
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dinesh Rokaya | + 2795 word(s) | 2795 | 2019-12-04 05:10:33 | | | |
| 2 | Nicole Yin | -1117 word(s) | 1678 | 2019-12-12 09:50:17 | | | | |
| 3 | Nicole Yin | Meta information modification | 1678 | 2020-07-30 12:08:14 | | | | |
| 4 | Nicole Yin | -12 word(s) | 1666 | 2020-10-22 11:27:09 | | |
Video Upload Options
Bioactive glass (BAG) consist of known biocompatible and bioactive minerals, including fluorapatite (FAP), wollastonite, diopside, and tricalcium phosphate. A bioactive material can interact with the biological environment to elicit a specific biological response and they can be osteoconductive or osteoinductive. Bioactive Glasses are amorphous solids with the irregular organization of atoms, optically transparent, and brittle consisting of silica networks. They possesses bioactivity and antimicrobial properties as presented n following sections. The bioactivity of BAGs involves several steps; BAGs immediately undergo ionic dissolution and glass degradation via the exchange of H+ ions in the solution and Na+ and Ca2+ from the glass network. BAG exhibits antimicrobial properties against various pathogens, e.g pathogens associated with sinusitis and osteomyelitis.
1. Introduction
A bioactive material can interact with the biological environment to elicit a specific biological response, such as the formation of a hydroxyapatite layer with a bond forming between the tissue and material. Bone and teeth, enamel and dentin, consist mainly of mineralized hard tissue in the form of hydroxyapatite , a crystalline calcium phosphate, Ca10(PO4)6(OH)2 [30]. In contrast, bioinert materials do not elicit any specific responses or interact with the biological environment. However, they can result in a foreign-body reaction and the formation of a fibrous capsule. The fibrous capsule may result in micromovements and eventual failure of a prosthesis. Bioactive materials may be osteoconductive or osteoinductive[1]. The most bioactive glass has a superior surface area with a higher dissolution rate and thus faster apatite formation[2]. In addition, they have shown to increase the mechanical properties of such composite for natural bones and provide biomimetic nano-structuration enhancing cell adhesion.
The bioactive properties are influenced by the structure and composition of the glass, manufacturing techniques, and the rate of ionic dissolution. This is clearly illustrated when comparing BAGs to the traditional Bioglass® 45S5. Bioglass® 45S5 possesses several shortcomings which include the possibility of gap formation between the material and host tissues due to a rapid degradation rate[3][4]. The lack of porosity should not only be assigned to the composition but also to the applied process and the degree of particle aggregation[5][6] . Also, a Bioglass® 45S5 may induce cytotoxic effects due to a high rise in pH due to high Na+ and Ca2+ leakage; this may additionally cause delayed hydroxyapatite formation[5][6][7]. The glass composition may not be favorable for the fabrication of porous scaffolds due to poor mechanical properties, such as too fragile[8][9]. Future research needs to improve the mechanical properties of the BAG.
2. Bioactivity of Bioactive Glass
Glasses are amorphous solids with the irregular organization of atoms, optically transparent, and brittle consisting of silica networks and are, therefore, often termed supercooled liquids[10]. Conventional glasses and BAGs differ in one aspect, their rate of dissolution. Conventional glasses, in general, are expected to have high durability and thus low dissolution rates. BAGs require specific dissolution rates for bioactivity. This is achieved by the addition of network modifiers, such as CaO and Na2O, to make the surface and silica network more reactive. From glass dissolution to the formation of hydroxyapatite , bioactivity involves several steps.
Once in contact with body fluids (BF) or simulated body fluid (SBF), BAGs immediately undergo ionic dissolution and glass degradation via the exchange of H+ ions in the solution and Na+ and Ca2+ from the glass network. The ion exchange results in the formation of silanol groups (Si–O–H) due to the hydrolysis of the silica groups. An increased alkaline local environment develops due to the increase in OH- concentration. The silica network is further degraded as the pH rises, forming orthosilicic acid and Si(OH)4 on the surface in the form of a negatively charged gel. The gel layer functions as a matrix for hydroxyapatite with precipitation sites[11]. Beneath the gel layer is a depleted alkaline surface layer on top of the bulk glass. On top of the gel layer, a layer of amorphous calcium phosphate forms. Precipitation and further mineralization occur due to the incorporated carbonate ions from the now supersaturated solution, thus the concentration of Ca- and Si-ions in solution are critical; about 88–100 ppm and 17–20 ppm of the respective ions are required. The newly formed hydroxyapatite enables growth factors to adsorb to the surface, as well as attachment, proliferation, and differentiation of osteoprogenitor cells by cytokines and extracellular matrix components expressed by the upregulation of several genes[11]. Although the tissue bonding properties of the BAG are still not precise, collagen and glycoproteins are believed to incorporate the surrounding bone tissue into the hydroxyapatite layer. As the hydroxyapatite grows inwards, the BAG starts to resorb and gets replaced by growing bone tissue[12]. The glass particles usually have a size of 90–170 μm, which affects their resorption rate. Particle sizes < 150 μm readily degrade as orthosilicic acid is released during the formation of the gel layer. Osteoclasts, once incorporated in the growing bone, break down larger particles[13] resulting in a more extended period of resorption and stronger bone[14].
3. Antimicrobial Properties
Dental implants or prosthetic joints are surgically inserted to replace lost tissue and increase the function and quality of life of the patients[15]. However, implants carry a risk of developing infections such as peri-implantitis (PI) or periprosthetic joint diseases (PJI). These infections result in increased morbidity and mortality, as well as resorption of surrounding bone tissue and eventual loosening of the implant. Tomasi and Derks[16] estimated a weighted mean prevalence of 22% for PI. Any artificial joint may develop PJI. PJI occurs in 0.2–9% of prostheses and is one of the most frequent indications of revision and replacement of the joint prosthesis making up for 15% of hip prostheses and 25% of knee prostheses[17]. Infection occurs due to the establishment of bacterial biofilm on the surface of the implant. Biofilm is a layer of microbial communities adhering to a surface via a robust polysaccharide matrix and is known to be about a thousand times more resistant to antibiotic (AB) therapy compared to planktonic bacteria. Bone infections pose an additional challenge of the reduced local effect of antibiotic treatment owing to insufficient vasculature[18] or areas of devitalized bone[19]. Additionally, the increasing prevalence of antibiotic-resistant bacteria, including multidrug-resistant bacteria, results in the ineffective treatment of bacterial infections with antibiotic therapy, including AB-loaded bone substitutes as carriers[20]. Surgical debridement or osseous resection followed by placement of a bone substitute in the defect has shown positive treatment outcomes for established PI cases. PJI can be managed by surgical debridement and AB therapy. However, for resistant pathogens or loosened prosthesis, revision is necessary, which results in reduced quality of life[21].
BAGs, specifically BAG-S53P4, have shown to possess broad-spectrum antimicrobial properties with no observed resistance to date[22]. S. aureus is among the most common bacterial strains implicated in PJIs and a major biofilm contributor. However, S53P4 has proved to reduce the biofilm mass in vitro conditions[23][24]. The antibiofilm activity has further been observed to affect several multi drug resistant strains isolated from PJIs[23]. Additionally, an increased antibiofilm effect is observed with the incorporation of antimicrobial molecules into the BAG[25][26][27]. S53P4 has successfully been used in the treatment of osteomyelitis[28]. The antimicrobial and antibiofilm effects of bioactive glasses differ from that of conventional Abs, as once embedded in the body, bioactive glasses increase the pH and osmolarity locally, which creates an environment unfriendly for bacterial growth and adhesion[25][29]. Bari et al.[26]developed Cu-doped mesoporous SiO2-CaO glass (Cu-MBG) by an ultrasound assisted one-pot synthesis (Figure 1). Cu-MBG nanoparticles showed antibacterial effects against 3 bacterial strains (E. coli, S. aureus, and S. epidermidis). The Cu-MBG can be a promising and versatile platform for bone and soft tissue regeneration.
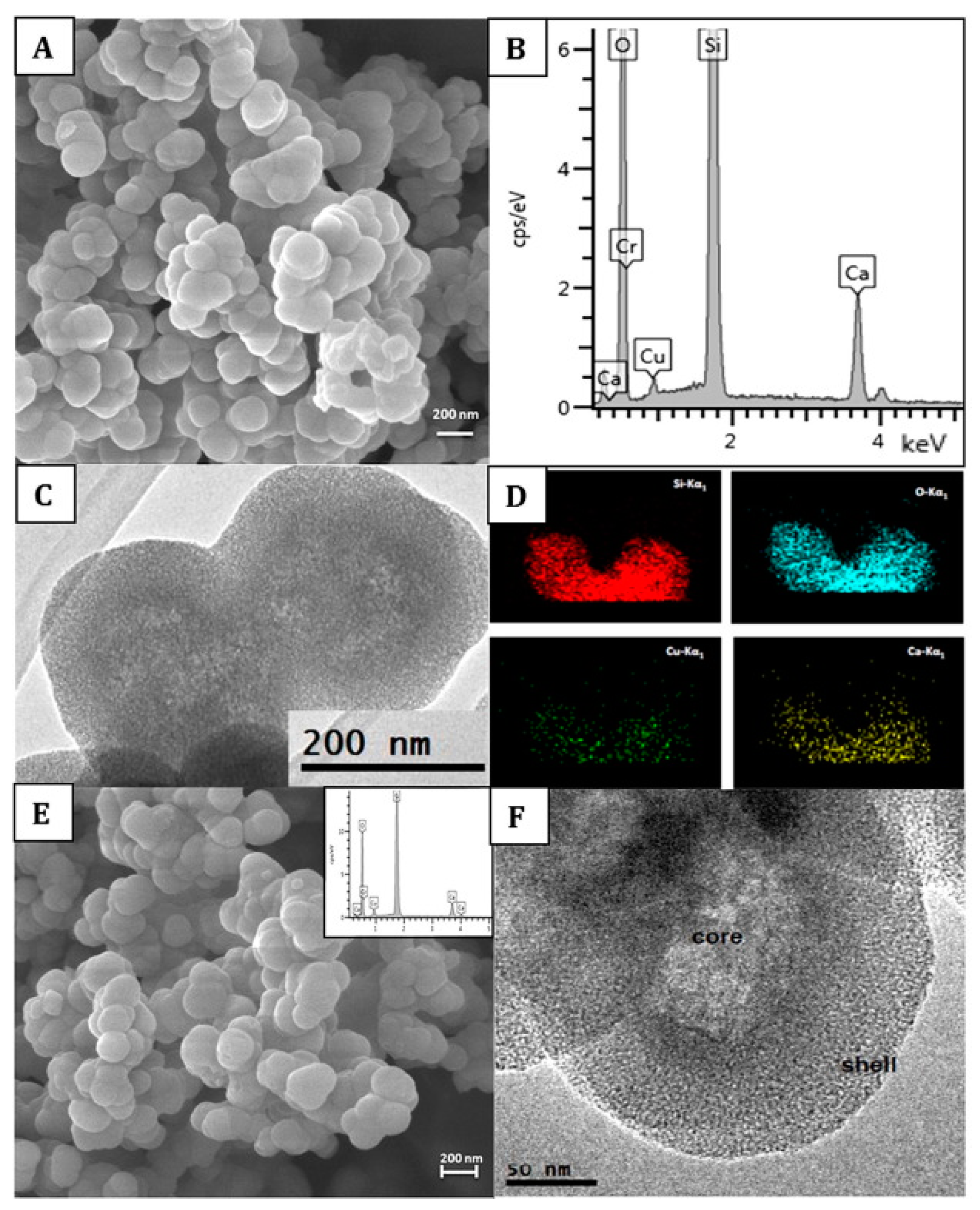
Figure 1. Characterization of Cu-doped mesoporous SiO2-CaO glass (Cu-MBG) 2%: SEM image (A), EDS spectrum (B), TEM image (C), and EDS mapping showing uniformly distributed in the nanoparticles of Si (red), O (blue), Cu (green), and Ca (yellow) (D). Characterization of Cu-MBG 5%: SEM image (E), related EDS spectrum (inset), and TEM image (F). Reproduced from [16] with permission from Elsevier.
As alkalinity is considered the primary antimicrobial mechanism, Bioglass® 45S5 is considered more effective. However, S53P4 presents a more delicate balance between antimicrobial properties, alkalinity with a pH of 7.9, and osteogenicity[30]. Particle size influences the antimicrobial properties as well; small particle sizes increase the surface area and the antimicrobial effect[24][31]. Alkali-free BAG doped with ZnO and SrO synthesized by melt quenching exhibited antimicrobial properties against strains of Staphylococcus aureus and Escherichia coli. The results suggest that BAGs can still provide antimicrobial properties in the absence of alkalinity[32]. These properties make BAG perhaps the ideal bone substitute in the treatment of bone infections such as osteomyelitis and peri-implant infections[33][34]. The US Food and Drug Administration (FDA) has approved Bioglass® 45S5 and S53P4 for clinical applications where antimicrobial properties are desired[35].
BAG also exhibits antimicrobial properties against pathogens associated with sinusitis, which makes it excellent for sinus augmentation and repairing of the orbital floor defects. A communication often exists between these anatomical structures, and infection from maxillary sinus can quickly spread to an orbital floor implant, necessitating implant removal[36]. Hence, BAG S53P4 can be used for these applications due to slow resorption and antimicrobial effect.
BAG-coated dental implants have shown promising results with reduced bone loss in experimentally induced PI in beagle dogs[37]. A recent in vitro study showed reduced biofilm formation of putative periodontal pathogen strains, in addition to S. mutans[38]. Besides, reduced growth of periodontitis-associated and cariogenic bacteria, as well as Enterococcus facials, has been reported using BAG containing propolis, a naturally occurring compound in beehives[39]. Additionally, BAGs can incorporate hydrophilic as well as hydrophobic compounds into their structure suggesting several undiscovered combinations of compounds may be achievable to increase antimicrobial efficiency[25], strengthening a future antimicrobial role of bioactive glass in dental applications. Another assessment was done on machined Ti6Al4V threaded dental implant coated with hydroxyapatite and bioactive glasses in human jaws, the outcome is very promising and futuristic. The observed BAGs are safe and effective like hydroxyapatite for enhancing osseointegration[40].
The details can is available at https://www.mdpi.com/1422-0067/20/23/5960
References
- T. Albrektsson; C. Johansson; Osteoinduction, osteoconduction and osseointegration. European Spine Journal 2001, 10, S96-S101, 10.1007/s005860100282.
- Charlotte Vichery; Jean-Marie Nedelec; Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials 2016, 9, 288, 10.3390/ma9040288.
- P. Sepulveda; J. R. Jones; L. L. Hench; In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. Journal of Biomedical Materials Research 2002, 61, 301-311, 10.1002/jbm.10207.
- Martin Vogel; Christian Voigt; Ulrich M. Gross; Christian M. Müller-Mai; In vivo comparison of bioactive glass particles in rabbits.. Biomaterials 2001, 22, 357-362, 10.1016/s0142-9612(00)00191-5.
- J.J.M. Damen; J.M. Ten Cate; Silica-induced Precipitation of Calcium Phosphate in the Presence of Inhibitors of Hydroxyapatite Formation. Journal of Dental Research 1992, 71, 453-457, 10.1177/00220345920710030601.
- Salonen J.I.,Arjasmaa M.,Tuominen U.,Behbehani M J.,Zaatar E I.; Bioactive glass in dentistry. Journal Of Minimum Intervention In Dentistry 2009, 2, 208-219, https://pdfs.semanticscholar.org/e86b/d05c9364f523591703b68c078b4d4c9ebdcc.pdf.
- Saqib Ali; Imran Farooq; Kefi Iqbal; A review of the effect of various ions on the properties and the clinical applications of novel bioactive glasses in medicine and dentistry.. The Saudi Dental Journal 2013, 26, 1-5, 10.1016/j.sdentj.2013.12.001.
- Qizhi Z. Chen; Ian D. Thompson; Aldo R. Boccaccini; 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414-2425, 10.1016/j.biomaterials.2005.11.025.
- Qiang Chen; Francesco Baino; Silvia Spriano; Nicola M. Pugno; Chiara Vitale-Brovarone; Modelling of the strength–porosity relationship in glass-ceramic foam scaffolds for bone repair. Journal of the European Ceramic Society 2014, 34, 2663-2673, 10.1016/j.jeurceramsoc.2013.11.041.
- W. H. Zachariasen; THE ATOMIC ARRANGEMENT IN GLASS. Journal of the American Chemical Society 1932, 54, 3841-3851, 10.1021/ja01349a006.
- Larry L. Hench; The story of Bioglass®. Journal of Materials Science: Materials in Electronics 2006, 17, 967-978, 10.1007/s10856-006-0432-z.
- Qiang Fu; Mohamed N. Rahaman; Hailuo Fu; Xin Liu; Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rate for bone tissue engineering applications. I. Preparation and in vitro degradation. Journal of Biomedical Materials Research Part A 2010, 95, 164-171, 10.1002/jbm.a.32824.
- Arun K. Gosain; Bioactive Glass for Bone Replacement in Craniomaxillofacial Reconstruction. Plastic and Reconstructive Surgery 2004, 114, 590-593, 10.1097/01.prs.0000128355.95900.dd.
- P Ducheyne; Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999, 20, 2287-2303, 10.1016/s0142-9612(99)00181-7.
- Pokpong Amornvit; Sahana Bajracharya; Dinesh Rokaya; Konrawee Keawcharoen; Walop Supavanich; Management of Obstructive Sleep Apnea with Implant Retained Mandibular Advancement Device. World Journal of Dentistry 2014, 5, 184-189, 10.5005/jp-journals-10015-1285.
- Jan Derks; Cristiano Tomasi; Peri-implant health and disease. A systematic review of current epidemiology. Journal of Clinical Periodontology 2015, 42, S158–S171, 10.1111/jcpe.12334.
- Kevin Bozic; Steven M. Kurtz; Edmund Lau; Kevin Ong; Thomas P. Vail; Harry E. Rubash; Daniel J. Berry; The Epidemiology of Revision Total Knee Arthroplasty in the United States. The Journal of Arthroplasty 2009, 24, e49, 10.1016/j.arth.2008.11.063.
- Rebecca A. Brady; Jeff G. Leid; Jason H. Calhoun; J. William Costerton; Mark E. Shirtliff; Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunology & Medical Microbiology 2008, 52, 13-22, 10.1111/j.1574-695x.2007.00357.x.
- Brendan Healy; Andrew Freedman; ABC of wound healing:Infections. BMJ 2006, 333, 0612454, 10.1136/sbmj.0612454.
- Ana C. Matos; Lídia M. Gonçalves; Patrícia Dias De Mendonça Rijo; Mário A. Vaz; António J. Almeida; Ana F. Bettencourt; A novel modified acrylic bone cement matrix. A step forward on antibiotic delivery against multiresistant bacteria responsible for prosthetic joint infections. Materials Science and Engineering: C 2014, 38, 218-226, 10.1016/j.msec.2014.02.002.
- Ninna R. Poulsen; Inger Mechlenburg; Kjeld Søballe; Jeppe Lange; Patient-reported quality of life and hip function after 2-stage revision of chronic periprosthetic hip joint infection: a cross-sectional study. HIP International 2017, 28, 407-414, 10.5301/hipint.5000584.
- Lorenzo Drago; Elena De Vecchi; Monica Bortolin; Marco Toscano; Roberto Mattina; Carlo Luca Romanò; Antimicrobial activity and resistance selection of different bioglass S53P4 formulations against multidrug resistant strains. Future Microbiology 2015, 10, 1293-1299, 10.2217/fmb.15.57.
- Monica Bortolin; Elena De Vecchi; Carlo Luca Romanò; Marco Toscano; Roberto Mattina; Lorenzo Drago; Antibiofilm agents against MDR bacterial strains: is bioactive glass BAG-S53P4 also effective?. Journal of Antimicrobial Chemotherapy 2015, 71, 123-127, 10.1093/jac/dkv327.
- Débora C. Coraça-Huber; Manfred Fille; Johann Hausdorfer; David Putzer; Michael Nogler; Efficacy of antibacterial bioactive glass S53P4 againstS. aureusbiofilms grown on titanium discs in vitro. Journal of Orthopaedic Research 2013, 32, 175-177, 10.1002/jor.22463.
- R. S. Magini; J. C. M. Souza; M. C. Fredel; M. E. Galarraga-Vinueza; Joana Mesquita-Guimaraes; A. R. Boccaccini; Anti-biofilm properties of bioactive glasses embedding organic active compounds. Journal of Biomedical Materials Research Part A 2016, 105, 672-679, 10.1002/jbm.a.35934.
- Alessandra Bari; Nora Bloise; Sonia Fiorilli; Giorgia Novajra; Maria Vallet-Regí; Giovanna Bruni; Almudena Torres-Pardo; José M. González-Calbet; Livia Visai; Chiara Vitale-Brovarone; et al. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomaterialia 2017, 55, 493-504, 10.1016/j.actbio.2017.04.012.
- Yu-Ting Xu; Qiong Wu; Ya-Ming Chen; Roger J. Smales; Shu-Ya Shi; Meng-Ting Wang; Antimicrobial effects of a bioactive glass combined with fluoride or triclosan on Streptococcus mutans biofilm. Archives of Oral Biology 2015, 60, 1059-1065, 10.1016/j.archoralbio.2015.03.007.
- N.C. Lindfors; P. Hyvönen; M. Nyyssönen; M. Kirjavainen; J. Kankare; E. Gullichsen; J. Salo; Bioactive glass S53P4 as bone graft substitute in treatment of osteomyelitis. Bone 2010, 47, 212-218, 10.1016/j.bone.2010.05.030.
- Saima Begum; William E Johnson; Tony Worthington; Richard A Martin; The influence of pH and fluid dynamics on the antibacterial efficacy of 45S5 Bioglass. Biomedical Materials 2016, 11, 15006, 10.1088/1748-6041/11/1/015006.
- Pekka K. Vallittu; Timo O. Närhi; Leena Hupa; Fiber glass–bioactive glass composite for bone replacing and bone anchoring implants. Dental Materials 2015, 31, 371-381, 10.1016/j.dental.2015.01.003.
- T. Waltimo; T.J. Brunner; M. Vollenweider; W.J. Stark; M. Zehnder; Antimicrobial effect of nanometric bioactive glass 45S5.. Journal of Dental Research 2007, 86, 754-757, 10.1177/154405910708600813.
- Saurabh Kapoor; Ashutosh Goel; Antonio Tilocca; Vikram Dhuna; Gaurav Bhatia; Kshitija Dhuna; José M.F. Ferreira; Role of glass structure in defining the chemical dissolution behavior, bioactivity and antioxidant properties of zinc and strontium co-doped alkali-free phosphosilicate glasses. Acta Biomaterialia 2014, 10, 3264-3278, 10.1016/j.actbio.2014.03.033.
- Lorenzo Drago; Christian Vassena; Simone Fenu; Elena De Vecchi; Valentina Signori; Raffaele De Francesco; Carlo Luca Romanò; In vitro antibiofilm activity of bioactive glass S53P4. Future Microbiology 2014, 9, 593-601, 10.2217/fmb.14.20.
- Mohamed N. Rahaman; B. Sonny Bal; Wenhai Huang; Review: Emerging developments in the use of bioactive glasses for treating infected prosthetic joints. Materials Science and Engineering: C 2014, 41, 224-231, 10.1016/j.msec.2014.04.055.
- Pekka K. Vallittu; Bioactive glass-containing cranial implants: an overview. Journal of Materials Science 2017, 52, 8772-8784, 10.1007/s10853-017-0888-x.
- Patricia Stoor; Eva Söderling; Jukka I. Salonen; Antibacterial effects of a bioactive glass paste on oral microorganisms.. Acta Odontologica Scandinavica 1998, 56, 161-165, 10.1080/000163598422901.
- Roberto Lopez-Piriz; Eva Solá-Linares; Mercedes Rodriguez-Portugal; Beatriz Malpica; Idoia Diaz-Güemes; Silvia Enciso; Leticia Esteban-Tejeda; Belén Cabal; Juan José Granizo; José Serafín Moya; et al.Ramon Torrecillas Evaluation in a Dog Model of Three Antimicrobial Glassy Coatings: Prevention of Bone Loss around Implants and Microbial Assessments. PLOS ONE 2015, 10, e0140374, 10.1371/journal.pone.0140374.
- Faleh Abushahba; Eva Söderling; Laura Aalto-Setälä; Johan Sangder; Leena Hupa; Timo O Närhi; Antibacterial properties of bioactive glass particle abraded titanium against Streptococcus mutans. Biomedical Physics & Engineering Express 2018, 4, 045002, 10.1088/2057-1976/aabeee.
- Rfa Bonfim; Vr Chitarra; Rt Gomes; Rd Zacarias; Vr Santos; Wa Vasconcelos; Laboratório de Microbiologia e Biomateriais – Faculdade de Odontologia – Universidade Federal de Minas Gerais Belo Horizonte Brazil; Antimicrobial activity of bioactive glass associated to Brazilian red and green propolis. Planta Medica 2009, 75, PJ194, 10.1055/s-0029-1234999.
- Surajit Mistry; Rajiv Roy; Biswanath Kundu; Someswar Datta; Manoj Kumar; Abhijit Chanda; Debabrata Kundu; Clinical Outcome of Hydroxyapatite Coated, Bioactive Glass Coated, and Machined Ti6Al4V Threaded Dental Implant in Human Jaws. Implant Dentistry 2016, 25, 252-260, 10.1097/id.0000000000000376.




