Distillery stillage has a high energy potential (13.6 MJ/kg TS, 10.4 MJ/kg COD), which indicates that it can be processed via anaerobic digestion and is a suitable substrate for conversion into energy.
1. Introduction
In alcohol distilleries, the amount of distillery stillage generated can be up to 15 times larger than the amount of alcohol produced. The stillage has high concentrations of organics and nitrogen, a low pH, and a dark brown color. Currently, stillage is mainly used for soil fertilization. For this purpose, it requires thickening and is used seasonally, which creates storage problems and transport costs. To reduce environmental pollution, physicochemical and biological processes have been employed for the treatment of distillery stillage. However, according to bioeconomy principles, the stillage should be transformed into value-added products.
2. Processing of Distillery Stillage—Bioethanol Production
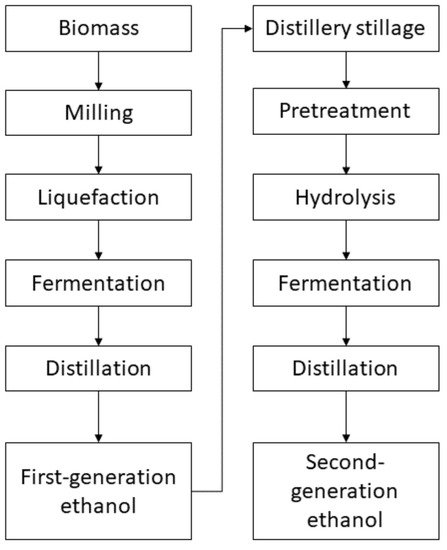
Due to the excess of distillery stillage on the market, it becomes necessary to develop an alternative concept of its management. Due to the high content of the polysaccharide fraction in the distillery stillage, low price, and widespread availability, an interesting direction is its use as a raw material source to produce second-generation ethanol (Figure 1).
Figure 1. First- and second-generation ethanol production.
The whole stillage includes the liquid remaining after distilling ethanol, containing, depending on the technology used, from 7 to 20% of dry weight (DW). It consists mainly of fiber, protein, fat, unhydrolyzed starch, and dead yeast cells. The biological transformation of the distillery stillage must be preceded by the depolymerization of hemicellulose and cellulose, leading to the obtaining of a liquid fraction of products rich in fermentable monosaccharides. However, the factor that limits the biotransformation of the distillery stillage to the second-generation ethanol is the presence of undesirable components in the hydrolysate, in particular, 2-furfural and 5-hydroxymethylfurfural. The presence of furfurals, byproducts of polysaccharide depolymerization, adversely affects the development of yeast, inhibiting or even stopping the alcohol fermentation of the remaining ingredients of the hydrolyzed distillery stillage. The examples of ethanol production from distillery stillage are presented in Table 1.
Table 1. The efficiency of the ethanol fermentation process using stillage.
| Type of Stillage |
Ethanol Production |
% of Theoretical Yield |
References |
| Corn grain |
60.97 L/kg starch |
84.80 |
[1] |
| Maize |
70.65% of starch |
98.76 |
[2] |
| Winter triticale BOGO |
68.87 L/kg starch |
95.78 |
[3] |
Conversion of the hemicellulose and cellulose to fermentable sugars, and then to ethanol has the potential to significantly increase the efficiency of the process. Mikulski and Kłosowski
[4] evaluated the effectiveness of various parameters of low-temperature pretreatment with dilute sulfuric acid (121 or 131 °C, 30 or 60 min, 0.1 or 0.2 M H
2SO
4) for production of cellulosic ethanol. Optimal conditions for dilute acid pretreatment of rye and wheat distillery stillage were 121 °C, 0.2 M H
2SO
4, and 60 min, whereas those of maize stillage were 131 °C, 0.2 M H
2SO
4, and 60 min. The highest efficiency of enzymatic hydrolysis was achieved for rye and wheat stillage using 1 g DW and the concentration of cellulolytic enzyme of 24%
w/w, and, for maize stillage, 3 g DW and enzyme concentration of 24%
w/w. The use of rye and wheat stillage for the production of ethanol did not require a detoxification process and enabled full attenuation of glucose after 48 h of the process. However, the use of maize stillage as a raw material must be preceded by a detoxification process to reduce 5-hydroxymethylfurfural concentration in the fermentation medium.
To increase the pretreatment efficiency of bioethanol production, microwaves can be used. Mikulski et al.
[5] tested the microwave-assisted pretreatment method of distillery stillage in the production of cellulosic bioethanol from maize distillery stillage. High glucose concentration (104.4 mg/g DW) and the highest yield of enzymatic cellulose hydrolysis (75.8%) were obtained for microwave pretreatment (300 W, 54 PSI, 15 min). These conditions allowed not only a high concentration of glucose to be obtained, but also a low concentration of fermentation inhibitors, i.e., 5-hydroxymethylfurfural (6.8 mg/g DW) and furfural (6.0 mg/g DW). The optimal dose of yeast,
Saccharomyces cerevisiae strain Ethanol Red, which gave a high attenuation, was 2 g/L of cellulose fermentation medium. Detoxification of cellulose hydrolysates with activated carbon enabled a high fermentation yield (approximately 77% of the theoretical yield) to be achieved. Microwave processing can be an effective pretreatment method in the production of cellulosic ethanol from maize distillery stillage, but this process requires a careful selection of parameters. The same research group proposed the hydrotropic delignification using sodium cumene sulfonate for pretreatment of rye, wheat, and maize stillage in the production of bioethanol. The highest stillage biomass extractives were obtained for a biomass particle size < 1.0 mm when exposed to 131 °C for 1 h at 20%
v/
v hydrotrope concentration. It has been shown that hydrotropic treatment causes changes in the stillage biomass structure (increase in porosity) and reduces the lignin content in biomass by 7–17%. Delignification with a hydrotrope also increased the concentration of fermentable sugars in the media prepared with stillage biomass, which led to a higher final ethanol concentration (up to ca. 3.5 g/L). Hydrotropic treatment is an effective way of pretreating stillage biomass. It provides a high degree of biomass bioconversion and creates the prospect of integrating the first- and second-generation ethanol production process to utilize the raw material more fully
[6].
The hydrolysis of distillery stillage to fermentable sugars was optimized using 2% (
v/v) H
2SO
4 at 100 °C for 5.5 h and produced 18 g/L xylose, 11.5 g/L arabinose, and 6.5 g/L glucose from 120 g/L stillage
[7]. Further enzymatic hydrolysis increased the release of glucose by 61%. Furfural, acetate, and lactate were the main inhibitors present in the acid hydrolysate of stillage. The lignin-derived inhibitors hydroxymethylfuraldehyde, hydroxybenzaldehyde, vanillin, and syringaldehyde were not detected. Neutralization of the hydrolysate with lime to pH 5 decreased the concentration of furfural by 50%. Fermentation of hydrolysate by recombinant
Zymomonas mobilis ZM4(pZB5) supplemented with 10 g/L of glucose produced 11 g/L of ethanol after 70 h, with residual xylose 12 g/L. Supplementation of the hydrolysate with 5 g/L yeast extract and 40 g/L of glucose produced 28 g/L of ethanol with 2.6 g/L residual xylose after 18 h. Arabinose was not utilized by this recombinant strain. It could be concluded that
Z. mobilis ZM4(pZB5) may be a suitable candidate for the fermentation of both glucose and xylose in stillage acid hydrolysates.