrome (CFS), has a more serious impact on health-related quality of life than brain stroke, angina pectoris, or schizophrenia [4].
Additionally, central fatigue is a neuromuscular dysfunction, a notable feature of chronic fatigue, that is caused by biochemical alterations in the brain [5]. Unlike peripheral fatigue, which is caused by energy-associated disturbances, mainly in muscles, central fatigue results from dysfunction of synaptic transmission in the central nervous system (CNS) [6]. Clinically, prolonged sleep disturbance and chronic stress are presumed to be inducers of central fatigue and are also major symptoms of pathologic central fatigue and chronic fatigue [7]. Important hypotheses on the pathophysiologic mechanisms of pathologic fatigue like CFS involve disruption of neuroendocrinological homeostasis resulting, for example, from chronic sleep deprivation (SD) [8].
Sleep is believed to play a key role in the maintenance of brain function and health, as well as in protection against and recovery from central fatigue [5][9]. Approximately 60% of subjects with chronic fatigue have comorbid sleep disorders, including insomnia, sleep apnea, and periodic limb movement disorder [10]. It is well known that chronic sleep restriction affects neuronal activity within the brain, altering serotonin (5-hydroxytryptamine, 5-HT) and dopamine (DA) levels, which are closely associated with the sleep/wake cycle [11]. While cortisol levels in the blood are elevated in the context of acute stress and general fatigue, this stress hormone is frequently depleted by prolonged SD and in subjects with severe central fatigue [12].
Panax ginseng (P. ginseng) is one of the most frequently employed herbs for various health issues, and it has shown moderate effects in treating fatigue [13][14]. Especially, Korean red ginseng (KRG), which is manufactured by repeated steaming and drying of raw ginseng, is known to exert pharmacologic activities, including antifatigue, antioxidative, and immunomodulatory effects [15][16]. To date, studies on the anti-fatigue effects of P. ginseng and KRG have focused on general or peripheral fatigue and have mainly involved measurement of energy metabolite- and oxidative stress-related marker levels in blood and tissues from clinical patients and animal models [17]. However, regarding central fatigue, evidence of the effects of P. ginseng and KRG is lacking.
2. Impaired Exercise Capability
Impaired exercise capability is a common complaint of subjects with chronic fatigue. In particular, sufferers of CFS are vulnerable to becoming fatigued after performing daily tasks in the home without exhibiting any muscular defects, which is referred to as postexertional malaise (PEM) [18]. The low force generated by motor units in CFS subjects is known to result from muscle membrane dysfunction caused by central neural dysregulation [19]. The 5-HT hyperactivity in the CNS is the most well-known alteration in neurotransmitters that inhibits proprioceptive sensory and muscular contractions [20]. Ginseng improves physical function in both healthy individuals and subjects complaining of fatigue [14][21]. Likewise, in the present study, KRG improved exercise performance in the rotarod test under both normal conditions and after SD. Rotarod performance reflects integrated motor functions, including muscle endurance, balance, and coordination [22].
The activity of 5-HT in the brain is strongly and positively correlated with a lack of sleep [23]. A human study revealed that a single day of SD elevated the plasma level of tryptophan, a precursor of 5-HT, by 20% [24]. As expected, 6 cycles of SD markedly elevated the 5-HT level and that of its synthetase (TPH2), but these alterations were significantly normalized by KRG treatment. In the context of chronic SD, overproduced 5-HT spills into the extrasynaptic space and activates 5HT1AR (a representative inhibitory receptor) on the initial axon segments of motor neurons, leading to inhibition of neuronal output [6]. The 5-HT level is controlled by the balance between its release and reuptake, which are determined by the p-GR/GR ratio (desensitizing 5HT1AR results in release and activation) and the expression of its reuptake transporter (5-HTT) in the RN [25][26]. Six cycles of SD increased the p-GR/GR ratio without changing the expression of 5HT1AR in the RN. A large amount of previous data showed that 5HT1AR desensitization (loss of the inhibitory function of 5-HT release) usually occurs without a change in the protein level of 5HT1AR, as shown in our results [25]. KRG treatment did not affect the release of 5-HT (GR activity or HT1AR expression) but increased its reuptake (increase in 5-HTT level). This suggests that administration of KRG might regulate serotonergic activity by promoting the reuptake of 5-HT in the synaptic space and that this may be the main mechanism of its anti-central fatigue properties.
Sufferers of chronic fatigue rarely experience restorative sleep and exhibit disrupted neuroendocrinological homeostasis [8]. A total of 87–95% of patients with CFS experience unrefreshing sleep [27]. Prolonged sleep disturbance dysregulates the hypothalamic–pituitary–adrenal (HPA) axis, especially in the metabolism of cortisol, which plays an important role in the stress response and the formation of sleep architecture [28]. Unlike acute stress, chronic stress induces low levels of serum cortisol and dysfunction of GR dysfunction in the hypothalamus by impeding its translocation and phosphorylation, resulting in a condition that is sometimes called adrenal fatigue [29]. In our SD model, serum corticosterone was almost completely depleted, and the GR ratio in the hypothalamus was increased; however, KRG treatment significantly attenuated these alterations in serum corticosterone levels and the p-GR/GR ratio. Several clinical studies have shown that serum corticosterone levels can be normalized by therapeutic agents, resulting in positive outcomes for patients with chronic fatigue [30]. The hypothalamus is a central region that responds to stress, sleep, and fatigue, and hypothalamic neurons can be damaged under extremely unfavorable conditions [31]. BDNF supports neuronal survival, growth, and differentiation and may indicate the health status of the hypothalamus [32]. In our study, KRG treatment significantly attenuated the SD-induced downregulation of the expression of both BDNF and its transcription factor (as indicated by the p-CREB/CREB ratio).
In modern society, it is common for individuals to have trouble sleeping in terms of both quantitative and qualitative parameters, as approximately 8–18% of the general population worldwide experience such issues [33]. An increase in 5-HT levels within the CNS resulting from sleep disturbance acts as a homeostatic pressure that causes symptoms such as drowsiness, lethargy, and loss of strength, leading the individual to rest [23]. In contrast with decreased 5-HT levels, decreased DA levels in the RN under chronic SD conditions lead to less activity and less arousal [34]. In our study, KRG treatment reversed the SD-induced reduction in the levels of DA and TH, which are enzymes involved in DA synthesis, in the RN (Figure 1), indicating that KRG balanced the levels of these neurochemicals. Some RCTs have proven that AA exerts antifatigue effects [35][36], so we used it as a positive control in this study. Treatment with 100 mg/kg AA modulated the levels of 5-HT (resulting in a much lower level than that induced by any dose of KRG, p < 0.01, data not shown) and DA (exhibiting a similar effect as KRG); however, we did not observe improvements in exercise performance or corticosterone levels in the blood. Presumably, exercise performance was not improved because the 5-HT level was 2-fold higher than that under normal conditions, and the changes in corticosterone levels and GR function in the hypothalamus were not significantly ameliorated. Considering the results obtained for AA, further pathophysiological studies on 5-HT- and corticosterone-related mechanisms of central fatigue are needed.
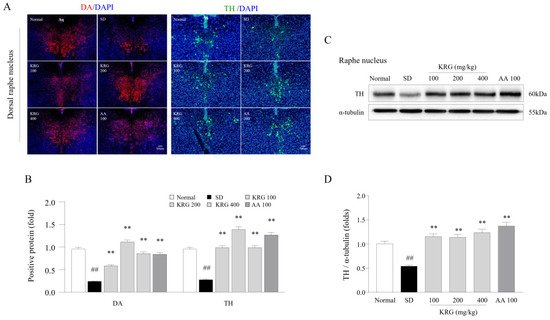
Figure 1. Effects of KRG on the levels of dopaminergic activity-related molecules in the raphe nucleus. The relative DA and TH levels in the DRN were measured using immunofluorescence (A), and the relative DA and TH levels were semiquantified (B) (n = 3). Representative photomicrographs were taken at a magnification of 100×. Western blot analysis was used to evaluate TH protein levels in the RN (C), which were semiquantified (D) (n = 5). The data are expressed as the means ± standard deviations (n = 3 or 5). ##, p < 0.01 compared with the normal group; **, p < 0.01 compared with the SD group.
No conventional therapeutics for chronic fatigue are available yet; thus, many nonresponders to rest use complementary therapies, including herbal medicines [37]. Traditionally, P. ginseng and KRG have been used as tonics for increasing vital energy and recovering from fatigue in eastern Asia. A few studies revealed the anti-fatigue effects of KRG by clinical (180 Chinese participants or 50 Korean patients) and preclinical (mice) investigation; however, their focus of anti-fatigue effect was not based on neurobiological underlying mechanism [38][39][40]. The present study is the first evidence for the anti-fatigue effect of KRG focusing on neurochemical alterations related to fatigue. The dose of KRG used in the present study was chosen based on the dose commonly used in the clinic, i.e., 2 g in adults. As limitations, KRG generally contains more ginsenosides, including Rg3, than P. ginseng [41]. We currently do not know which components of KRG are mainly responsible for its effects and whether it has similar effects on severe central fatigue in humans, such as in those suffering from CFS. Additionally, owing to the mechanism of central fatigue related to CNS factors, precise electrophysiological and micro histological studies are needed to support whether motor dysfunctions occurred due to peripheral factors such as muscular defects.
3. Conclusions
KRG can protect against or alleviate fatigue related to chronic SD which could contribute to central fatigue. The underlying mechanisms may involve the modulation of neurotransmitters, including serotonin in the brain, and corticosterone. Our data suggest that the therapeutic potential of KRG for patients suffering from severe fatigue should be evaluated clinically in the future.
References
- Finsterer, J.; Mahjoub, S.Z. Fatigue in healthy and diseased individuals. Am. J. Hosp. Palliat. Med. 2014, 31, 562–575.
- Son, C.-G. Differential diagnosis between “chronic fatigue” and “chronic fatigue syndrome”. Integr. Med. Res. 2019, 8, 89.
- Wessely, S.; Chalder, T.; Hirsch, S.; Wallace, P.; Wright, D. The prevalence and morbidity of chronic fatigue and chronic fa-tigue syndrome: A prospective primary care study. Am. J. Public Health 1997, 87, 1449–1455.
- Hvidberg, M.F.; Brinth, L.; Olesen, A.V.; Petersen, K.D.; Ehlers, L.H. The Health-Related Quality of Life for Patients with Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS). PLoS ONE 2015, 10, e0132421.
- Meeusen, R.; Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F. Central fatigue. Sports Med. 2006, 36, 881–909.
- Cotel, F.; Exley, R.; Cragg, S.J.; Perrier, J.-F. Serotonin spillover onto the axon initial segment of motoneurons induces cen-tral fatigue by inhibiting action potential initiation. Proc. Natl. Acad. Sci. USA 2013, 110, 4774–4779.
- Yamashita, M.; Yamamoto, T. Tryptophan and Kynurenic Acid May Produce an Amplified Effect in Central Fatigue Induced by Chronic Sleep Disorder. Int. J. Tryptophan Res. 2014, 7, 9–14.
- Cleare, A.J. The Neuroendocrinology of Chronic Fatigue Syndrome. Endocr. Rev. 2003, 24, 236–252.
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377.
- Fossey, M.; Libman, E.; Bailes, S.; Baltzan, M.; Schondorf, R.; Amsel, R.; Fichten, C.S. Sleep quality and psychological ad-justment in chronic fatigue syndrome. J. Behav. Med. 2004, 27, 581–605.
- Saper, C.B.; Scammell, T.E.; Lu, J. Hypothalamic regulation of sleep and circadian rhythms. Nature 2005, 437, 1257–1263.
- Papadopoulos, A.S.; Cleare, A.J. Hypothalamic–pituitary–adrenal axis dysfunction in chronic fatigue syndrome. Nat. Rev. Endocrinol. 2012, 8, 22–32.
- Shergis, J.; Zhang, A.L.; Zhou, W.; Xue, C.C. Panax ginseng in Randomised Controlled Trials: A Systematic Review. Phytother. Res. 2012, 27, 949–965.
- Arring, N.M.; Millstine, D.; Marks, L.A.; Nail, L.M. Ginseng as a treatment for fatigue: A systematic review. J. Altern. Complementary Med. 2018, 24, 624–633.
- He, M.; Huang, X.; Liu, S.; Guo, C.; Xie, Y.; Meijer, A.H.; Wang, M. The Difference between White and Red Ginseng: Variations in Ginsenosides and Immunomodulation. Planta Med. 2018, 84, 845–854.
- Lee, Y.-M.; Yoon, H.; Park, H.-M.; Song, B.C.; Yeum, K.-J. Implications of red Panax ginseng in oxidative stress associated chronic diseases. J. Ginseng Res. 2017, 41, 113–119.
- Jin, T.-Y.; Rong, P.-Q.; Liang, H.-Y.; Zhang, P.-P.; Zheng, G.-Q.; Lin, Y. Clinical and Preclinical Systematic Review of Panax ginseng CA Mey and Its Compounds for Fatigue. Front. Pharmacol. 2020, 11, 1031.
- Clayton, E.W. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: An IOM report on redefining an illness. JAMA 2015, 313, 1101–1102.
- Klaver-Krol, E.; Hermens, H.; Vermeulen, R.; Klaver, M.; Luyten, H.; Henriquez, N.; Zwarts, M. Chronic fatigue syndrome: Abnormally fast muscle fiber conduction in the membranes of motor units at low static force load. Clin. Neurophysiol. 2021, 132, 967–974.
- Nagata, A.; Nakayama, K.; Nakamura, S.; Mochizuki, A.; Gemba, C.; Aoki, R.; Dantsuji, M.; Maki, K.; Inoue, T. Serotonin1B receptor-mediated presynaptic inhibition of proprioceptive sensory inputs to jaw-closing motoneurons. Brain Res. Bull. 2019, 149, 260–267.
- Cristina-Souza, G.; Santos-Mariano, A.C.; Lima-Silva, A.E.; Costa, P.L.; Domingos, P.R.; Silva, S.F.; Abreu, W.C.; De-Oliveira, F.R.; Osiecki, R. Panax ginseng Supplementation Increases Muscle Recruitment, Attenuates Perceived Effort, and Accelerates Muscle Force Recovery After an Eccentric-Based Exercise in Athletes. J. Strength Cond. Res. 2020.
- Jones, B.J.; Roberts, D.J. The quantitative measurement of motor inco-ordination in naive mice using an accelerating rotarod. J. Pharm. Pharmacol. 1968, 20, 302–304.
- Oikonomou, G.; Altermatt, M.; Zhang, R.-W.; Coughlin, G.M.; Montz, C.; Gradinaru, V.; Prober, D.A. The Serotonergic Raphe Promote Sleep in Zebrafish and Mice. Neuron 2019, 103, 686–701.e8.
- Davies, S.K.; Ang, J.E.; Revell, V.L.; Holmes, B.; Mann, A.; Robertson, F.P.; Cui, N.; Middleton, B.; Ackermann, K.; Kayser, M. Effect of sleep deprivation on the human metabolome. Proc. Natl. Acad. Sci. USA 2014, 111, 10761–10766.
- Evrard, A.; Barden, N.; Hamon, M.; Adrien, J. Glucocorticoid Receptor-Dependent Desensitization of 5-HT1A Autoreceptors by Sleep Deprivation: Studies in GR-i Transgenic Mice. Sleep 2006, 29, 31–36.
- Rudnick, G.; Sandtner, W. Serotonin transport in the 21st century. J. Gen. Physiol. 2019, 151, 1248–1264.
- Mariman, A.N.; Vogelaers, D.P.; Tobback, E.; Delesie, L.M.; Hanoulle, I.P.; Pevernagie, D.A. Sleep in the chronic fatigue syndrome. Sleep Med. Rev. 2013, 17, 193–199.
- Henry, M.; Ross, I.L.; Thomas, K.G.F. Reduced slow-wave sleep and altered diurnal cortisol rhythms in patients with Addi-son’s disease. Eur. J. Endocrinol. 2018, 179, 319–330.
- Russell, G.; Lightman, S. The human stress response. Nat. Rev. Endocrinol. 2019, 15, 525–534.
- Nijhof, S.L.; Rutten, J.M.; Uiterwaal, C.S.; Bleijenberg, G.; Kimpen, J.L.; van de Putte, E.M. The role of hypocortisolism in chronic fatigue syndrome. Psychoneuroendocrinology 2014, 42, 199–206.
- Bains, J.S.; Cusulin, J.I.W.; Inoue, W. Stress-related synaptic plasticity in the hypothalamus. Nat. Rev. Neurosci. 2015, 16, 377–388.
- Tapia-Arancibia, L.; Rage, F.; Givalois, L.; Arancibia, S. Physiology of BDNF: Focus on hypothalamic function. Front. Neuroendocr. 2004.
- Ohayon, M.M. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med. Rev. 2002, 6, 97–111.
- Cho, J.R.; Treweek, J.B.; Robinson, J.E.; Xiao, C.; Bremner, L.R.; Greenbaum, A.; Gradinaru, V. Dorsal raphe dopamine neu-rons modulate arousal and promote wakefulness by salient stimuli. Neuron 2017, 94, 1205–1219.e1208.
- Suh, S.-Y.; Bae, W.K.; Ahn, H.-Y.; Choi, S.-E.; Jung, G.-C.; Yeom, C.H. Intravenous Vitamin C administration reduces fatigue in office workers: A double-blind randomized controlled trial. Nutr. J. 2012, 11, 7.
- Shafat, A.; Butler, P.; Jensen, R.L.; Donnelly, A. Effects of dietary supplementation with vitamins C and E on muscle function during and after eccentric contractions in humans. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 93, 196–202.
- Adams, D.; Wu, T.; Yang, X.; Tai, S.; Vohra, S. Traditional Chinese medicinal herbs for the treatment of idiopathic chronic fatigue and chronic fatigue syndrome. Cochrane Database Syst. Rev. 2009.
- Zhang, L.; Chen, X.; Cheng, Y.; Chen, Q.; Tan, H.; Son, D.; Chang, D.; Bian, Z.-X.; Fang, H.; Xu, H. Safety and antifatigue effect of Korean Red Ginseng: A randomized, double-blind, and placebo-controlled clinical trial. J. Ginseng Res. 2019, 43, 676–683.
- Sung, W.S.; Kang, H.R.; Jung, C.Y.; Park, S.S.; Lee, S.H.; Kim, E.J. Efficacy of Korean red ginseng (Panax ginseng) for mid-dle-aged and moderate level of chronic fatigue patients: A randomized, double-blind, placebo-controlled trial. Complementary Ther. Med. 2020, 48, 102246.
- Kim, D.; Lee, B.; Kim, H.; Kim, M. Effects of Red Ginseng on Exercise Capacity and Peripheral Fatigue in Mice. Phys. Ther. Rehabil. Sci. 2021, 10, 175–184.
- Lee, S.M.; Bae, B.-S.; Park, H.-W.; Ahn, N.-G.; Cho, B.-G.; Cho, Y.-L.; Kwak, Y.-S. Characterization of Korean Red Ginseng (Panax ginseng Meyer): History, preparation method, and chemical composition. J. Ginseng Res. 2015, 39, 384–391.
More
Information
Subjects:
Others
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.0K
Revisions:
2 times
(View History)
Update Date:
10 Sep 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




