
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Michal Zalecki | + 2599 word(s) | 2599 | 2021-07-20 08:33:35 | | | |
| 2 | Amina Yu | Meta information modification | 2599 | 2021-07-29 05:57:32 | | |
Video Upload Options
Cocaine- and amphetamine-regulated transcript (CART) is a peptide suggested to play a role in gastrointestinal tract tissue reaction to pathology. Gastric ulceration is a common disorder affecting huge number of people, and additionally, it contributes to the loss of pig livestock production. Importantly, ulceration as a focal disruption affecting deeper layers of the stomach wall differs from other gastrointestinal pathologies and should be studied individually. The pig’s gastrointestinal tract, due to its many similarities to the human counterpart, provides a valuable experimental model for studying digestive system pathologies. To date, the role of CART in gastric ulceration and the expression of the gene encoding CART in porcine gastrointestinal tube are completely unknown.
1. Introduction
2. CART-Immunofluorescence in the Structures of Enteric Nervous System
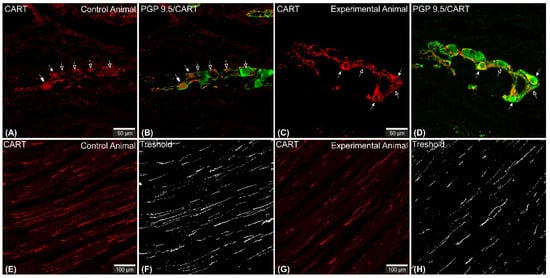
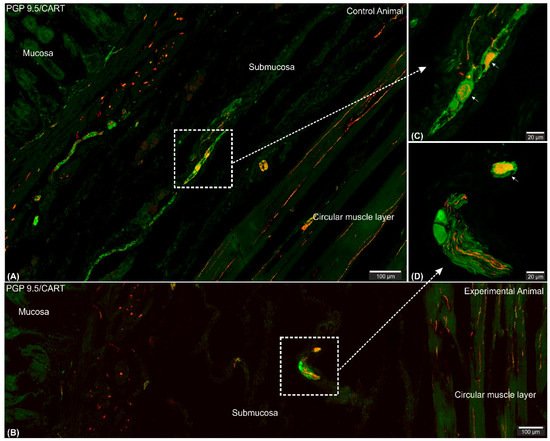
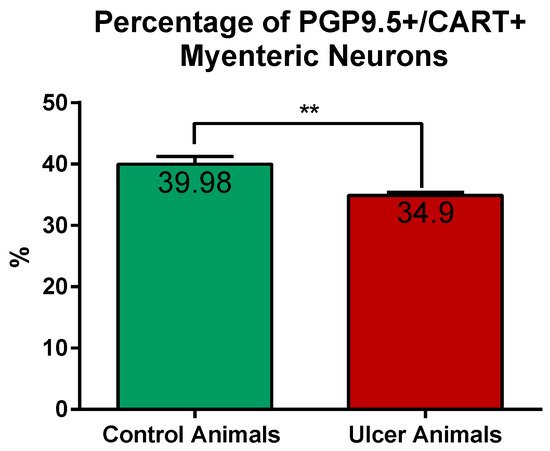
2.1. CART-Immunoreactive Myenteric Plexus Perikarya
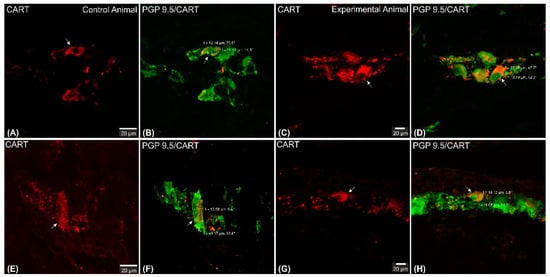
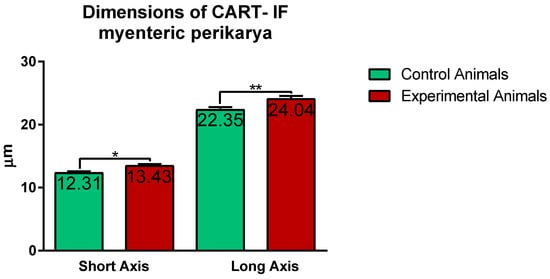
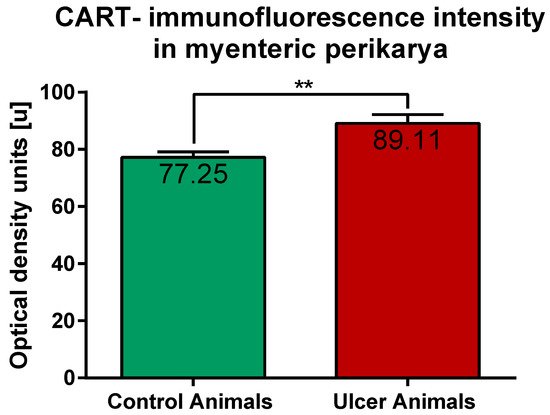
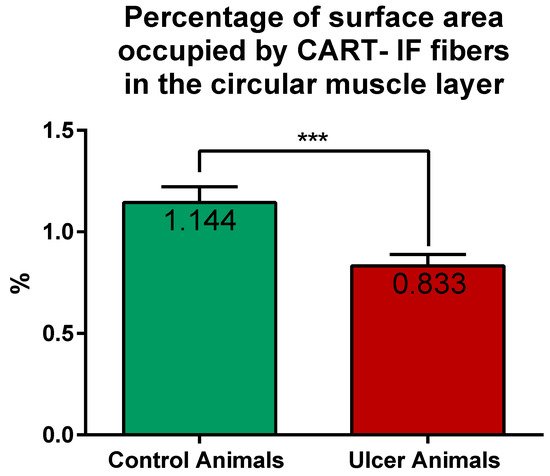
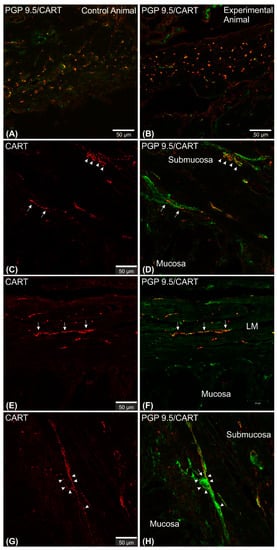
2.2. CART-Immunoreactive Nerve Fibers
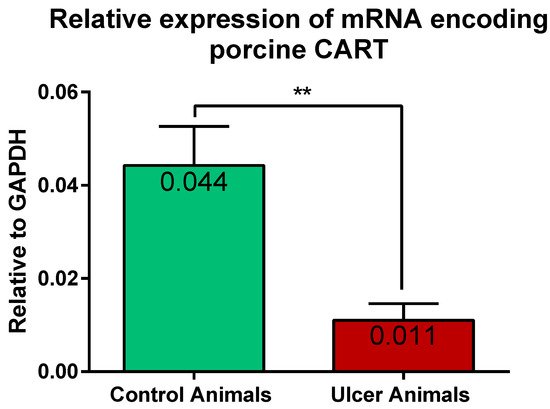
2.3. Expression of mRNA Encoding CART in the Stomach Wall
3. Discussion
References
- Furness, J.B. The Enteric Nervous System; Blackwell: Oxford, UK, 2006.
- Furness, J.B. The enteric nervous system: Normal functions and enteric neuropathies. Neurogastroenterol. Motil. 2008, 20 (Suppl. 1), 32–38.
- Oponowicz, A.; Kozłowska, A.; Gonkowski, S.; Godlewski, J.; Majewski, M. Changes in the distribution of cocaine- and amphetamine-regulated transcript-containing neural structures in the human colon affected by the neoplastic process. Int. J. Mol. Sci. 2018, 19, 414.
- Rząp, D.; Czajkowska, M.; Całka, J. Neurochemical plasticity of nnos-, vip-and cart-immunoreactive neurons following prolonged acetylsalicylic acid supplementation in the porcine jejunum. Int. J. Mol. Sci. 2020, 21, 2157.
- Burliński, P.J. Inflammation- and axotomy-induced changes in cocaine- and amphetamine-regulated transcript peptide-like immunoreactive (CART-LI) nervous structures in the porcine descending colon. Pol. J. Vet. Sci. 2012, 15, 517–524.
- Bulc, M.; Całka, J.; Zielonka, Ł.; Dabrowski, M.; Palus, K. Effect of chemically-induced diabetes mellitus on phenotypic variability of the enteric neurons in the descending colon in the pig. Ann. Anim. Sci. 2020.
- Rytel, L.; Wojtkiewicz, J.; Snarska, A.; Mikołajczyk, A. Changes in the Neurochemical Characterization of Enteric Neurons in the Porcine Duodenum After Administration of Low-Dose Salmonella Enteritidis Lipopolysaccharides. J. Mol. Neurosci. 2020, 1–11.
- Mikolajczyk, A.; Makowska, K. Cocaine- and amphetamine-regulated transcript (CART) peptide in the nerve fibres of the porcine gallbladder wall under physiological conditions and after Salmonella Enteritidis lipopolysaccharides administration. Folia Morphol. 2017, 76, 596–602.
- Gonkowski, S.; Kamińska, B.; Burliński, P.; Kroll, A.; Całka, J. The influence of drug-resistant ulcerative colitis on the number of cocaine- and amphetamine-regulated transcript peptide-like immunoreactive (CART-LI) mucosal nerve fibres of the descending colon in children. Prz. Gastroenterol. 2009, 4, 147–151.
- Kasacka, I.; Piotrowska, Z. Evaluation of density and distribution of CART-immunoreactive structures in gastrointestinal tract of hypertensive rats. BioFactors 2012, 38, 407–415.
- Bulc, M.; Gonkowski, S.; Calka, J. Expression of Cocaine and Amphetamine Regulated Transcript (CART) in the Porcine Intramural Neurons of Stomach in the Course of Experimentally Induced Diabetes Mellitus. J. Mol. Neurosci. 2015, 57, 376–385.
- Makowska, K.; Gonkowski, S.; Zielonka, L.; Dabrowski, M.; Calka, J. T2 Toxin-Induced Changes in Cocaine- and Amphetamine-Regulated Transcript (CART)-Like Immunoreactivity in the Enteric Nervous System Within Selected Fragments of the Porcine Digestive Tract. Neurotox. Res. 2017, 31, 136–147.
- Palus, K.; Bulc, M.; Całka, J. Effect of acrylamide supplementation on the CART-, VAChT-, and nNOS-immunoreactive nervous structures in the porcine stomach. Animals 2020, 10, 555.
- Makowska, K.; Gonkowski, S. Bisphenol a (Bpa) affects the enteric nervous system in the porcine stomach. Animals 2020, 10, 2445.
- Zalecki, M. The Influence of Antral Ulcers on Intramural Gastric Nerve Projections Supplying the Pyloric Sphincter in the Pig (Sus scrofa domestica)—Neuronal Tracing Studies. PLoS ONE 2015, 10, e0126958.
- Zalecki, M. Gastric ulcer induced changes in substance P and Nk1, Nk2, Nk3 receptors expression in different stomach localizations with regard to intrinsic neuronal system. Histochem. Cell Biol. 2019, 151, 29–42.
- Zalecki, M.; Pidsudko, Z.; Franke-Radowiecka, A.; Wojtkiewicz, J.; Kaleczyc, J. Galaninergic intramural nerve and tissue reaction to antral ulcerations. Neurogastroenterol. Motil. 2018, 30, e13360.
- Zalecki, M.; Sienkiewicz, W.; Franke-Radowiecka, A.; Klimczuk, M.; Kaleczyc, J. The influence of gastric antral ulcerations on the expression of Galanin and GalR1, GalR2, GalR3 receptors in the pylorus with regard to gastric intrinsic innervation of the pyloric sphincter. PLoS ONE 2016, 11, e0155658.
- Liebermann-Meffert, D.; Allgower, M. Neuromuscular tissue defects and antropyloric dysfunction in peptic ulcer. Scand. J. Gastroenterol. Suppl. 1981, 67, 111–113.
- Liebermann-Meffert, D.; Muller, C.; Allgower, M. Gastric hypermotility and antropyloric dysfunction in gastric ulcer patients. Br. J. Surg. 1982, 69, 11–13.
- Murray, G.F.; Ballinger, W.F.; Stafford, E.S. Ulcers of the pyloric channel. Am. J. Surg. 1967, 113, 199–203.
- Friendship, R.M. Gastric ulceration in swine. J. Swine Health Prod. 2004, 12, 34–35.
- Melnichouk, S.I. Mortality associated with gastric ulceration in swine. Can. Vet. J. 2002, 43, 223.
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793.
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J., Jr.; Frazier, K.S. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356.
- Swindle, M.M. The development of swine models in drug discovery and development. Future Med. Chem. 2012, 4, 1771–1772.
- Douglass, J.; McKinzie, A.A.; Couceyro, P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 1995, 15, 2471–2481.
- Murphy, K.G.; Abbott, C.R.; Mahmoudi, M.; Hunter, R.; Gardiner, J.V.; Rossi, M.; Stanley, S.A.; Ghatei, M.A.; Kuhar, M.J.; Bloom, S.R. Quantification and synthesis of cocaine- and amphetamine-regulated transcript peptide (79-102)-like immunoreactivity and mRNA in rat tissues. J. Endocrinol. 2000, 166, 659–668.
- Makowska, K.; Gonkowski, S. Cocaine- and Amphetamine-Regulated Transcript (CART) Peptide in Mammals Gastrointestinal System—A Review. Ann. Anim. Sci. 2017, 17, 3–21.
- Ekblad, E. CART in the enteric nervous system. Peptides 2006, 27, 2024–2030.
- Zacharko-Siembida, A.; Arciszewski, M.B. Immunoreactivity to cocaine- and amphetamine-regulated transcript in the enteric nervous system of the pig and wild boar stomach. Anat. Histol. Embryol. 2014, 43, 48–55.
- Rekawek, W.; Sobiech, P.; Gonkowski, S.; Zarczynska, K.; Snarska, A.; Wasniewski, T.; Wojtkiewicz, J. Distribution and chemical coding patterns of cocaine- and amphetamine-regulated transcript-like immunoreactive (CART-LI) neurons in the enteric nervous system of the porcine stomach cardia. Pol. J. Vet. Sci. 2015, 18, 515–522.
- Kasacka, I.; Piotrowska, Z.; Car, H.; Janiuk, I.; Lebkowski, W. Cocaine- and amphetamine-regulated transcript: Identification and distribution in human gastrointestinal tract. J. Biol. Regul. Homeost. Agents 2012, 26, 419–428.
- Wierup, N.; Gunnarsdóttir, A.; Ekblad, E.; Sundler, F. Characterisation of CART-containing neurons and cells in the porcine pancreas, gastro-intestinal tract, adrenal and thyroid glands. BMC Neurosci. 2007, 8, 51.
- Ekblad, E.; Kuhar, M.; Wierup, N.; Sundler, F. Cocaine- and amphetamine-regulated transcript: Distribution and function in rat gastrointestinal tract. Neurogastroenterol. Motil. 2003, 15, 545–557.
- Armitage, A.K.; Dean, A.C.B. Function of the pylorus and pyloric antrum in gastric emptying. Gut 1963, 4, 174–178.
- Zacharko-Siembida, A.; Kulik, P.; Szalak, R.; Lalak, R.; Arciszewski, M.B. Co-expression patterns of cocaine- and amphetamine-regulated transcript (CART) with neuropeptides in dorsal root ganglia of the pig. Acta Histochem. 2014, 116, 390–398.
- Zalecki, M. Extrinsic primary afferent neurons projecting to the pylorus in the domestic pig—Localization and neurochemical characteristics. J. Mol. Neurosci. 2014, 52, 82–89.
- Kozłowska, A.; Godlewski, J.; Majewski, M. Distribution patterns of cocaine-and amphetamine-regulated transcript-and/or galanin-containing neurons and nerve fibers located in the human stomach wall affected by tumor. Int. J. Mol. Sci. 2018, 19, 3357.
- Rychlik, A.; Gonkowski, S.; Nowicki, M.; Calka, J. Cocaine- and amphetamine-regulated transcript immunoreactive nerve fibres in the mucosal layer of the canine gastrointestinal tract under physiological conditions and in inflammatory bowel disease. Vet. Med. 2015, 60, 361–367.
- Wojtkiewicz, J.; Gonkowski, S.; Bladowski, M.; Majewski, M. Characterisation of cocaine-and amphetamine-regulated transcript-like immunoreactive (CART-LI) enteric neurons in the porcine small intestine. Acta Vet. Hung. 2012, 60, 371–381.
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232.




