
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Melvin R Hayden | + 2140 word(s) | 2140 | 2021-09-09 06:18:44 | | | |
| 2 | Jessie Wu | Meta information modification | 2140 | 2021-09-24 09:52:59 | | | | |
| 3 | Jessie Wu | Meta information modification | 2140 | 2021-09-24 09:59:47 | | | | |
| 4 | Jessie Wu | Meta information modification | 2140 | 2021-09-24 10:01:20 | | |
Video Upload Options
The COVID-19 pandemic has paralleled the great Spanish flu pandemic of 1918–1919 in the United States. Previous historical accounts have strongly suggested a post-viral syndrome and, currently, a post-COVID-19 viral syndrome is unquestionable, which shares many of the characteristics of myalgic encephalomyelitis/chronic fatigue syndrome that is present globally. The original term for this post-acute sequela of SARS-CoV-2 (PASC) was termed long haulers by those who were affected with this syndrome and it is now termed long COVID (LC) or PASC. International researchers and clinicians are desperately trying to better understand the pathobiological mechanisms possibly involved in this syndrome. This review aims to summarize many of the cumulated findings associated with LC/PASC and provides supportive and representative illustrations and transmission electron micrographic remodeling changes within brain tissues associated with a stress type of injury as occurs in the classic db/db and novel BTBR ob/ob obesity and diabetes mellitus mice models. These models are utilized to merely provide a response to metabolic stress injury wound healing mechanisms that are also present in humans. This review posits that neuroglial activation and chronic neuroinflammation may be a common denominator for the development of the complex LC/PASC syndrome following acute COVID-19 due to SARS-CoV-2.
This review was written with the intent of describing the mounting problem of Long COVID or PASC referred to by patients as Long-Haulers and the authors' personal experience with treating patients with the post-viral syndrome of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) due to HSV-1 encephalitis and a cluster of patients with human cytomegalic virus (hCMV) infections who were suffering from what is now termed Long COVID- Post-Acute Sequela of Coronavirus-2 or PASC.
1. Introduction
2. COVID-19 Effects on the Hypothalamic-Pituitary-Adrenal Axis (HPA) and Adrenal Gland with Residual Effects in LC/PASC
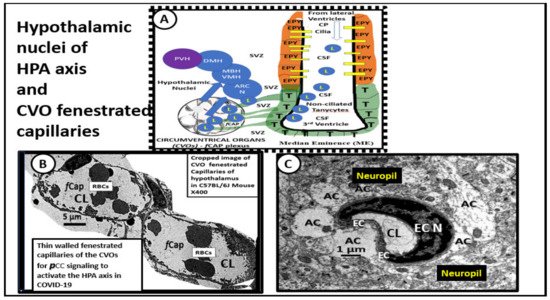
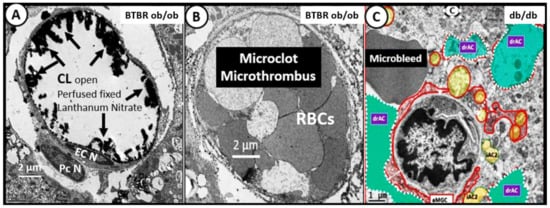
3. Central Nervous System Inflammatory Induced Thromboembolism: Microclots and Microbleeds in Acute COVID-19 and Possibly LC/PASC Due to Increased PCC and CNSCC
3. Repurposing—Repositioning Approved Medications for LC/PASC
References
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms and functional impairment assessed 8 months after mild COVID-19 among health care workers. JAMA 2021, 325, 2015.
- Lerner, A.M.; Robinson, D.A.; Yang, L.; Williams, C.F.; Newman, L.M.; Breen, J.J.; Eisinger, R.W.; Schneider, J.S.; Adimora, A.A.; Erbelding, E.J. Toward Understanding COVID-19 Recovery: National Institutes of Health Workshop on Postacute COVID-19. Ann. Intern. Med. 2021, 174, 999–1003.
- Bellavance, M.-A.; Rivest, S. The HPA—Immune axis and the immunomodulatory actions of glucocorticoids in the brain. Front. Immunol. 2014, 5, 136.
- Siejka, A.; Barabutis, N. Adrenal insufficiency in the COVID-19 era. Am. J. Physiol. Metab. 2021, 320, E784–E785.
- Zinserling, V.A.; Semenova, N.; Markov, A.G.; Rybalchenko, O.V.; Wang, J.; Rodionov, R.N.; Bornstein, S.R. Inflammatory cell infiltration of adrenals in COVID-19. Horm. Metab. Res. 2020, 52, 639–641.
- Santana, M.F.; Borba, M.G.S.; Baía-Da-Silva, D.C.; Val, F.; Alexandre, M.A.A.; Brito-Sousa, J.D.; Melo, G.C.; Queiroga, M.V.O.; Farias, M.E.L.; Camilo, C.C.; et al. Case report: Adrenal pathology findings in severe COVID-19: An autopsy study. Am. J. Trop. Med. Hyg. 2020, 103, 1604–1607.
- Loriaux, D.L.; Fleseriu, M. Relative adrenal insufficiency. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 392–400.
- Annane, D.; Pastores, S.M.; Rochwerg, B.; Arlt, W.; Balk, R.A.; Beishuizen, A.; Briegel, J.; Carcillo, J.; Christ-Crain, M.; Cooper, M.S.; et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (part I): Society of critical care medicine (SCCM) and european society of intensive care medicine (ESICM) 2017. Intensive Care Med. 2017, 43, 1751–1763.
- Pal, R. COVID-19, hypothalamo-pituitary-adrenal axis and clinical implications. Endocrine 2020, 68, 251–252.
- McFadyen, J.D.; Stevens, H.; Peter, K. The emerging threat of (Micro)thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020, 127, 571–587.
- Poggesi, A.; Pasi, M.; Pescini, F.; Pantoni, L.; Inzitari, D. Circulating biologic markers of endothelial dysfunction in cerebral small vessel disease: A review. J. Cereb. Blood Flow Metab. 2015, 36, 72–94.
- Hayden, M.R. Type 2 diabetes mellitus increases the risk of late-onset Alzheimer’s disease: Ultrastructural remodeling of the neurovascular unit and diabetic gliopathy. Brain Sci. 2019, 9, 262.
- Hayden, M.; Banks, W. Deficient leptin cellular signaling plays a key role in brain ultrastructural remodeling in obesity and type 2 diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 5427.
- Hayden, M.R. Endothelial activation and dysfunction in metabolic syndrome, type 2 diabetes and coronavirus disease 2019. J. Int. Med. Res. 2020, 48.
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. medRxiv 2021.
- Page, M.; Thomson, G.J.A.; Nunes, J.M.; Engelbrecht, A.-M.; Nell, T.A.; De Villiers, W.J.S.; De Beer, M.C.; Engelbrecht, L.; Kell, D.B.; Pretorius, E. Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation. Sci. Rep. 2019, 9, 3102.
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418.
- Chioh, F.W.; Fong, S.-W.; E Young, B.; Wu, K.-X.; Siau, A.; Krishnan, S.; Chan, Y.-H.; Carissimo, G.; Teo, L.L.; Gao, F.; et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. eLife 2021, 10, e64909.
- Xue, H.; Li, J.; Xie, H.; Wang, Y. Review of drug repositioning approaches and resources. Int. J. Biol. Sci. 2018, 14, 1232–1244.
- Regland, B.; Forsmark, S.; Halaouate, L.; Matousek, M.; Peilot, B.; Zachrisson, O.; Gottfries, C.-G. Response to vitamin B12 and folic acid in myalgic encephalomyelitis and fibromyalgia. PLoS ONE 2015, 10, e0124648.




