
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Camilo Chiang | + 2218 word(s) | 2218 | 2020-10-08 07:45:44 | | | |
| 2 | Nora Tang | -25 word(s) | 2193 | 2020-10-26 05:27:04 | | |
Video Upload Options
Effect of light quality on indoor experiments, aiming to reach near to natural growth.
1. History and Introduction
When investigating the effect of light quality in plant development, previous studies were mainly focused on single species, and they generally did not directly compare findings with natural conditions. In the present study, we deliberately investigated a suite of species from different functional plant types to determine if, and how, they react to the different treatments. Through application of the same mean climatic conditions indoors, as in the initial field trial, we could better assess which LED light conditions are generating the most natural-like plant performance. Our results showed clear differences within and between the light treatments when compared to the field trial on most measured plant traits. The effect sizes were highly species-specific, while effect directions were similar among species, with the clear exception of SLA and root biomass production. As expected, light treatments with very extreme blue: red (B:R) ratios (6 and 62% B) induced more extreme (‘unnatural’) values in most plant traits than treatments with a more balanced B:R ratio (25 and 35% B).
2. Light Quality Effects on Morphology
Studies that compared indoor with outdoor plant growth were previously often biased by a higher plant density in the indoor condition [1]. In our study, we deliberately kept the exact same plant densities between the field and the phytotron trials to avoid any stand density bias on plant morphology. The effects of B light percentages on plant morphology have been previously reported in several studies [2][3][4][5][6][7][8][9]. In general, B light is sensed by the cryptochrome system, where under high irradiances or high levels of B light, plants exhibit shorter and stunted growth (For example [2][10][11]). It is also known that a total lack of B or R light negatively affects plant performance, including growth rate, height, photosynthesis and several other parameters. For example, Hernandez et al. [12] found that tomato plants grew shorter under either B or R light mixtures compared with only B or R light.
Previous studies have shown that under high levels of B light, there is an increase in the palisade cell area, which can lead to an increase in leaf thickness (For example [2][4][12]). However, this B light-induced increase in leaf thickness does not necessarily have to translate into a lower SLA [13]. Dougher and Budgee [6] identified that the direction of the effect of B light on SLA is very species dependent. Independent of the applied light quality, Poorter et al. [1] found that on average, indoor experiments tend to produce plants with higher SLA compared to field grown plants, mainly due to higher temperatures and lower light quantity in indoor facilities. In our study, which applied the average temperature and light quantity as in the field trial, the SLA of most species was similar between plants growing in the phytotrons and in the field.
Under the different treatments stem, leaf, root, and total dry biomass largely followed the trend in plant height. The lower biomass at high B% can thus be explained by a stronger inhibition of stem elongation by B light due to an increased cryptochrome activity [10], exposing the plants to lower irradiance due to larger distances to the light source compared with plants treated under a lower percentage of B light. In addition, the stunted growth of plants at high B% leads to an increased self-shading of leaves and decrease in light interception, which has been proposed to result in negative consequences for the whole plant productivity [5]. Although the individual species reacted differently between phytotrons and the field trial, on average, a significantly higher plant biomass within our phytotron treatments compared with the field was found (except for the 62% B treatment). In contrast, Poorter et al. [1] reported lower biomass under indoor conditions compared with field grown plants depending on species and functional group. Again, this apparent contradiction could be explained by the fact that in contrast to other indoor experiments, we deliberately applied the same average temperatures and light strength in the phytotrons as were measured in the field trial. Poorter et al. [1] demonstrated that indoor experiments often use low levels of light, which might reduce plant biomass in comparison with outdoor-grown plants.
While the effect of light quality on the aboveground organs was quite similar among species in the current study, the direction of the effect on roots was clearly species dependent. With species such as Alnus and Ocimum exhibiting higher root growth at very low and high B%, and species such as Raphanus and Ulmus showing increased root production at intermediate B percentages (25 and 35% B). To date, scarce information is available on the effects of light quality on belowground plant productivity. A previous study by Yorio et al. [14] reported that under 10% B mixed with 90% R light there was a higher root production in Lactuca, Raphanus, and Spinacia, compared with plants grown under pure R light. Nhut et al. [15] found that mixtures of B and R light stimulate the production of roots compared with pure R light in strawberry plantlets. Independent of light quality, we found a significantly enhanced root production in the phytotron treatments compared to the field grown plants, except for the 62% B treatment. As indicated by Poorter et al. [1], indoor climatization might induce root zone conditions that differ markedly from field conditions, leading to altered root production and consequently profoundly changed plant growth. As all plants in our experiment were regularly watered in both field and phytotron treatments, we can exclude that the observed higher root productivity in the phytotrons results from different water availability between indoor and field trials. However, pot soil temperature was not monitored, and it is possible that it differed significantly between indoor and field conditions, partly due to the lack of infrared radiation from the LED lamps.
3. Light Quality Effect on Leaf Pigmentation
The concentration of chlorophyll and carotenoids changed strongly with light quality in our study. Under natural sunlight, cryptochrome activity is reduced at high radiation, thereby signalling strong light conditions in the plant. The same effect can be achieved under experimental conditions by exposing plants to high percentages of B light [16]. The high proportion of B light in our 62% B treatment thus triggered the enhanced production of photosynthetic pigments despite the fact that the other treatments with lower B% had the same PPFD. In fact, the low concentrations of Chl a and b in plants that have been treated with low levels of B light or monochromatic R light in previous studies, have even led to photo-oxidative stress in plants due to an increase of O2- and H2O2 radicals that induce cellular damage [2][17]. Barnes and Bugbee [16] proposed that a minimum of 20−30 μmol m−2 s−1 of B light is necessary to reach natural-like growth and morphologies, even if such a minimum requirement for B light appears to be highly species-specific [18]. It is likely that due to all of our light treatments including at least 6% of B light, we did not observe light quality related stress effects in our experiment. However, we identify that even with over 30 μmol m−2 s−1 of B light (at 6% B), higher percentages of B can increase the photosynthetic maximum capacity in several species, indicating that it is not just the quantity of B light, but also its relationship with other wavebands in the spectrum. Interestingly, most species showed higher Chl a:b ratios in the phytotrons compared to the field trial. This effect has been observed previously in indoor-grown plants [19], where it is attributed to the lack of fluctuating light conditions in indoor facilities.
Like chlorophyll, the production of carotenoids was also significantly increased with 62% of B light compared to 6% B (and 35% B), yet only the 25% B and the 62% B treatments induced higher carotenoid concentrations than in the field trial. Hogewoning et al. [2] reported an increase of carotenoids in cucumber plants when B was increased to 50% in the light spectra. An increase of carotenoids has been shown to work as an accumulative protection mechanism correlating with high light intensities or high B ratios. For example, the authors of [4] found that Fv/Fm of rapeseed leaves was reduced under monochromatic B or R light treatments, compared with mixtures of B and R. They attributed this to a higher PS II damage and linked the higher concentrations of carotenoids to a protection mechanism against oxygen radical formation. This is in line with our Fv/Fm results, where lower percentages of B in the applied spectra induce small but significant differences of the Fv/Fm values in almost all investigated species.
4. Light Quality Effects on Photosynthesis
When Amax was measured under the same standardised light conditions (30% B and 70% R) in the current study, plants under 63% B showed, on average, significantly higher Amax compared to plants under 25% B and the field trial. This could be partially explained by the increased chlorophyll concentrations in 63% B treated plants (see above). Previously, higher Amax have been linked to higher levels of stomatal conductance and nitrogen concentration, where the latter is correlated to Rubisco, cytochrome, proteins and chlorophyll content [20]. A higher Amax has also been suggested to partially derive from an instantaneous stimulation of photosynthesis (i.e., during the exposure to the light within the gas-exchange chamber) due to the lack of adaptation to the standardised light condition [2]. In our case, using 70% R in plants adapted to 62% B may promote a higher Amax, meanwhile this may not be the case in plants adapted to lower percentages of B light, and therefore higher percentages of R light. Kim et al. [21] have shown that in Pisum sativum about four days were necessary to reach full photosynthetic acclimation after a transition from a PSI to a PSII stimulating light environment and vice versa. Similarly, Hogewoning et al. [22] showed in duckweed, that six days were needed to fully acclimate to different light conditions, using the Chl a:b ratio as the control parameter.
In contrast to the measurements of standardised light, when measured under the respective in situ light conditions, Amax was significantly lower at very low (6%) or very high (62%) B light conditions, despite the higher concentration of chlorophyll at 62% B or small differences in SLA (Figure 1B). In a similar but more extreme experiment, several long-term studies reported lower net photosynthesis or Amax in plants raised under monochromatic B or R light [2][3][4]. Hogewoning et al. [2], also reported dysfunctional photosynthesis in cucumber plants, grown under pure R light and a dose response curve in Amax when the B% was increased up to 50% B, with no further increase of Amax beyond 50% B. The increase of Amax with B percentages was associated with a reduction of the SLA, an increase of N and chlorophyll per leaf area, and higher stomatal conductance under mixtures of B and R light compared with only B or R [2]. Matsuda et al. [23] reported an increase of Amax in spinach plants exposed to a 1:1 B: R radiation compared with just B light, associated with increased leaf N concentration. Shengxin et al. [4] showed that dark adapted Fv/Fm values were higher (as an indicator for less photo-stress) under mixtures of B and R light compared with monochromatic B or R light.
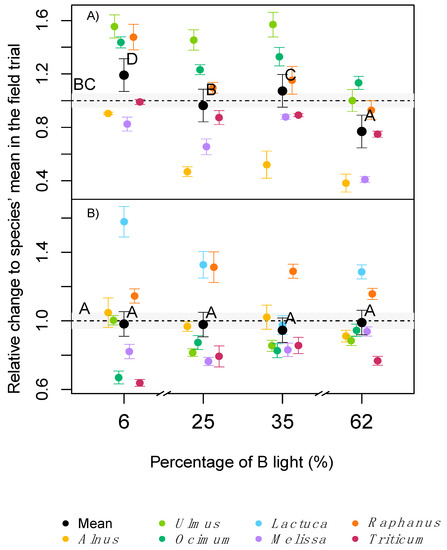
The effects of treatments on photosynthesis were also visible in the quantum yield of the CO2 fixation curve (α) of the investigated species. Similar to Amax, a more natural level of B light may explain a higher efficiency when an ‘in situ’ light was used for our gas-exchange measurements, with significantly higher values indoor than in the field trial. Similar results have been reported at 15–30% B compared with 50% B [2]. This effect may indicate the evolutionary adaption of species to the natural sunlight spectrum, with higher quantum yield under a more natural B:R ratio (circa 33% of B in the sunlight spectrum [24]). Other conditions with extreme levels of B or R light may require the adaptation to each light condition, where CO2 fixation may have a wavelength dependence related to absorption properties of the different pigments involved. Terashima et al. [25], described three major causes for the wavelength dependency of the quantum yield: absorption by photosynthetic carotenoids, absorption by non-photosynthetic pigments and an imbalanced excitation of the two photosystems, where an imbalance in excitation will result in quantum yield losses [13][26]. It has been shown that a correct light stimulus, with light qualities matching the species-specific ratio of PSII and PSI, is key to high quantum efficiency of photosynthesis [27]. The light compensation point of photosynthesis (CP) was generally not affected by light quality. Similar results have been observed in previous cases [28][4].
In the current study, the average dark respiration (DR) using the standardised light, independent of the species, was relatively lower at 62% B compared with the other light treatments or the field trial. Atkin et al. [29] described in tobacco that observed changes in DR were dependent on the previously applied irradiance (tested between 0 to 300 μmol photons m−2 s−1). An instantaneous stimulation of the photosystems in low light adapted plants due the stimulus of an intensity radiation burst was hypothesised. Although the total photon flux was the same between treatments in our study, similar short time effects on DR might have occurred when plants were exposed to a high intensities and light spectrum that they were not adapted to.
References
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plant growing in controlled conditions and in the field. New Phytol. 2016, 213, 383–855.
- Hogewoning, S.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117.
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal red:blue ratio in led lighting for nutraceutical indoor horticulture. Sci. Hortic. 2015, 193, 202–208.
- Shengxin, C.; Chunxia, L.; Xuyang, Y.; Song, C.; Xuelei, J.; Xiaoyin, L.; Zhigang, X.; Rongzhan, G. Morphological, photosynthetic, and physiological responses of rapeseed leaf to different combinations of red and blue lights at the rosette stage. Front. Plant Sci. 2016, 7, 1144.
- Hogewoning, S.; Trouwborst, G.; Meinen, E.; van Ieperen, W. Finding the optimal growth-light spectrum for greenhouse crops. Acta Hortic. 2012, 956, 357–363.
- Dougher, T.; Bugbee, B. Differences in the response of Wheat, Soybean and Lettuce to reduced Blue radiation. Photochem. Photobiol. 2001, 73, 199–207.
- Hogewoning, S.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Harbison, J. An artificial solar spectrum substantially alters plants development compared with usual climate room irradiance spectra. J. Exp. Bot. 2010, 61, 1267–1276.
- Terfa, M.; Solhaug, K.; Gislerød, H.; Olsem, J.; Torre, S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa x hybrida but does not affect time to flower opening. Physiol. Plant. 2013, 148, 146–159.
- Gautam, P.; Terfa, M.; Olsen, J.; Torre, S. Red and blue light effects on morphology and flowering of Petunia x hybrida. Sci. Hortic. 2015, 184, 171–178.
- Hernandez, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74.
- Lin, C.; Yang, H.; Guo, H.; Modckler, T.; Chen, J.; Cashmore, A. Enhancement of blue-light sensitivity of Arabidopsis seedlings by a blue light receptor cryptochrome 2. Proc. Natl. Acad. Sci. USA 1998, 95, 2686–2690.
- Hernandez, R.; Eguchi, T.; Deveci, M.; Kubota, C. Tomato seedlings physiological respones under different percentages of blue and red photon flux ratios using LEDs and cool white fluorescent lamps. Sci. Hortic. 2016, 213, 270–280.
- Zheng, L.; Van Labeke, M. Long-term effects of Red- and Blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917.
- Yorio, N.; Goins, G.; Kagie, H. Improving spinach, radish and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. Hortscience 2001, 36, 380–383.
- Nhut, D.; Takamura, T.; Watanabe, H.; Okamoto, K.; Tanaka, M. Responses of strawberry plantlets in vitro under super bright red and blue light-emitting (LEDs). Plant Celltissue Organ Cult. 2003, 73, 43–52.
- Barnes, C.; Bugbee, B. Morphological responses of wheat to blue light. J. Plant Physiol. 1992, 139, 339–342.
- Bae, G.; Choi, G. Decoding of lights signals by plants phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311.
- Dougher, T.; Bugbee, B. Is blue light good or bad for plants? Life Support Biosph. Sci. 1998, 5, 129–136.
- Vialet-Chabrand, S.; Matthews, J.; Simkin, A.; Raines, C.; Lawson, T. Importance of fluctuations in light on plants photosynthetic acclimation. Plant Physiol. 2017, 173, 2163–2179.
- Matsuda, R.; Ohashi-kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthesis characteristics of rice leaves grown under red light with or without supplemental blue light. Plant Cell Physiol. 2004, 45, 1870–1874.
- Kim, J.; Glick, R.; Melis, A. Dynamic of photosystem stoichiometry adjustment by light quality in chloroplasts. Plant Physiol. 1993, 102, 181–190.
- Hogewoning, S.; Trouwborst, G.; Engbers, G.; Harbison, J.; van Ieperen, W.; Ruijsch, J.; Schapendok, A.; Pot, C.; van Kooten, O. Plant physiological acclimation to irradiation by light-emitting diodes (LEDs). Acta Hortic. 2007, 761, 183–191.
- Matsuda, R.; Ohashi-kaneko, K.; Fujiwara, K.; Kurata, K. Analysis of the relationship between blue-light photon flux density and the photosynthetic properties of spinach (Spinacia oleracea L.) leaves with regard to the acclimation of photosynthesis to growth irradiance. Soil Sci. Plant Nutr. 2007, 53, 459–465.
- Chiang, C.; Olsen, J.; Basler, D.; Bånkestad, D.; Hoch, G. Latitude and weather influences on sun light quality and the relationship to tree growth. Forest 2019, 10, 610.
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic quesion of why the leaves are green. Plant Cell Physiol. 2009, 50, 684–697.
- Pfannschmidt, T. Acclimation to varying light qualities: Toward the functional relationship of state transitions and adjustment of photosystem stoichiometry. J. Phycol. 2005, 41, 723–725.
- Chow, W.; Melis, A.; Anderson, J. Adjustments of photo-system stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc. Natl. Acad. Sci. USA 1990, 87, 7502–7506.
- Furuyama, S.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue/ red ratio and light intensity on photomorphogenesis and photosynthesis of red leaf lettuce. Acta Hortic. 2014, 1037, 317–322.
- Atkin, O.; Evans, J.; Siebke, K. Relationship between the inhibition of leaf respiration by light and enhancement of leaf dark respiration following light treatment. Aust. J. Plant Physiol. 1998, 25, 437–443.




