| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shiro Jimi | + 2591 word(s) | 2591 | 2020-10-10 05:41:40 | | | |
| 2 | Catherine Yang | -1040 word(s) | 1551 | 2020-10-21 06:17:15 | | |
Video Upload Options
The treatment of biofilm infection is difficult in clinical practice. Therefore, the eradication mechanisms of action of silver sulfadiazine, which is a well-utilized antibacterial compound, in a biofilm were investigated. Its unique mode of action in biofilms is defined.
Biofilm-forming drug-resistant microbes are frequently detected in hospitals. Therefore, we examined the effects of silver sulfadiazine (SSD), a well-utilized antibacterial compound, and clarified the mechanisms underlying its activity against biofilms formed by methicillin-resistant Staphylococcus aureus (MRSA). The effects of SSD were a result of silver ions rather than sulfadiazine. SSD had a lesser revelation capacity of silver ions than silver nitrate; however, only SSD could eradicate mature biofilms by bacterial killing. Nevertheless, the effect was reduced by the addition of an ion chelator. Thus, the silver in SSD could be liberated after binding sulfadiazine to biofilms, and the use of SSD is reasonable in the eradication of biofilms in wounds.
1. Introduction
1.1 Biofilm (BF) infections
BFs cause chronic infections. Several types of symbiotic bacteria, such as Staphylococcus aureus and Pseudomonas aeruginosa, colonize our body and form BFs [1][2][3]. BFs contain massive extracellular polysaccharides/exopolymeric substances (EPS), and acquire an antibiotic-tolerant nature, which is explained by the decreased drug penetration [4] and the appearance of dormant cells (i.e., persister cells) [5]. Thus, the eradication of BFs has become a difficult task. In clinical practice, methicillin-resistant Staphylococcus aureus (MRSA) causes soft-tissue infections, indwelling catheter-associated infections, bacteremia, endocarditis, and osteomyelitis. Approximately 80% of chronic wound infections are attributed to bacteria or BFs [1]. BFs produce a subpopulation of drug-resistant cells called persister cells [6][7][8][9]. The BF matrix predominantly contains EPSs [10][11] and interacts with other molecules, including quorum-sensing signaling molecules/autoinducers, polypeptides, lectins, lipids, and extracellular DNA [12][13][14]. Specific molecules targeting BFs and effective drugs for BF eradication have not yet been identified. Therefore, BF is a serious exacerbating factor in antimicrobial resistance.
1.2 Silver sulfadiazine (SSD)
Among the many reported anti-biofilm antibodies and compounds, SSD is unique due to its complex with sulfadiazine and silver and has been clinically used for the treatment of patients with BFs [15][16]. Using the SSD mechanisms of action of BF eradication activity, light can be shed on the treatment of BFs formed by bacteria in our body.
SSD has been used as an exogenous antimicrobial agent since the 1960s [17][18] on partial and full-thickness burns to prevent infection [19][20]. It is registered on the World Health Organization’s List of Essential Medicines [21]. SSD possesses broad-spectrum antibacterial activity, reacting nonspecifically to Gram-negative and Gram-positive bacteria, causing distortion of the cell membrane and inhibition of DNA replication [18][22][23][24]. Nevertheless, common side effects of SSD include pruritus and pain at the site of administration [25] as well as decreased white blood cell counts, allergic reactions, bluish-gray skin discoloration, and liver inflammation [26][27][28]. However, the Cochrane systematic review (2010) [29] did not recommend the use of SSD due to the insufficient evidence on whether silver-containing dressings or topical agents promote wound healing or prevent wound infection. In addition, specific antibiotics are currently available.
The bactericidal actions of silver can be explained through three different mechanisms: (1) the production of dissolved oxygen-derived reactive oxygen species by its catalytic activity [32][33], (2) the cross-linkage with silver at the sites of hydrogen bonding between the double strands of DNA [31][32], and (3) the inhibition of enzyme activities by intracellular silver ions [31][33]. Sulfadiazine (SD), a sulfonamide, inhibits intracellular folate metabolism in bacteria, resulting in proliferation arrest. In the investigation of silver-containing agents, SD has been selected from various compounds due to its high and broad bactericidal effects [18][22][23][24]. SSD is a reliable antibacterial agent and has recently been used as a coating material for indwelling catheters [34][35]. However, the action mechanisms of SSD on BFs are still unclear.
2. Experimental discussion: SSD action on MRSA BFs
2.1 Primary setting for an in vitro study
To clarify the effect of SSD on BFs formed from MRSA, an in vitro study was performed. ATCC BAA-2856 (OJ-1) [36][37][38], a high-BF-forming strain of MRSA was used. The BF chip method was utilized as previously described [38][39]. The SSD concentration in medical drugs, such as Silvadene, is 1% (10,000 μg/mL); it could be diluted with tissue fluid exuded from wounds after application. Therefore, the highest concentration was set at 4,000 μg/mL (0.4%: 11,200 μM), which is an acceptable concentration for a clinical setting.
2.2 BF eradication effects from different compounds
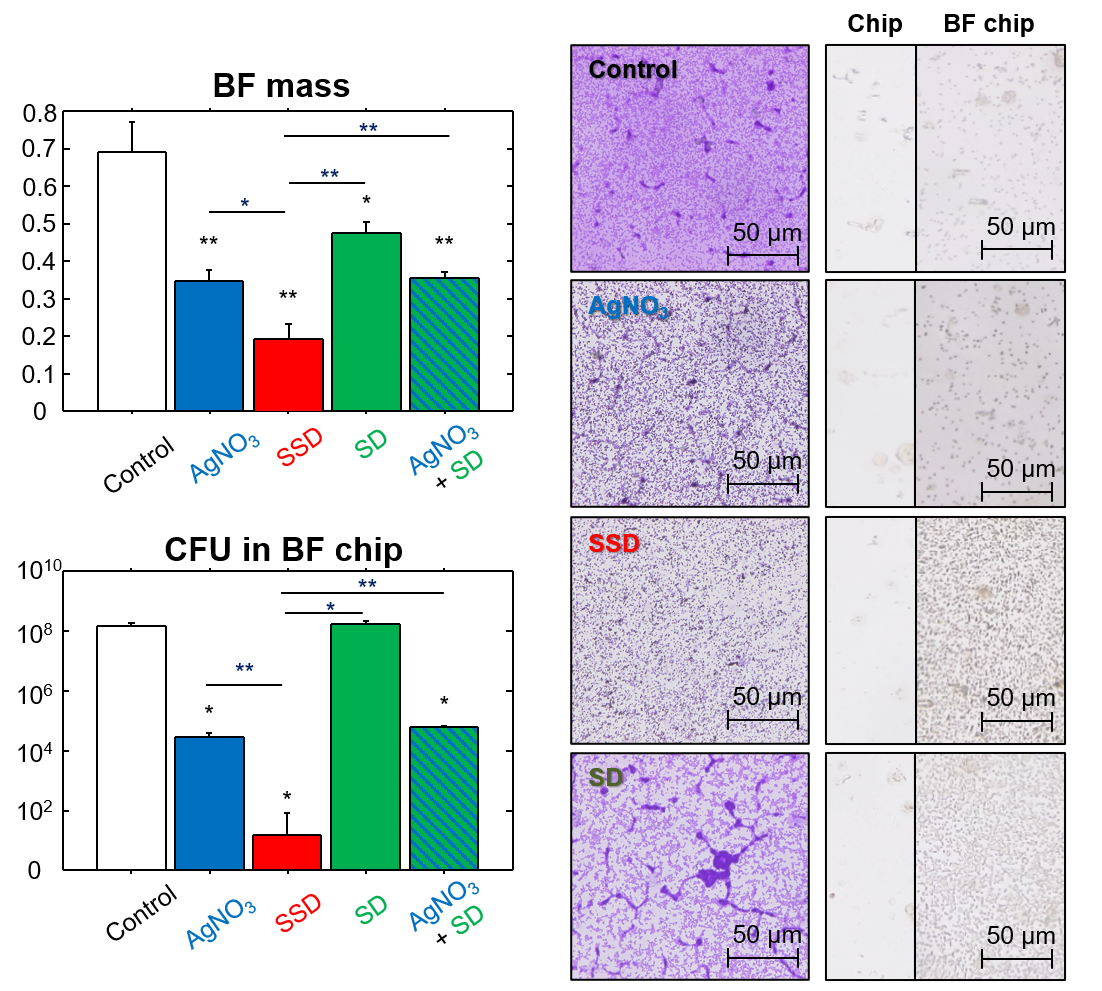
Among the compounds tested (SSD, SD, and AgNO3), SSD significantly decreased BF mass and live bacteria number in BFs. This decrease was considerably greater than that due to AgNO3. This phenomenon was supported by silver deposition on the BF chip. The efficacy of the simultaneous addition of AgNO3 and SD instead of SSD was also examined. These effects were equivalent to those of AgNO3 alone but were significantly lower than SSD, indicating that the molecular form of SSD is important for the induction of silver-related BF eradication (Figure 1).
Figure 1. Effects of SSD and other compounds on BFs.
2.3 Effects of silver ion on bacteria in different states
SSD and AgNO3 induced a similar antibacterial potency in the minimum inhibitory concentration for planktonic cells derived from BFs (bMIC) and the minimum bactericidal concentration for planktonic cells derived from BFs (bMBC), both of which were detected in the planktonic state derived from the BFs. In accordance with their threshold levels, silver ion concentrations reached more than 5 μM. In any case, such a concentration would be necessary for growth inhibition and killing planktonic bacteria. However, in the range of effective doses, silver ions were always generated at higher levels in AgNO3 than with SSD. This reflects the contradiction of the bactericidal effects of SSDs. The results suggest that some factor(s) other than simple diffusion of silver ions may be involved.
2.4 Bactericidal effects in BFs
The minimum BF eradication concentration (MBEC) level is a threshold for bacterial killing concentration in BFs. MBEC was detectable only in SSD, which was also confirmed by the CFU assay. This clearly shows that AgNO3 could not efficiently kill bacteria in BFs, and its effect remained within the level of growth inhibition. In contrast, SSD strongly and dose-dependently depressed the live cell number in matured BFs, in which the live cell density at a concentration of 700 μM (the equivalent level of bMIC) reached 1/10,000 of the control after 24 h of incubation. At a concentration of 2,800 μM (the equivalent level of MBEC), the live cell number was 1/300 of the initial cell density and 1/300,000 of the control after 24 h of incubation. These results suggest that silver ion concentration in solution does not directly influence SSD.
2.5 Mode of SSD action in solution
To clarify its mode of action, SSD was confined in a sealed chamber, which constrained it and prevented bacteria from penetrating beyond its membrane but allowed ionized silver to pass freely. Therefore, silver ion concentrations in the chambered condition were lower, i.e., 700 and 1,400 μM SSD, as compared with non-chambered conditions; however, at higher concentrations (> 2,800 μM SSD), they became similar. Upon confinement, SSD-induced bMBC and MBEC levels were no longer observed. Our study also showed that SD itself had the property of binding to BFs. Although the exact binding mechanisms of SSD on BFs are still unknown, the direct attachment of SSD to BFs is crucial in inducing bactericidal effects.
2.6 Role of silver ions released from SSD in BF eradication
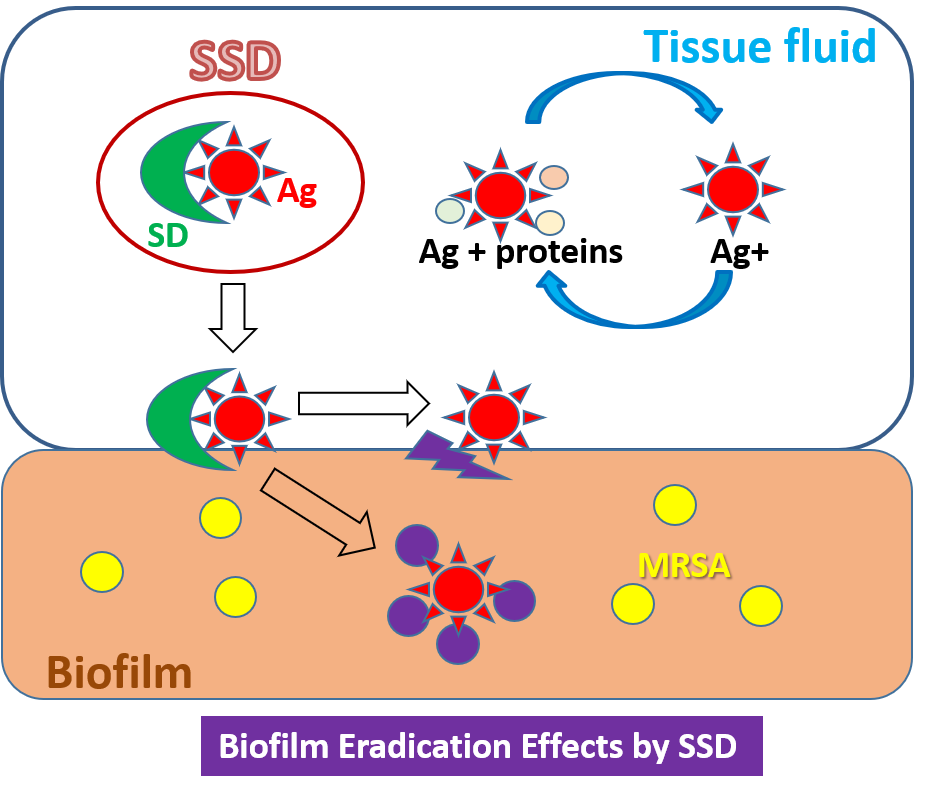
To validate the role of silver ions in the antibacterial properties of SSD, ethylenediamine-N,N,N',N’-tetraacetic acid (EDTA) was used as a chelator for cationic free ions at a concentration of 680 μM, which was approximately 40 to 100 times greater than that of the liberated silver ions in the media with AgNO3 and SSD. With the addition of EDTA, no changes in bMIC, bMBC, and MBEC were found in the culture with AgNO3. In contrast, the addition of EDTA increased all the values in the culture with SSD. The mechanisms of antibacterial action between AgNO3 and SSD are unknown; however, their mode of action might be different. Silver ions from AgNO3 could be more easily bound by negatively charged components in media as compared to SSD, and a bound–liberation cycle may be repeated. Moreover, the silver holding capacity by which SSD could reach BFs may be greater than that in AgNO3. This mechanism may act especially on the BFs due to the selective adhesion of SD on BFs, which was confirmed by the greater deposition of silver on the BF chip incubated with SSD, whereas sites of the deposition on BFs could not be identified. It therefore seems likely that SSD may act on the bacteria settled in the BFs. After deposition of SSD, silver ions could be released in a micro-environment in BFs, by which the opportunities to kill bacteria could be increased, resulting in strong distortions of the BF structure.
3. Conclusions and Future Perspectives
SSD was effective against MRSA, especially in the BF state, but SD itself had no effect. In a micro-environment, SSD preferably binds to BFs via the nature of SD, and then releases silver ions by which bacteria settled on the BFs are killed. In the future, further molecular investigations will be needed to identify the SSD binding site on BFs, which would lead to the discovery of target molecules of BFs formed by MRSA. Additionally, a systematic investigation of the use of SSD in BF-infected wounds should be performed in clinical practice rather than for infection prevention (figure 2).
Figure 2. The action of SSD on biofilms formed by MRSA.
References
- Kiedrowski, M.R.; Horswill, A.R. New approaches for treating staphylococcal biofilm infections. Ann N Y Acad Sci 2011, 1241, 104–121, doi:10.1111/j.1749-6632.2011.06281.x.
- Otto, M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 2013, 64, 175–188, doi:10.1146/annurev-med-042711-140023.
- Abdulhaq, N.; Nawaz, Z.; Zahoor, M.A.; Siddique, A.B. Association of biofilm formation with multi drug resistance in clinical isolates of. EXCLI J 2020, 19, 201–208, doi:10.17179/excli2019-2049.
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 2016, 14, 563-575, doi:10.1038/nrmicro.2016.94.
- Harms, A.; Maisonneuve, E.; Gerdes, K. Mechanisms of bacterial persistence during stress and antibiotic exposure. Science 2016, 354, doi:10.1126/science.aaf4268.
- Evans, R.C.; Holmes, C.J. Effect of vancomycin hydrochloride on Staphylococcus epidermidis biofilm associated with silicone elastomer. Antimicrob Agents Chemother 1987, 31, 889–894.
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol 2002, 292, 107-113, doi:10.1078/1438-4221-00196.
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Role of persisters and small-colony variants in antibiotic resistance of planktonic and biofilm-associated Staphylococcus aureus: an in vitro study. J Med Microbiol 2009, 58, 1067–1073, doi:10.1099/jmm.0.009720-0.
- Garcia, L.G.; Lemaire, S.; Kahl, B.C.; Becker, K.; Proctor, R.A.; Denis, O.; Tulkens, P.M.; Van Bambeke, F. Antibiotic activity against small-colony variants of Staphylococcus aureus: review of in vitro, animal and clinical data. J Antimicrob Chemother 2013, 68, 1455–1464, doi:10.1093/jac/dkt072.
- Flemming, H.C. The perfect slime. Colloids Surf B Biointerfaces 2011, 86, 251–259, doi:10.1016/j.colsurfb.2011.04.025.
- Dragoš, A.; Kovács, Á. The Peculiar Functions of the Bacterial Extracellular Matrix. Trends Microbiol 2017, 25, 257–266, doi:10.1016/j.tim.2016.12.010.
- Roche, F.M.; Downer, R.; Keane, F.; Speziale, P.; Park, P.W.; Foster, T.J. The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. J Biol Chem 2004, 279, 38433 – 38440, doi:10.1074/jbc.M402122200.
- Kline, K.A.; Fälker, S.; Dahlberg, S.; Normark, S.; Henriques-Normark, B. Bacterial adhesins in host-microbe interactions. Cell Host Microbe 2009, 5, 580–592, doi:10.1016/j.chom.2009.05.011.
- Lee, J.; Zhang, L. The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 2015, 6, 26–41, doi:10.1007/s13238-014-0100-x.
- Agostinho, A.M.; Hartman, A.; Lipp, C.; Parker, A.E.; Stewart, P.S.; James, G.A. An in vitro model for the growth and analysis of chronic wound MRSA biofilms. J Appl Microbiol 2011, 111, 1275-1282, doi:10.1111/j.1365-2672.2011.05138.x.
- Lemire, J.A.; Kalan, L.; Bradu, A.; Turner, R.J. Silver oxynitrate, an unexplored silver compound with antimicrobial and antibiofilm activity. Antimicrob Agents Chemother 2015, 59, 4031-4039, doi:10.1128/AAC.05177-14.
- Carr, H.S.; Wlodkowski, T.J.; Rosenkranz, H.S. Silver sulfadiazine: in vitro antibacterial activity. Antimicrob Agents Chemother 1973, 4, 585–587, doi:10.1128/aac.4.5.585.
- Fox, C.L.; Modak, S.M. Mechanism of silver sulfadiazine action on burn wound infections. Antimicrob Agents Chemother 1974, 5, 582–588, doi:10.1128/aac.5.6.582.
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial resistance to silver in wound care. J Hosp Infect 2005, 60, 1–7, doi:10.1016/j.jhin.2004.11.014.
- Heyneman, A.; Hoeksema, H.; Vandekerckhove, D.; Pirayesh, A.; Monstrey, S. The role of silver sulphadiazine in the conservative treatment of partial thickness burn wounds: A systematic review. Burns 2016, 42, 1377–1386, doi:10.1016/j.burns.2016.03.029.
- WHO. World Health Organization model list of essential medicines. Geneva, 2019.
- Hoffmann, S. Silver sulfadiazine: an antibacterial agent for topical use in burns. A review of the literature. Scand J Plast Reconstr Surg 1984, 18, 119–126, doi:10.3109/02844318409057413.
- Fuller, F.W.; Parrish, M.; Nance, F.C. A review of the dosimetry of 1% silver sulfadiazine cream in burn wound treatment. J Burn Care Rehabil 1994, 15, 213–223, doi:10.1097/00004630-199405000-00003.
- Miller, A.C.; Rashid, R.M.; Falzon, L.; Elamin, E.M.; Zehtabchi, S. Silver sulfadiazine for the treatment of partial-thickness burns and venous stasis ulcers. J Am Acad Dermatol 2012, 66, e159–165, doi:10.1016/j.jaad.2010.06.014.
- Fuller, F.W. The side effects of silver sulfadiazine. J Burn Care Res 2009, 30, 464–470, doi:10.1097/BCR.0b013e3181a28c9b.
- Muller, M.J.; Hollyoak, M.A.; Moaveni, Z.; Brown, T.L.; Herndon, D.N.; Heggers, J.P. Retardation of wound healing by silver sulfadiazine is reversed by Aloe vera and nystatin. Burns 2003, 29, 834–836, doi:10.1016/s0305-4179(03)00198-0.
- Chaby, G.; Viseux, V.; Poulain, J.F.; De Cagny, B.; Denoeux, J.P.; Lok, C. [Topical silver sulfadiazine-induced acute renal failure]. Ann Dermatol Venereol 2005, 132, 891–893, doi:10.1016/s0151-9638(05)79509-0.
- Abedini, F.; Ahmadi, A.; Yavari, A.; Hosseini, V.; Mousavi, S. Comparison of silver nylon wound dressing and silver sulfadiazine in partial burn wound therapy. Int Wound J 2013, 10, 573–578, doi:10.1111/j.1742-481X.2012.01024.x.
- Carter, M.J.; Tingley-Kelley, K.; Warriner, R.A. Silver treatments and silver-impregnated dressings for the healing of leg wounds and ulcers: a systematic review and meta-analysis. J Am Acad Dermatol 2010, 63, 668–679, doi:10.1016/j.jaad.2009.09.007.
- Lansdown, A.B. Silver. I: Its antibacterial properties and mechanism of action. J Wound Care 2002, 11, 125–130, doi:10.12968/jowc.2002.11.4.26389.
- Marx, D.E.; Barillo, D.J. Silver in medicine: the basic science. Burns 2014, 40 Suppl 1, S9–18, doi:10.1016/j.burns.2014.09.010.
- Russell, A.D.; Hugo, W.B. Antimicrobial activity and action of silver. Prog Med Chem 1994, 31, 351–370, doi:10.1016/s0079-6468(08)70024-9.
- Slawson, R.M.; Lee, H.; Trevors, J.T. Bacterial interactions with silver. Biol Met 1990, 3, 151–154, doi:10.1007/bf01140573.
- de Sousa, J.K.T.; Haddad, J.P.A.; de Oliveira, A.C.; Vieira, C.D.; Dos Santos, S.G. In vitro activity of antimicrobial-impregnated catheters against biofilms formed by KPC-producing Klebsiella pneumoniae. J Appl Microbiol 2019, 127, 1018-1027, doi:10.1111/jam.14372.
- Mohseni, M.; Shamloo, A.; Aghababaie, Z.; Afjoul, H.; Abdi, S.; Moravvej, H.; Vossoughi, M. A comparative study of wound dressings loaded with silver sulfadiazine and silver nanoparticles: In vitro and in vivo evaluation. Int J Pharm 2019, 564, 350-358, doi:10.1016/j.ijpharm.2019.04.068.
- Makino, T.; Jimi, S.; Oyama, T.; Nakano, Y.; Hamamoto, K.; Mamishin, K.; Yahiro, T.; Hara, S.; Takata, T.; Ohjimi, H. Infection mechanism of biofilm-forming Staphylococcus aureus on indwelling foreign materials in mice. Int Wound J 2015, 12, 122-131, doi:10.1111/iwj.12061.
- Haraga, I.; Abe, S.; Jimi, S.; Kiyomi, F.; Yamaura, K. Increased biofilm formation ability and accelerated transport of Staphylococcus aureus along a catheter during reciprocal movements. J Microbiol Methods 2017, 132, 63 – 68, doi:10.1016/j.mimet.2016.11.003.
- Ueda, Y.; Mashima, K.; Miyazaki, M.; Hara, S.; Takata, T.; Kamimura, H.; Takagi, S.; Jimi, S. Inhibitory effects of polysorbate 80 on MRSA biofilm formed on different substrates including dermal tissue. Sci Rep 2019, 9, 3128, doi:10.1038/s41598-019-39997-3.
- Jimi, S.; Miyazaki, M.; Takata, T.; Ohjimi, H.; Akita, S.; Hara, S. Increased drug resistance of meticillin-resistant Staphylococcus aureus biofilms formed on a mouse dermal chip model. J Med Microbiol 2017, 66, 542-550, doi:10.1099/jmm.0.000461.