
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Desiree Ha | + 4170 word(s) | 4170 | 2021-08-23 07:57:36 | | | |
| 2 | Amina Yu | -244 word(s) | 3926 | 2021-08-24 04:18:03 | | | | |
| 3 | Xiaoqiang Wang | + 120 word(s) | 4046 | 2021-08-24 19:31:36 | | | | |
| 4 | Amina Yu | Meta information modification | 4046 | 2021-08-25 10:49:40 | | | | |
| 5 | Amina Yu | + 13 word(s) | 4059 | 2021-08-25 10:50:47 | | |
Video Upload Options
According to Global Cancer Statistics 2020, the burden of cancer incidence and mortality is rapidly growing worldwide. The epidemiological features of cancer reflect both the aging and growth of the population and the changes in the prevalence and distribution of the main cancer risk factors, several of which are particularly associated with the environment. Exogenous estrogens, such as synthetic industrial estrogenic compounds (xenoestrogens) and estrogenic molecules from plants (phytoestrogens), are environmental factors that potentially cause various cancers through their interactions with cellular signaling processes involving estrogen signaling pathways.
1. Introduction
Estrogens are classified as either endogenous or exogenous, according to their origins [1]. Yet, both can bind to estrogen receptors (ERs), and/or many other nuclear receptors, simultaneously triggering genomic and transcriptomic changes in various organ systems. These changes can consequently contribute to the initiation and progression of multiple types of cancers, including the classical hormone-related breast and prostate cancer [2,3], as well as the non-classical hormone-related cancers, such as lung cancer [4], colorectal cancer [5], and gastric cancer [6].
Endogenous estrogens (estradiol/E2, estrone/E1, and estriol/E3) in humans are produced by endocrine glands and/or by extra-glandular tissues through steroidogenesis enzymes, such as cytochrome P450 oxidases (CYPs), hydroxysteroid dehydrogenases (HSDs), and aromatase (CYP19) [7]. Although the sex gonads (ovaries and testes) and adrenal cortex are the primary sites of estrogen synthesis, extra-gonadal estrogens are also produced in the mammary glands, lungs, liver, and intestines, and play an equally important role in controlling biological activities [8]. The important roles of endogenous estrogens in the etiology of breast cancer have been extensively studied, leading to the development of well-tolerated endocrine therapy for breast cancer [9].
At the molecular and cellular levels, xenoestrogens/phytoestrogens can imitate endogenous estrogens by enhancing and/or interrupting endogenous estrogen signaling pathways. They may exert either beneficial or harmful activities in humans depending on a set of complex factors such as exposure dose, time, intracellular signal transduction, and tissue complexity [16]. The binding of estrogens to ERs results in the activation of estrogen signaling pathways. There are intracellular ERs, including ER-alpha (ERα) and ER-beta (ERβ), as well as membrane-associated ERs, such as membrane ERs (mERs) and G protein-coupled estrogen receptors (GPER/GPR30) [17]. In addition to binding to ERs, exogenous estrogens can exert estrogenic activity by cross-talking with many other pathways, including pathways related to membrane-associated growth factor receptors, such as human epidermal growth factor receptor (EGFR/HER) and insulin-like growth factor 1-receptor (IGF1R) [18], as well as nuclear receptors, including aryl hydrocarbon receptor (AhR) [19], peroxisome proliferator-activated receptors (PPARs) [20], and estrogen-related receptor alpha/gamma (ERRα/γ) [21]. Multiple synergistic signaling pathways may contribute to the outcome of exogenous estrogen exposure on overall health and/or cancer cells. At the tissue level, exogenous estrogens may exhibit another dimension of complexity by influencing both cancer cells and cancer-associated stromal cells, including immune cells, fibroblasts, and adipocytes [22]. At the systemic level, exposure to exogenous estrogens has been linked to increased breast cancer risk during certain life stages known as the windows of susceptibility (WOS) that include the prenatal, pubertal, pregnancy, and menopausal transition periods, during which the mammary glands undergo anatomical and functional transformations. Therefore, environmental hormones (e.g., endocrine-disrupting chemicals/EDC) and certain therapeutics (e.g., prescribed for coexisting medical conditions or in the form of hormone replacement therapy) can influence breast cancer risk, development, or outcome [23]. Considering the spatial heterogeneity (variety of cell types) and temporal heterogeneity (various stages of differentiation) of cancer, xenoestrogens/phytoestrogens could display integrated activities in a tumor-selective and/or life stage(s)-specific manner.
The growing concerns of the exogenous estrogenic influence on health, especially towards cancer, have prompted considerable public attention and scientific interest. Knowledge of how these exogenous estrogens mimic endogenous estrogens, and how they exert their impacts on overall health, is crucial to resolve their impacts in the etiology of varying cancers. In this review, we conducted an exhaustive evaluation on the advanced research technology, molecular mechanisms, and ongoing translational studies in the development of prevention and therapeutic approaches towards human cancers. Here, we aim to provide a thorough, updated understanding of xenoestrogens/phytoestrogens and their biological activities and mechanisms in cancer.
2. Advanced Methodology in Studying the Biological Effects of Xenoestrogens and Phytoestrogens
While population-based studies have defined correlations with environmental estrogen exposure and cancer risk, and in vitro models with cultured cancer cells provide an advantageous method to interpret the single-agent causality of exposure and disease. However, these models fail to consider a multifactorial analysis to explore the causal relationship between exposure and cancer development/progression. A novel approach to investigate the complexity of cancer with advanced modes and emerging techniques will be helpful to interpret measurable environmental and biological parameters simultaneously. These emerging approaches include in vivo models with rodents, PDX models, multi-omics-based unbiased analyses, and single-cell analyses [256,257]. Using multidisciplinary approaches, the etiology of human cancer can be more thoroughly investigated.
In vivo models with rodents have been useful for studying the phenotypic changes and mechanisms of exogenous estrogen exposure. These models include carcinogenesis models and therapeutic models, with the former consisting of healthy or genetically engineered mice upon long-term exposure and the latter using established tumor xenografts. More specific and cancer-relevant PDX models, generated by the direct implantation of tumor fragments from human patients into immune-deficient mice, are increasingly being utilized for translational cancer research because they have been proven to maintain many of the biological properties of human cancers, such as genetic features, histology, and tumor cell population heterogeneity [118,119,120]. Many studies have reported that the response to treatments in PDX models correlated well with the treatment results of patients whose tumors supplied the PDX cancer. Therefore, PDX models provide a suitable option for studying the effects of exogenous estrogens on human cancers [121,122,123]. For example, the xenoestrogen methylparaben was shown to promote tumor growth and stem-like features using an ER+ breast cancer PDX model [124]. Our group has also recently performed bulk RNA-seq analysis on an ER+ breast cancer PDX treated with PBDEs, and concluded that PBDEs induced the expression of estrogen-responsive genes, especially those related to cell proliferation [125]. Other groups have also reported the effect of GEN [126,127] and DES [128] on prostate cancer PDX models. Additionally, another study investigated the potential chemo-enhancing effects and mechanisms of GEN and its analog AXP107-11, which showed an improved bioavailability of AXP107-11 for clinical use compared to GEN [129]. These findings suggest that PDX models would help further the understanding of the biological effects of exogenous estrogens as relevant models of human cancers. In addition to its advantage in mimicking the natural situation of tumor development, PDX models include all the cells in the surrounding tissues, rather than just the cancer cells, enabling the assessment of the biological effects on the whole population in a tissue and the specific cell-to-cell interactions [130].
Tumor development and progression are widely recognized as complicated processes in which tumor cells, and many other contributors such as fibroblasts, immune cells, and other stromal cells from the tumor microenvironment, play distinct roles by their interactions with one another. Thus, the heterogeneity of cell populations within tissues of interest has been one of the major limitations of previous, especially with in vitro models. Additionally, even in the in vivo models, it is sometimes a challenge to capture the effect of estrogenic compounds in each type of cell, especially when those cells are too minor to cause apparent phenotypic changes. The recent development of single cell RNA sequencing (scRNA-seq) provides transcriptomic information at a single-cell resolution, enabling the ability to profile each isolated cell’s characteristics from a given tissue or organ [134,135]. This unprecedented capability of scRNA-seq technology allows us to capture subtle changes caused by xenoestrogens/phytoestrogens and their targeted cells, not only in the tumor cells of interest but also in the surrounding stromal cells (e.g., fibroblasts or immune cells), furthering the understanding of the potential interactions between these heterogeneous cell populations. Thus, this information can greatly help to reveal the mechanisms of cancer-initiating and/or promoting the effects of exogenous estrogens.
We have demonstrated that this state-of-art technology can overcome some of the limitations of the pre-existing in vitro and in vivo models. We previously reported a study using scRNA-seq analysis on normal mouse mammary glands of a surgically menopaused mouse model treated with estrogen and PBDEs [136]. Our results suggest that PBDEs enhance estrogen-mediated mammary gland regrowth through the up-regulation of Areg expression in mammary epithelial cells, which in turn affects its cognate receptor, EGFR expressed on mammary fibroblasts and further modulates the recruitment of tumor-promoting M2 macrophages. These findings support the hypothesis that PBDE exposure with estrogen treatment increases the risk of breast cancer development during a critical period, menopause. scRNA-seq analysis also provides fundamental insights into the regulatory activity of PBDEs on distinct populations in normal mammary glands in the presence of estrogen. Furthermore, we expanded our scRNA-seq analysis to study the effect of PBDEs on the differentiation of mammary epithelial cells by integrating human and mouse datasets from our and others’ studies, thereby constructing a mammary cell gene expression atlas [137]. One group utilized scRNA-seq technology, although not directly related to cancer research, to investigate the transcriptomic changes induced by a known xenoestrogen, di (2-ethylhexyl) phthalate (DEHP), exposure. They revealed the reproductive toxicity of DEHP in murine germ cells and pre-granulosa cells at a single-cell level [138]. Although scRNA-seq has some limitations, such as technical noise from the cell preparation process, loss of spatial information, higher costs than other models, and requirement for freshly prepared samples [139,140,141], it serves as an excellent option for studying the complicated activity of xenoestrogens/phytoestrogens in heterogeneous cell populations of target tissues.
3. Biological Activities and Mechanisms of Xenoestrogens and Phytoestrogens in Cancers
In addition to regulating cell functions through interactions with estrogen signaling, xenoestrogens and phytoestrogens can affect cells through oxidative stress signaling by generating reactive oxygen species (ROS) within healthy cells or cancer cells (Figure 1). Oxidative stress-mediated signaling is a double-edged sword in cancer cell behavior. Oxidative stresses are suggested to play important roles in estrogen-induced breast carcinogenesis [183]. There is growing evidence that the induction of ROS by bisphenol A (BPA) may contribute significantly to its genomic toxicity and carcinogenic potential [184,185]. On the contrary, many chemotherapeutic strategies are designed to significantly increase cellular ROS levels, leading to tumor cell apoptosis [186]. As noted above, the phytoestrogen coumestrol (COU) is a potential chemotherapeutic agent for breast cancer. Evidence indicates that COU acts by inducing intracellular ROS, coupled with DNA fragmentation, up-regulation of p53/p21, cell cycle arrest, mitochondrial membrane depolarization, and caspases 9/3 activation [187].
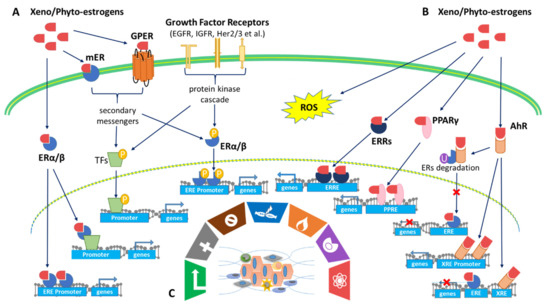
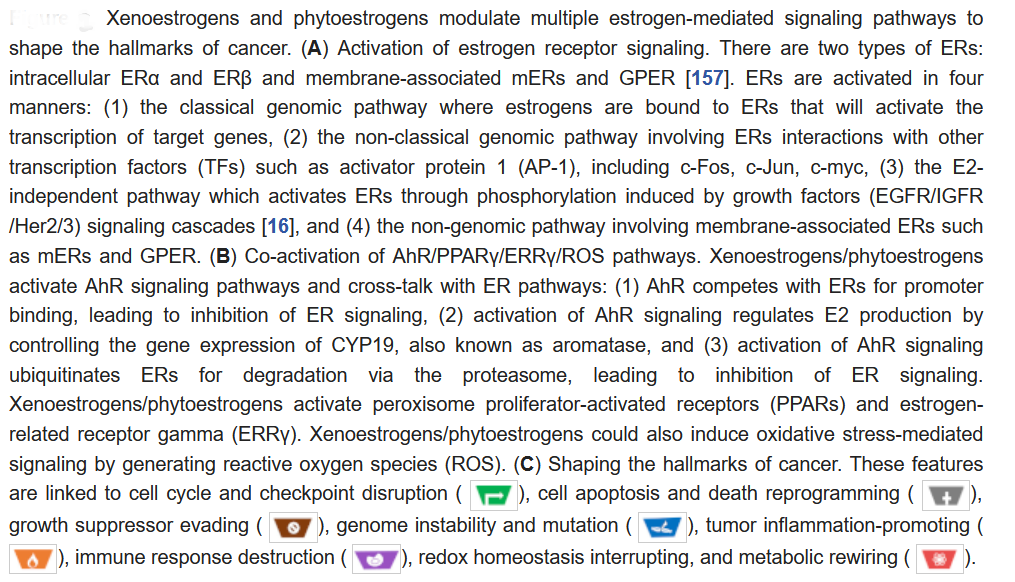
Xenoestrogens/phytoestrogens primarily modulate the hallmarks of cancer cells by inappropriately activating ERs, cross-talking with membrane-associated growth factor receptors (EGFR/IGFR/Her2/3), and many other nuclear receptors (AhR/PPARs/ERRα/γ). In the presence of active signaling, the hallmarks acquired by cancer cells are modulated and linked to cell cycle and checkpoint disruption, metabolic rewiring, regulation of apoptosis, and redox homeostasis [188]. In addition to cancer cells, tumors exhibit another dimension of complexity by recruiting heterogeneous cell types and creating a “tumor microenvironment”. These cells include tumor-infiltrating immune cells, cancer-associated fibroblasts (CAFs), cancer-associated adipocytes (CAAs), and more [189]. The impact of exogenous estrogens on tumor-associated cells is significant (Figure 2).
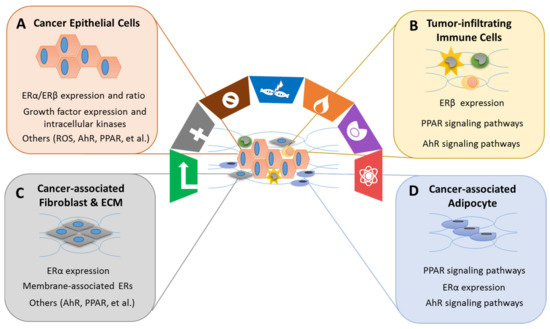
The effects of xenoestrogens and phytoestrogens on the tumor microenvironment are challenging to study. Traditional animal models that use homogeneous cancer cells do not mimic the actual dynamic, multicellular environment of a human tumor. Therefore, advanced research models, such as PDXs and scRNA-seq technology, allow scientists to capture changes caused by xenoestrogens/phytoestrogens in both cancer cells and the surrounding stromal cells, ultimately improving the understanding of the interactions among these heterogeneous cell populations.
The biological effects of phytoestrogens on breast cancer have also been linked to age and critical time points in a woman’s life [210]. In premenopausal women, who are at a high risk for early breast cancer, dietary isoflavone intake has been associated with an increased breast cancer risk by promoting cancer cell growth. However, isoflavone intake appears to have a protective impact on later breast cancer recurrence and mortality among postmenopausal breast cancer patients [211]. On the other hand, some phytoestrogens appear to reduce breast cancer risk throughout life. Asian diets, inclusive of abundant soy products, include phytoestrogens that appear to act as chemo-preventives for breast cancer in Asian women who consume more soy than women who consume a Western diet [212]. However, the relevant research on phytoestrogens in breast cancer is complicated, inconsistent, and inconclusive [213].
4. Application of Phytoestrogens in the Prevention or Treatment of Cancers: Evidence from Clinical Trials
Phytoestrogens such as soy isoflavones daidzein (DAI), genistein (GEN), and glycitein are dietary components that are thought to reduce the incidence and severity of various cancers [226]. The assumed benefits of this soy diet have led to numerous clinical studies on phytoestrogen efficacies to determine a suitable amount for human consumption without any adverse effects. Additionally, clinical studies of phytoestrogens combined with cancer treatments are underway to observe if there is a synergistic effect to treat cancer. Here, we have reviewed 18 clinical trials [61,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241,242,243,244], conducted between 2002 to present, focused on breast cancer (seven trials) [227,228,229,230,231,232,233,234], prostate cancer (eight trials) [61,235,236,237,238,239,240,241], endometrial cancer (two trials) [242,243], and colon cancer (two trials) [240,244], combined with two categories of phytoestrogens treatments: fruits/whole grains/seeds such as resveratrol and curcumin (eight trials) and soy isoflavones such as GEN (10 trials) (Table 1 and Supplementary Table S1).
Table 1. Clinical trials of phytoestrogens used as cancer prevention and/or cancer treatments.
| Identifier | Cancer Type/Prevention | Chemicals | Date | Participants/ Type of Study | Aims | Results |
|---|---|---|---|---|---|---|
| NCT00597532 [2] |
Breast | Genistein + Daidzein | 8/2002–4/2016 | 140 women/ R P controlled study | To examine the effects of soy supplementation on breast cancer-related genes and pathways | Tumors- PRE vs POST = altered EXP of 21 out of 202 genes. ↑ FANCC & UGT2A1 EXP in TG vs. PG (p < 0.05) Over-EXP of FGFR2, E2F5, BUB1, CCNB2, MYBL2, CDK1, and CDC20 (p < 0.01) in tumors with high-genistein signature |
| NCT00513916 [3][4] |
Breast | Isoflavones | 7/2006–2/2012 | 82 multiethnic PR/ R, crossover ‡ | To study the effects of dietary soy on estrogens in breast fluid, blood, and urine samples from healthy women | High-soy diet resulted in a modest trend of a lower cytological subclass in breast epithelial cells ↑isoprostane excretion in high-soy diet (p = 0.02) |
| NCT00612560 [5] |
Breast | Ground flaxseed (FS) ± anastrozole (AI) | 11/2007–4/2014 | 24 PO; 2 x 2 factorial R intervention | To examine the effect of flaxseed consumption compared to AI, and the effect of combined flaxseed and AI therapy on breast cancer treatment | ↓ serum steroid hormone DHEA w/ AI treatment (p = 0.009) PRE vs POST in FS + AI = ~40% ↓ EXP of estrogen receptorβ Lower Enterolactone excretion in FS + AI vs FS |
| NCT00290758 [6] |
Breast | Mixed soy isoflavones | 1/2006–7/2009 | 126 women (≥ 25 years)/ R *B | To study how well genistein works in preventing breast cancer in women at high risk for breast cancer | ↑ Ki-67 labeling index within PR TG (p = 0.04) Within TG, ↑ EXP of 14/28 genes (p = 0.017–0.052), but no S changes in PG TG vs PG = ↑ ESR1, FAS, FOXA1, MYB (p = NS) |
| NCT01219075 [7] |
Breast | Daidzein, genistein, glycitein | 7/2010–present | 85 women (30–75 years)/ D-B, R, P-controlled | To study soy isoflavones supplement in treating women at high risk or with breast cancer | NS differences in breast density area (p = 0.23) and mammogrpahic density % (p = 0.38) in TG vs PG |
| University of North Dakota School of Medicine and Health Sciences [3] | Breast | Trans- resveratrol | N/A | 39 women/D-B, R, P-controlled | To determine if trans-resveratrol had a dose-related effect on DNA methylation and prostaglandin expression in humans | ↑ levels of trans-resveratrol & resveratrol-glucuronide in serum = ↓ RASSF-1α methylation (p = 0.047) & ↓ PGE2 EXP in breast (p = 0.045) |
| National Center of Oncology, Yerevan, Armenia (Ministry of Health Republic of Armenia Registration No.: Nr 5592-17-02-23) [4] |
Breast | Curcumin + Paclitaxel | 3/2017–9/2018 | 150 women (18–75 years)/ *, single-institution, R, P-controlled, D-B, parallel group, two-arm trial | To assess the efficacy and safety of intravenous curcumin infusion in combination with paclitaxel in patients with metastatic and advanced breast cancer | ↑ objective response rate (ORR) of TG vs PG (16 weeks after beginning treatment, p < 0.05; completed treatment, p < 0.01) 3 months after treatment, ↑ ORR TG vs. PG (p < 0.07) ↑ fatigue in TG vs. PG (p = 0.05), but the overall physical performance was significantly higher with curcumin than the placebo during treatment and at the end of follow-up |
| NCT00596895 [8] |
Prostate | soy milk containing isoflavonoid | 11/2003–11/2007 | 20 men/ O-L, * nonrandomized trial | To evaluate the efficacy of isoflavone in patients with PSA recurrent prostate cancer after prior therapy. | Slope of PSA level (after vs. before study entry): ↓ in 6 men (p < 0.05), ↑ in 2 men (p < 0.05), and NS changes in 12 men A decline in the rise of serum PSA after the initiation of soy milk. |
| NCT01009736 [9] |
Prostate | Tomato-soy juice | 1/2008–7/2009 | 60 men/ * dose-escalating | To study the side effects of tomato-soy juice and its effect on biomarkers in patients with prostate cancer undergoing prostatectomy | High TG vs PG, ↓prostate-specific antigen slope (p = 0.078) |
| NCT00255125 [10] |
Prostate | Soy isoflavone capsules | 9/2005–10/2009 | 86 men (≥18 years)/ D-B, R, P-controlled | To evaluate the effects of soy isoflavone consumption on prostate specific antigen, hormone levels, total cholesterol, and apoptosis in men with localized prostate cancer. | TG vs PG in malignant prostate tissue = down-regulated 12 genes involved in cell cycle control and 9 genes involved in apoptosis No significant changes in serum total testosterone, free testosterone, total estrogen, estradiol, PSA, and total cholesterol |
| NCT00765479 [11] |
Prostate | Soy protein isolate | 9/2011–7/2013 | 284 men (40–75 years)/ R, P-controlled | Secondary analysis of body weight, blood pressure, thyroid hormones, iron status, and clinical chemistry in a 2-y trial of soy protein supplementation in middle-aged to older men. | Soy supplementation did not affect body weight, blood pressure, serum total cholesterol, iron status parameters, calcium, phosphorus, and thyroid hormones. |
| NCT00546039 [12] |
Prostate | Synthetic genistein | 4/2007–1/2009 | 47 Norwegian men/ * P-controlled, R, D-B | To evaluate safety and mechanisms of possible chemopreventive effects of synthetic genistein (BONISTEIN) in patients with localized prostate cancer undergoing laparoscopic radical prostatectomy | Genistein intervention significantly reduced the mRNA level of KLK4 in tumor cells (p = 0.033) and p27Kip1 In genistein intervention, no significant effects on proliferation-, cell cycle-, apoptosis-, or neuroendocrine biomarkers |
| NCT02724618 [13] |
Prostate | Nanocurcumin | 3/2016–present | 64 men/ R, D-B, * P-controlled | To determine the efficacy of oral nanocurcumin in prostate cancer patients undergoing radiotherapy. | Nanocurcumin was well tolerated. No significant difference was found between two groups regarding tumor response. |
| NCT02138955 [14] |
Prostate, Colon | Curcumin | 3/2014–6/2017 | 32 participants (18–85 years)/ ∞, single-center, O-L | To investigate the safety and tolerability of increasing doses of liposomal curcumin in patients with metastatic cancer | 300 mg/m2 liposomal curcumin over 6 h was the maximum tolerated dose, and a recommended starting dose for anti-cancer trials Anti-tumor activity was not detected |
| NCT01917890 [15] |
Prostate | Curcumin | 3/2011–10/2013 | 40 men (50–80 years)/ R, D-B, P-controlled | To evaluate the effect of curcumin supplementation on oxidative status of patients with prostate cancer who undergo radiotherapy | In TG: ↓ activity of superoxide dismutase (SOD) (p = 0.026), and ↑ plasma total antioxidant capacity (TAC) (p = 0.014) ↓ PSA level in both TG and PG No significant differences in treatment outcomes were observed between TG and PG |
| NCT00118846 [16] |
Endometria | Genistein, daidzein, glycitein, | 3/2004–3/2009 | 350 women (45-92 years)/ R, D-B, P-controlled | To determine whether long-term isoflavone soy protein (ISP) supplementation affects endometrial thickness and rates of endometrial hyperplasia and cancer in postmenopausal women | Soy-treated group did not significantly differ on the mean baseline or on-trial changes in endometrial thickness ISP has been found to predominantly act on the beta-type estrogen receptor because of its structure similar to 17β-estradiol and selective estrogen receptor modulator (SERM)-like activity. |
| NCT02017353 [17] |
Endometrial | Curcumin Phytosome (CP) | 10/2013–10/2016 | 7 women (≥18 years)/ O-L, * non-randomized | To determine whether curcumin can inhibit tumor induced inflammation in patients with endometrial carcinoma. In addition, curcumin could possibly induce a better functioning of chemotherapy and a decrease in toxicity from chemotherapy. | In TG, downregulated MHC expression levels on leukocytes (p = 0.0313), frequency of monocytes (p= 0.0114), and ICOS expression by CD8+ T cells (p = 0.0002), but upregulated CD69 levels on CD16- NK cells (0.0313). |
| NCT00256334 [18] |
Colon | Trans-resveratrol + quercetin | 7/2005–4/2009 | 11 participants (≥18 years)/∞ pilot, O-L | To evaluate the effects of a low dose of plant-derived resveratrol formulation and resveratrol-containing freeze-dried grape powder on Wnt signaling in the colon | Resveratrol did not inhibit Wnt pathway in colon cancer, but did inhibit Wnt pathway in normal colonic mucosa (p < 0.03) |
R, randomized; D-B, double-blind; P, placebo; O-L, open-label; ∞, phase I; *, phase II; ‡, phase III; TG, treatment group; PG, placebo group; PRE, pre-treatment; POST, post-treatment; PR, premenopausal women; PO, postmenopausal women; B, baseline; NS, non-significant; S, significant; EXP, expression.
Of the 18 trials, in terms of safety, four trials have shown that phytoestrogens are well-tolerated, safe to use, and/or have no major safety concerns. One trial studies prostate and colon cancer in phase 1 (NCT02138955) while two trials studies breast cancer in phase 2 (Nr 5592-17-02-23) and 3 (NCT00513916). In terms of the efficacy, seven trials showed little or no evidence that phytoestrogens were antagonistic to breast cancer (four trials, NCT01219075, NCT00597532, NCT00612560, and NCT00290758), prostate cancer (two trials, NCT00255125 and NCT02724618), or endometrial cancer (one trial, NCT00118846). Meanwhile, a total of six clinical trials have shown no significant differences between the treatment and placebo groups, including two breast cancer trials (NCT00290758, NCT00597532), three prostate cancer trials (NCT01009736, NCT01917890, NCT02724618), and one endometrial cancer trial (NCT00118846). Additionally, four clinical trials stated that the conclusions were not statistically significant, including one breast cancer trial (NCT00597532), two prostate cancer trials (NCT00255125, NCT0191789), and one endometrial cancer trial (NCT00118846). Lastly, five clinical trials consisting of two breast cancer studies (University of North Dakota School of Medicine and Health Sciences and NCT00513916), one prostate cancer study (NCT00546039), one endometrial cancer study (NCT02017353, phase 2), and one colon cancer study (NCT00256334, phase 1) suggested the need for larger and/or longer studies.
While the clinical trials of phytoestrogens noted above gave few promising results, combinations of a phytoestrogen with an established chemotherapy drug may be a more promising approach. For example, patients receiving curcumin (CUR) and Paclitaxel to treat metastatic breast cancers had a greater objective response rate ( p < 0.05 16 weeks after starting treatment, and p < 0.01 after completed treatment) compared to patients receiving Paclitaxel alone (Ministry of Health Republic of Armenia Registration No.: Nr 5592-17-02-23). Moreover, some men observed a slow rise of serum PSA after consuming 141 mg of isoflavones per day (NCT00596895). This prostate cancer trial has also shown that GEN may have an inhibitory effect on androgen-related biomarkers and supports GEN as a chemo-preventive agent in prostate cancer (NCT00546039).
While tumor response has been used to evaluate the effectiveness of phytoestrogens in cancer treatment, more recent clinical trials have added gene expression analysis. Phytoestrogens alter cancer-related gene expression profiles in breast cancer (NCT00597532, NCT00290758, and University of North Dakota School of Medicine and Health Sciences trail), prostate cancer (NCT00546039), endometrial cancer (NCT02017353), and colon cancer (NCT00256334). More interestingly, some of the trials have shown that phytoestrogens are altering the cancer-related gene expression profiles [227,233,238,243,244]. Under the concept of personalized medicine, gene expression analyses could be an alternative and cost-effective way to predict the effectiveness of phytoestrogens in cancer prevention and treatments. However, a larger number of clinical trial participants and more studies of phytoestrogens and their impact on cancers are still needed to better define their anti-cancer potentials.
5. Future Directions and Conclusions
In conclusion, exogenous estrogens, particularly xenoestrogens and phytoestrogens are an important contributor to the development and progression of cancers. Future studies on etiology of human cancers related to environmental exogenous estrogen exposure should focus on synthesizing various perspectives: (1) at the molecular and cellular level, looking at different types of ERs (ERα, ERβ, mER, and GPER) and cross-talk with other signaling pathways, (2) at the tissue level, considering the spatial heterogeneity of tissue composition and temporal heterogeneity of cancer progression, (3) at the systematic level, studying the exposure time at critical developmental windows, and (4) at the individual or population level, considering gene-environment interactions. Incorporated analysis of all the data in a clearly understood fashion allows for the modeling of prevention and therapy on an individual basis and the potential for developing new diagnostic biomarkers and drugs. Moreover, in the future, closer collaboration among oncology, systems biology, and environmental health may provide a significant qualitative and quantitative leap forward in the elucidation of human cancer etiology. The information gained from such collaborations could be applied in the introduction of preventive measures, personalized medicine, and more relevant public health intervention, ultimately, improving the knowledge and management of the complex environmental interactions underlying this life-threatening disease.
References
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170.
- Zaheer, K.; Akhtar, M.H. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293.
- Wu, A.H.; Spicer, D.; Garcia, A.; Tseng, C.-C.; Hovanessian-Larsen, L.; Sheth, P.; Martin, S.E.; Hawes, D.; Russell, C.; MacDonald, H.; et al. Double-Blind Randomized 12-Month Soy Intervention Had No Effects on Breast MRI Fibroglandular Tissue Density or Mammographic Density. Cancer Prev. Res. (Phila) 2015, 8, 942–951.
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-Resveratrol Alters Mammary Promoter Hypermethylation in Women at Increased Risk for Breast Cancer. Nutr. Cancer 2012, 64, 393–400.
- Sen, C.; Morimoto, Y.; Heak, S.; Cooney, R.V.; Franke, A.A.; Maskarinec, G. Soy Foods and Urinary Isoprostanes: Results from a Randomized Study in Premenopausal Women. Food Funct. 2012, 3, 517.
- McCann, S.E.; Edge, S.B.; Hicks, D.G.; Thompson, L.U.; Morrison, C.D.; Fetterly, G.; Andrews, C.; Clark, K.; Wilton, J.; Kulkarni, S. A Pilot Study Comparing the Effect of Flaxseed, Aromatase Inhibitor, and the Combination on Breast Tumor Biomarkers. Nutr. Cancer 2014, 66, 566–575.
- Khan, S.A.; Chatterton, R.T.; Michel, N.; Bryk, M.; Lee, O.; Ivancic, D.; Heinz, R.; Zalles, C.M.; Helenowski, I.B.; Jovanovic, B.D.; et al. Soy Isoflavone Supplementation for Breast Cancer Risk Reduction: A Randomized Phase II Trial. Cancer Prev. Res. (Phila) 2012, 5, 309–319.
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.-R.; et al. Efficacy and Safety of Curcumin in Combination with Paclitaxel in Patients with Advanced, Metastatic Breast Cancer: A Comparative, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytomedicine 2020, 70, 153218.
- Pendleton, J.M.; Tan, W.W.; Anai, S.; Chang, M.; Hou, W.; Shiverick, K.T.; Rosser, C.J. Phase II Trial of Isoflavone in Prostate-Specific Antigen Recurrent Prostate Cancer after Previous Local Therapy. BMC Cancer 2008, 8, 1–10.
- Grainger, E.M.; Moran, N.E.; Francis, D.M.; Schwartz, S.J.; Wan, L.; Thomas-Ahner, J.; Kopec, R.E.; Riedl, K.M.; Young, G.S.; Abaza, R.; et al. A Novel Tomato-Soy Juice Induces a Dose-Response Increase in Urinary and Plasma Phytochemical Biomarkers in Men with Prostate Cancer. J. Nutr. 2019, 149, 26–35.
- Hamilton-Reeves, J.M.; Banerjee, S.; Banerjee, S.K.; Holzbeierlein, J.M.; Thrasher, J.B.; Kambhampati, S.; Keighley, J.; Van Veldhuizen, P. Short-Term Soy Isoflavone Intervention in Patients with Localized Prostate Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. PLoS ONE 2013, 8, e68331.
- Bosland, M.C.; Enk, E.; Schmoll, J.; Schlicht, M.J.; Randolph, C.; Deaton, R.J.; Xie, H.; Zeleniuch-Jacquotte, A.; Kato, I. Soy Protein Supplementation in Men Following Radical Prostatectomy: A 2-Year Randomized, Placebo-Controlled Clinical Trial. Am. J. Clin. Nutr. 2021, 113, 821–831.
- Lazarevic, B.; Hammarström, C.; Yang, J.; Ramberg, H.; Diep, L.M.; Karlsen, S.J.; Kucuk, O.; Saatcioglu, F.; Taskèn, K.A.; Svindland, A. The Effects of Short-Term Genistein Intervention on Prostate Biomarker Expression in Patients with Localised Prostate Cancer before Radical Prostatectomy. Br. J. Nutr. 2012, 108, 2138–2147.
- Saadipoor, A.; Razzaghdoust, A.; Simforoosh, N.; Mahdavi, A.; Bakhshandeh, M.; Moghadam, M.; Abdollahi, H.; Mofid, B. Randomized, Double-Blind, Placebo-Controlled Phase II Trial of Nanocurcumin in Prostate Cancer Patients Undergoing Radiotherapy: Nanocurcumin for Prostate Cancer Patients Undergoing Radiotherapy. Phytother. Res. 2019, 33, 370–378.
- Greil, R.; Greil-Ressler, S.; Weiss, L.; Schönlieb, C.; Magnes, T.; Radl, B.; Bolger, G.T.; Vcelar, B.; Sordillo, P.P. A Phase 1 Dose-Escalation Study on the Safety, Tolerability and Activity of Liposomal Curcumin (LipocurcTM) in Patients with Locally Advanced or Metastatic Cancer. Cancer Chemother. Pharmacol. 2018, 82, 695–706.
- Hejazi, J.; Rastmanesh, R.; Taleban, F.-A.; Molana, S.-H.; Hejazi, E.; Ehtejab, G.; Hara, N. Effect of Curcumin Supplementation during Radiotherapy on Oxidative Status of Patients with Prostate Cancer: A Double Blinded, Randomized, Placebo-Controlled Study. Nutr. Cancer 2016, 68, 77–85.
- Quaas, A.M.; Kono, N.; Mack, W.J.; Hodis, H.N.; Felix, J.C.; Paulson, R.J.; Shoupe, D. Effect of Isoflavone Soy Protein Supplementation on Endometrial Thickness, Hyperplasia, and Endometrial Cancer Risk in Postmenopausal Women: A Randomized Controlled Trial. Menopause 2013, 20, 840–844.
- Tuyaerts, S.; Rombauts, K.; Everaert, T.; Van Nuffel, A.M.T.; Amant, F. A Phase 2 Study to Assess the Immunomodulatory Capacity of a Lecithin-Based Delivery System of Curcumin in Endometrial Cancer. Front. Nutr. 2019, 5, 138.




