
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Dawei Jiang | + 2019 word(s) | 2019 | 2021-08-18 12:29:53 | | | |
| 2 | Beatrix Zheng | + 76 word(s) | 2095 | 2021-08-27 10:46:44 | | |
Video Upload Options
Escherichia coli Nissle 1917 (EcN) is a commonly used probiotic in clinical practice. Its facultative anaerobic property drives it to selectively colonize in the hypoxic area of the tumor for survival and reproduction. EcN can be engineered as a bacteria-based microrobot for molecular imaging, drug delivery, and gene delivery.
1. Characteristics of EcN
EcN is a facultative anaerobic organism that proliferates mainly in the interface between the necrotic and hypoxic regions of tumors [1] and exists in rich oxygen areas [2], expanding their potential applications for various tumor types. Moreover, the special serum-sensitive lipopolysaccharide on the membrane of EcN promotes quick clearance from normal organs [3]. Researchers have systematically studied the biodistribution and quantitative tumor colonization of EcN in vivo [4]. It was found that the tumor/liver ratio of EcN colonization after intravenous injection was at least 10,000:1 in 4T1 tumor-bearing BALB/c mice, giving EcN a massive edge over traditional nanomedicine in terms of tumor accumulation and overall biodistribution profile. Interestingly, the average number of EcN found in tumors was significantly higher than the injected dose due to the colonization and proliferation of EcN. The minimum bacterial dose for successful colonization was 20,000 CFU, at which the average number in the tumor reached 10 8 CFU. As the injection dose increases, the number of bacteria colonized in the tumor increases, but the bacteria in the liver and spleen grow accordingly. Moreover, the route of EcN injection, such as intravenous (i.v.), intraperitoneal (i.p.), and intertumoral (i.t.) injection, did not influence the tumor targeting and tumor-to-organ ratios. Oral administration of EcN confirmed that the bacteria crossed the gastrointestinal tract and colonized hepatic metastases [5]. Therefore, preferential tumor colonization of EcN may allow for flexible administration choices to meet specific clinical needs.
EcN has multiple peritrichous flagella that may drive it forward as a bio-engine [6]. Therefore, EcN can be developed into a self-propelled microrobot to break through the biological or pathological barriers to deliver therapeutic payloads [7]. The whole-genome sequencing of EcN has been completed [8], and methods for genetic modification of genomes and transformation of plasmid have been established to engineer EcN for heterologous gene expression [9][10][11]. Therefore, the therapeutic payloads can be drugs, expressed proteins, antigens, and immunoregulatory factors. However, constitutive expression of therapeutic factors may cause undesirable adverse effects, such as hepatic and splenic injury, so it is necessary to control the heterologous gene expression temporally and quantitatively. Weiss’s group established an in vivo remote control (IVRC) system to deliberate the external control of gene expression [12]. Three inducible promoter systems enabled EcN to remotely control and precisely regulate the kinetics of gene expression, and the L-arabinose–ParaBAD system showed the highest induce efficiency [13]. After oral administration or intraperitoneal injection of inducer L-arabinose, the expression of reporter gene luciferase in EcN colonized tumor reached its maximum after 6 h and stopped when L-arabinose was removed. Therefore, the controllable expression of EcN may provide a highly flexible and suitable treatment for individualized therapy. Precise regulation of the ECN number to control its proliferation in the tumor and expression of therapeutic agents will be of great significance to achieve spatiotemporal and quantitative imaging and treatment response. However, the underlying mechanisms of tumor targeting, and colonization of bacteria are complex and remain unclear. The influential factors may include the bacterial species used, types of tumor treated, and the tumor microenvironment [14]. Therefore, regulating the expression of bacteria may be a more practical means.
2. EcN-Mediated Tumor Imaging
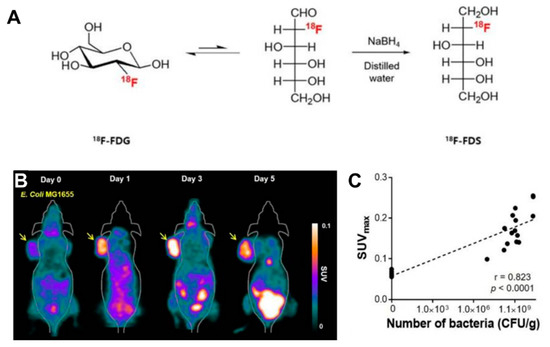
Radionuclide-based molecular imaging, namely, PET and SPECT, is a powerful tool to assess physiological and pathological processes in vivo without penetration depth limitation. Currently, radiopharmaceuticals for bacterial imaging focus on tracking bacteria to differentiate sterile inflammation from infection [15]. Based on the tumor-specific colonization nature of EcN, radiopharmaceuticals monitoring EcN can be used for tumor imaging. The living body itself contains various background bacteria, so radiotracers must be highly specific to target injected EcN. The endogenous bacterial thymidine kinase gene (TK gene) of EcN has been shown to be an effective reporter gene for nuclear medicine imaging using radiolabeled pyrimidine nucleoside analogs, such as [18F]-FEAU, [124I]-FIAU, and [125I]-FIAU [16][17]. Since the substrate of bacterial TK presents poorly binding affinity with mammalian TK, the radiotracers mentioned above can selectively identify and locate bacteria in vivo [18]. PET Imaging with [18F]-FEAU exhibited high accumulation in tumors and a linear correlation with the number of colonized EcNs, offering precise information about the survival, proliferation, and number of the bacteria. A strategy of engineered EcN with exogenous reporter genes hSSTR2 has been reported for in vivo tumor visualization [19]. The outer membrane protein receptor FyuA of EcN can selectively recognize the 64Cu and 89Zr labeled metallophore yersiniabactin (YbT), which has a high affinity for transition metals [20]. A substantially higher PET signal was also observed in the EcN colonized tumor than that without the bacterial injection. PET tracers targeting bacteria-specific sugar metabolism have also been developed, and [18F]-FDS is the most representative one. [18F]-FDS, a synthetic analog of [18F]-FDG, has been shown to accumulate in Gram-negative Enterobacteriaceae selectively but not in mammalian or cancer cells. In PET imaging, the uptake of radioactivity in the tumor had a positive relationship with the number of viable bacteria, allowing a semiquantitative measure of bacterial density in the tumor [21] ( Figure 1 ). The successful visualization and quantification of therapeutic E. coli by [18F]-FDS will make it possible to predict the therapeutic response, which could facilitate the clinical translation of bacteria-mediated tumor therapy.
3. EcN-Mediated Tumor Therapy
3.1. Direct Drug Delivery
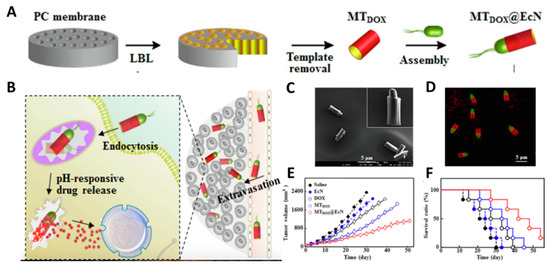
3.2. Gene Therapy
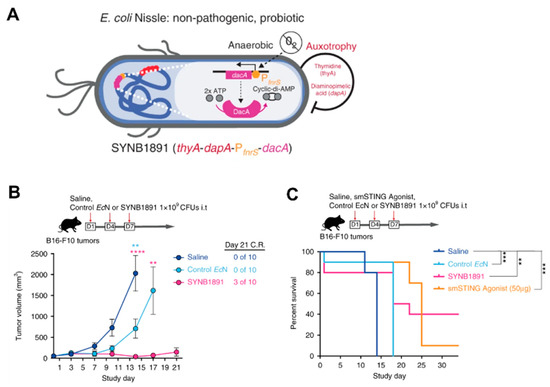
3.3. Immunotherapy
References
- Li, R.; Helbig, L.; Fu, J.; Bian, X.; Herrmann, J.; Baumann, M.; Stewart, A.F.; Müller, R.; Li, A.; Zips, D. Expressing cytotoxic compounds in Escherichia coli Nissle 1917 for tumor-targeting therapy. Res. Microbiol. 2019, 170, 74–79.
- Xie, S.; Zhao, L.; Song, X.; Tang, M.; Mo, C.; Li, X. Doxorubicin-conjugated Escherichia coli Nissle 1917 swimmers to achieve tumor targeting and responsive drug release. J. Control. Release 2017, 268, 390–399.
- Sonnenborn, U.; Schulze, J. The non-pathogenic Escherichia coli strain Nissle 1917–features of a versatile probiotic. Microb. Ecol. Health Dis. 2009, 21, 122–158.
- Stritzker, J.; Weibel, S.; Hill, P.J.; Oelschlaeger, T.A.; Goebel, W.; Szalay, A.A. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int. J. Med. Microbiol. 2007, 297, 151–162.
- Danino, T.; Prindle, A.; Kwong, G.A.; Skalak, M.; Li, H.; Allen, K.; Hasty, J.; Bhatia, S.N. Programmable probiotics for detection of cancer in urine. Sci. Transl. Med. 2015, 7, ra284–ra289.
- Berg, H.C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003, 72, 19–54.
- Erkoc, P.; Yasa, I.C.; Ceylan, H.; Yasa, O.; Alapan, Y.; Sitti, M. Mobile microrobots for active therapeutic delivery. Adv. Ther. 2019, 2, 1800064.
- Reister, M.; Hoffmeier, K.; Krezdorn, N.; Rotter, B.; Liang, C.; Rund, S.; Dandekar, T.; Sonnenborn, U.; Oelschlaeger, T.A. Complete genome sequence of the gram-negative probiotic Escherichia coli strain Nissle 1917. J. Biotechnol. 2014, 187, 106–107.
- Yu, X.; Lin, C.; Yu, J.; Qi, Q.; Wang, Q. Bioengineered Escherichia coli Nissle 1917 for tumour-targeting therapy. Microb. Biotechnol. 2020, 13, 629–636.
- Zainuddin, H.S.; Bai, Y.; Mansell, T.J. CRISPR-based curing and analysis of metabolic burden of cryptic plasmids in Escherichia coli Nissle 1917. Eng. Life Sci. 2019, 19, 478–485.
- Lan, Y.-J.; Tan, S.-I.; Cheng, S.-Y.; Ting, W.-W.; Xue, C.; Lin, T.-H.; Cai, M.-Z.; Chen, P.-T.; Ng, I.-S. Development of Escherichia coli Nissle 1917 derivative by CRISPR/Cas9 and application for gamma-aminobutyric acid (GABA) production in antibiotic-free system. Biochem. Eng. J. 2021, 168, 107952.
- Loessner, H.; Endmann, A.; Leschner, S.; Westphal, K.; Rohde, M.; Miloud, T.; Hämmerling, G.; Neuhaus, K.; Weiss, S. Remote control of tumour-targeted Salmonella enterica serovar Typhimurium by the use of l-arabinose as inducer of bacterial gene expression in vivo. Cell. Microbiol. 2007, 9, 1529–1537.
- Loessner, H.; Leschner, S.; Endmann, A.; Westphal, K.; Wolf, K.; Kochruebe, K.; Miloud, T.; Altenbuchner, J.; Weiss, S. Drug-inducible remote control of gene expression by probiotic Escherichia coli Nissle 1917 in intestine, tumor and gall bladder of mice. Microbes Infect. 2009, 11, 1097–1105.
- Zhou, S.; Gravekamp, C.; Bermudes, D.; Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 2018, 18, 727–743.
- Signore, A.; Artiko, V.; Conserva, M.; Ferro-Flores, G.; Welling, M.M.; Jain, S.K.; Hess, S.; Sathekge, M. Imaging bacteria with radiolabelled probes: Is it feasible? J. Clin. Med. 2020, 9, 2372.
- Brader, P.; Stritzker, J.; Riedl, C.C.; Zanzonico, P.; Cai, S.; Burnazi, E.M.; Ghani, E.R.; Hricak, H.; Szalay, A.A.; Fong, Y. Escherichia coli Nissle 1917 facilitates tumor detection by positron emission tomography and optical imaging. Clin. Cancer Res. 2008, 14, 2295–2302.
- Jang, S.J.; Lee, Y.J.; Lim, S.; Kim, K.I.; Lee, K.C.; An, G.I.; Lee, T.S.; Cheon, G.J.; Lim, S.M.; Kang, J.H. Imaging of a localized bacterial infection with endogenous thymidine kinase using radioisotope-labeled nucleosides. Int. J. Med. Microbiol. 2012, 302, 101–107.
- Bettegowda, C.; Foss, C.A.; Cheong, I.; Wang, Y.; Diaz, L.; Agrawal, N.; Fox, J.; Dick, J.; Dang, L.H.; Zhou, S. Imaging bacterial infections with radiolabeled 1-(2′-deoxy-2′-fluoro-β-D-arabinofuranosyl)-5-iodouracil. Proc. Natl. Acad. Sci. USA 2005, 102, 1145–1150.
- Liu, Q.; Lan, X. Evaluation of bacteria Nissle 1917 for tumor targeting imaging. J. Nucl. Med. 2020, 61, 444.
- Siddiqui, N.A.; Houson, H.A.; Thomas, S.C.; Blanco, J.R.; ODonnell, R.E.; Hassett, D.J.; Lapi, S.E.; Kotagiri, N. Radiolabeled bacterial metallophores as targeted PET imaging contrast agents for accurate identification of bacteria and outer membrane vesicles in vivo. bioRxiv 2020.
- Kang, S.-R.; Jo, E.J.; Nguyen, V.H.; Zhang, Y.; Yoon, H.S.; Pyo, A.; Kim, D.-Y.; Hong, Y.; Bom, H.-S.; Min, J.-J. Imaging of tumor colonization by Escherichia coli using 18F-FDS PET. Theranostics 2020, 10, 4958.
- Xie, S.; Chen, M.; Song, X.; Zhang, Z.; Zhang, Z.; Chen, Z.; Li, X. Bacterial microbots for acid-labile release of hybrid micelles to promote the synergistic antitumor efficacy. Acta Biomater. 2018, 78, 198–210.
- Xie, S.; Xia, T.; Li, S.; Mo, C.; Chen, M.; Li, X. Bacteria-propelled microrockets to promote the tumor accumulation and intracellular drug uptake. Chem. Eng. J. 2020, 392, 123786.
- MacDiarmid, J.A.; Mugridge, N.B.; Weiss, J.C.; Phillips, L.; Burn, A.L.; Paulin, R.P.; Haasdyk, J.E.; Dickson, K.-A.; Brahmbhatt, V.N.; Pattison, S.T. Bacterially derived 400 nm particles for encapsulation and cancer cell targeting of chemotherapeutics. Cancer Cell 2007, 11, 431–445.
- Paukner, S.; Stiedl, T.; Kudela, P.; Bizik, J.; Al Laham, F.; Lubitz, W. Bacterial ghosts as a novel advanced targeting system for drug and DNA delivery. Expert Opin. Drug Deliv. 2006, 3, 11–22.
- Zhang, Y.; Ji, W.; He, L.; Chen, Y.; Ding, X.; Sun, Y.; Hu, S.; Yang, H.; Huang, W.; Zhang, Y.E. coli Nissle 1917-derived minicells for targeted delivery of chemotherapeutic drug to hypoxic regions for cancer therapy. Theranostics 2018, 8, 1690.
- Montanaro, J.; Inic-Kanada, A.; Ladurner, A.; Stein, E.; Belij, S.; Bintner, N.; Schlacher, S.; Schuerer, N.; Mayr, U.B.; Lubitz, W. Escherichia coli Nissle 1917 bacterial ghosts retain crucial surface properties and express chlamydial antigen: An imaging study of a delivery system for the ocular surface. Drug Des. Dev. Ther. 2015, 9, 3741.
- He, L.; Yang, H.; Liu, F.; Chen, Y.; Tang, S.; Ji, W.; Tang, J.; Liu, Z.; Sun, Y.; Hu, S. Escherichia coli Nissle 1917 engineered to express Tum-5 can restrain murine melanoma growth. Oncotarget 2017, 8, 85772.
- He, L.; Yang, H.; Tang, J.; Liu, Z.; Chen, Y.; Lu, B.; He, H.; Tang, S.; Sun, Y.; Liu, F. Intestinal probiotics E. coli Nissle 1917 as a targeted vehicle for delivery of p53 and Tum-5 to solid tumors for cancer therapy. J. Biol. Eng. 2019, 13, 58.
- Song, H.; Liu, D.; Dong, S.; Zeng, L.; Wu, Z.; Zhao, P.; Zhang, L.; Chen, Z.-S.; Zou, C. Epitranscriptomics and epiproteomics in cancer drug resistance: Therapeutic implications. Signal Transduct. Target. Ther. 2020, 5, 193.
- Naidoo, J.; Page, D.; Li, B.; Connell, L.; Schindler, K.; Lacouture, M.; Postow, M.; Wolchok, J. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391.
- Gurbatri, C.R.; Lia, I.; Vincent, R.; Coker, C.; Castro, S.; Treuting, P.M.; Hinchliffe, T.E.; Arpaia, N.; Danino, T. Engineered probiotics for local tumor delivery of checkpoint blockade nanobodies. Sci. Transl. Med. 2020, 12, eaax0876.
- Leventhal, D.S.; Sokolovska, A.; Li, N.; Plescia, C.; Kolodziej, S.A.; Gallant, C.W.; Christmas, R.; Gao, J.-R.; James, M.J.; Abin-Fuentes, A. Immunotherapy with engineered bacteria by targeting the STING pathway for anti-tumor immunity. Nat. Commun. 2020, 11, 2739.




