Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bratosin Bogdan | + 3502 word(s) | 3502 | 2021-08-25 03:58:53 | | | |
| 2 | Peter Tang | Meta information modification | 3502 | 2021-08-30 03:48:38 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bogdan, B. Single Cell Protein. Encyclopedia. Available online: https://encyclopedia.pub/entry/13675 (accessed on 08 February 2026).
Bogdan B. Single Cell Protein. Encyclopedia. Available at: https://encyclopedia.pub/entry/13675. Accessed February 08, 2026.
Bogdan, Bratosin. "Single Cell Protein" Encyclopedia, https://encyclopedia.pub/entry/13675 (accessed February 08, 2026).
Bogdan, B. (2021, August 29). Single Cell Protein. In Encyclopedia. https://encyclopedia.pub/entry/13675
Bogdan, Bratosin. "Single Cell Protein." Encyclopedia. Web. 29 August, 2021.
Copy Citation
Single cell protein (SCP) is the first product of the fermentation process and has proven to be a good protein alternative. Food competition is becoming more intense as the world’s population continues to grow. Soon, SCP may be able to compensate for a protein deficit. Various global businesses are focusing on SCP production, and the scope of its application is expanding as time and knowledge increases.
single cell protein
algae
yeast
fungi
bacteria
mechanism of production
nutritional benefits
1. Introduction
The world population is continuously increasing. By 2050, the world population could increase to 9.3 billion [1][2] which at current consumption levels would cause the global demand for animal-derivative protein to reach 1250 million tons per year [3]. On the other hand, recent evidence has shown that almost 690 million people in the world (8.9 percent of the world population) are estimated to have been undernourished in 2019, and this is estimated to exceed 840 million by 2030. The most affected regions are Africa, Asia, Latin America and (to a lesser extent) the Caribbean [4]. Forecasting studies have shown that population increase will also be accompanied by economic progress, and consequently the increase in the living standard of about 3 billion people. It means that not only will water and food demands increase, but also the number of people who have more exigencies for food quality [5].
The United Nations Food and Agriculture Organization (FAO) describes proteins as macromolecules that make up the structural components of cells, tissues, muscles and organs. Proteins are required for metabolic function and are the nitrogen source for both humans and animals to build the structural and functional units required for living. The composition of a protein’s constituent amino acids reveals its nutritional worth. The most frequent are necessary amino acids, which humans and animals cannot synthesize. As a result, we consume meat or a protein sources to meet these needs [6].
Single cell proteins (SCPs) are isolated from the cells of microorganisms with high protein content as dried cells and/or as purified proteins. SCPs show very attractive features as a nutrient supplement for humans as they have a high protein content with wide amino acid spectrum, low fat content and a higher protein:carbohydrate ratio than forages [7]. SCPs contain vitamins, e.g., thiamine, riboflavin, pyridoxine, nicotinic acid, pantothenic acid, folic acid, biotin, cyanocobalamin, ascorbic acid, β-carotene and α-tocopherol; essential amino acids, represented by lysine and methionine; minerals; nucleic acids and lipids [8][9]. Until now, SCPs have been used for a wide range of purposes, from food (aroma carriers, vitamin carriers, emulsifying acids, etc.) and feed (pigs, poultry, cattle, fish) production, to the paper and lead industry [9][10].
Common sources for SCPs are represented by waste and raw materials (starch, fruit, fruit waste, molasses, etc.), combustible and/or combustible waste and/or byproducts (natural gas, petroleum byproducts, ethanol, methanol, biomass, etc.) [8]. Methanol is soluble in its aqueous phase at all concentrations, and it can be easily removed from harvested biomass. Ethanol is a good substrate, but the process is not economically feasible. There are several advantages to utilizing waste for SCP production; these include the conversion of low-cost organic waste to useful products and a reduction in environment pollution. The cellulose, hemicellulose and lignin of natural waste wood originating from agriculture and forestry sources are attractive natural sources of SCP. However, they must be pretreated chemically (acid hydrolysis) or enzymatically (cellulases) to transform cellulose into fermentable sugars. Domestic sewage and waste retained from industrial cellulose processing, starch production and food processing can also be utilized. Agricultural waste has been reported to be an excellent substrate for cost-effective SCP production, the resulting protein-enriched product being of good quality and suitable for animal feed. It can even be consumed by humans after further processing [11].
The production of SCP from non-waste sources achieved industrial-scale production in the 1970s but was not economically competitive with other protein supplements Recently, interest in SCP has been renewed, partly because of the identification of new, less expensive production processes, but largely due to the realization that SCP production has vast potential environmental benefits over traditional protein supplements in animal feed [12].
1.1. Historical Background of SCP
Many microorganisms have been directly utilized as food. SCPs can be lifesaving in less privileged areas where malnutrition is a real and life-threatening problem. A species of alga called Spirulina was grown many years ago in Africa’s Lake Chad, and was subsequently used as food to compensate for local people’s protein shortfall [6]. Germans reportedly utilized a certain species of Candida in their meals during World War I, including sausages and soups. Proteins generated from bacterial, fungal and algal cultures were widely used in food and as food from then on. The concept of SCP arose from this method, and these proteins are now widely used [13].
1.2. Applications of SCP
SCP produced on commercial scale is used [13]:
-
In animal feed and nutrition, for the stuffing and fattening of poultry, laying hens, calves and pigs.
-
As food additives (vitamin and aroma carriers and emulsifying agents), to enhance nutritional value (of baked food items, ready-made meals, soups, etc.) and as starter cultures (baker’s, brewer’s and wine yeast).
-
In industrial processes, as a foam-stabilizing agent and in paper and leather processing.
1.3. Mechanism of Production
There are different kinds of substrates, as categorized in [14]:
-
High-energy resources: gas oil, natural gas, ethanol, methanol, n-alkanes and acetic acid
-
Renewable plant resources: starch, sugar and cellulose
-
Various wastes: sulfite waste liquor, molasses, whey, milk and fruit waste
-
Carbon dioxide
The choice of substrate is made according to cost, availability, oxygen required during fermentation, quantity of heat produced and cooling capacity of the fermenter, but also the cost related to post-treatment processing [8][14]. Selected substrates are used as a growth medium by microorganisms such as bacteria, algae, fungi and yeast for increasing their cell mass, which is made up of SCP [15]. Fermentation is the main process responsible for SCP production [16], as shown is Figure 1.
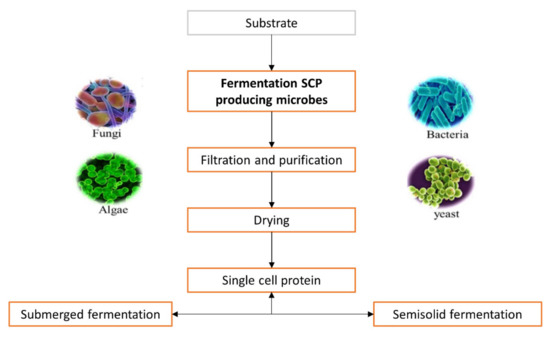
Figure 1. General steps during industrial production of single cell proteins.
The available biomass is harvested when the fermentation process is completed and can be used as a protein source [17]. The biomass is processed further by purification, cell disruption, washing and protein extraction [18] to provide high production rates with high yields.
The demand for protein is increasing due to changes in food consumption patterns. However, the reliance on animal and dairy production to meet the growing demand for protein is ultimately unsustainable [3]. Hence, alternative sources should be explored to supply food for humans and animals [19].
SCP is widely viewed as a potential co-product that could boost the economic potential of a biorefinery process that is otherwise unprofitable and lower the downstream processing costs associated with the disposal of process waste. It is preferable to sell residual biomass as feed rather than as fertilizer. This can be seen in numerous publications in which specific waste products are converted to SCP and are assessed as food for specific animals [3].
2. Bacterial Metabolism
Bacteria have a wide range of metabolic processes characterized by enzymatic assimilation (the intake and use of organic and inorganic chemicals required for cellular growth and maintenance) and dissimilation reactions (the oxidation and breakdown of substrate). Assimilation reactions are endergonic, which means they require energy, whereas dissimilation reactions are exergonic, which means they generate energy. These reactions form the basis of the bacterial cell’s self-replication and are involved in critical functions of the cell [20].
Vasdekis and Stephanopoulos (2015) describe bacteria as cells that can value the energy they capture from the environment to accomplish their essential operations, thanks to their particular energy-transforming ability [21]. Chemical energy is conserved in adenosine triphosphate (ATP), adenosine diphosphate (ADP) and/or molecules with a thioester link (e.g., succinyl SCoA and acetyl SCoA). These compounds have high energy phosphate bonds, which are used by enzymatic systems to synthesize new compounds needed for cell existence and development.
Bacterial enzymatic systems include B-complex vitamins as functional coenzymes that are involved in cell growth and energy transformation processes by catalyzing many oxidation–reduction reactions [22].
SCP metabolism involves the biological oxidation of organic compounds and yields simple organic and/or inorganic compounds together with ATP. The bacterial cell needs these compounds for anabolic pathways. Two modes of energy production are known in bacteria within heterotrophic metabolism: anaerobic respiration or fermentation (Figure 2A) and aerobic respiration (Figure 2B). Energy production may occur in both aerobic and anaerobic environments.
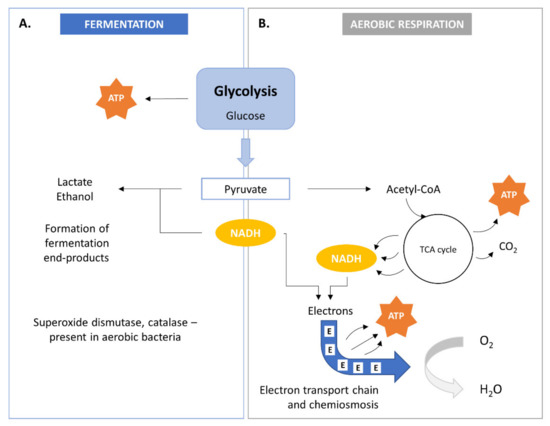
Figure 2. Bacterial metabolism by fermentation (A) and aerobic aspiration (B). TCA, tricarboxylic acid.
3. Algal Metabolism
The micro-algal metabolism refers to processes that include both biochemical mechanisms and nutrient transport. Intake nutrients are converted through a metabolic pathway into nutritional principles needed for vital processes, such as growth, reproduction and defense mechanisms. The mechanisms for the acquisition of carbon supplies, light capture, assimilation of nutrients (nitrogen and sulfur) and synthesis of the unique secondary metabolites make the difference between micro-algal metabolism and the metabolisms of other organisms [23].
The micro-algae display oxygen-evolving photosynthesis, which is a key feature that distinguishes them from other lower eukaryotes. This metabolic process is characterized by specific reactions often produced in the presence of light, at thylakoid membrane level. In algae, the basic chemo-organotrophic metabolism is similar to that encountered in bacteria. In algae, anabolic processes may occur both in the presence of light (photolithotrophic) or in the dark. The photorespiration process is catalyzed by specific enzymatic systems, including ATP syntheses, the cytochrome b6–f complex or enzymes specific to the photosynthetic carbon-reduction cycle. In micro-algae, the photosynthetic reactions yield carbohydrates as phosphates. The algae photolithotrophic metabolism involves pathways that cannot take place in the dark. There are known linear pathways that produce the synthesis of essential C skeletons, and they use photons, nitrogen, ammonium nitrate, ammonium sulfate, ammonium di-hydrogen phosphate and carbon dioxide for growth. Micro-algae that grow in the dark have only organic carbon as a source [24].
Figure 3 outlines the pathways of energy, carbon and oxygen in photosynthesis, photorespiration, dark respiration and the growth of an alga. No attempt is made to represent stoichiometries.
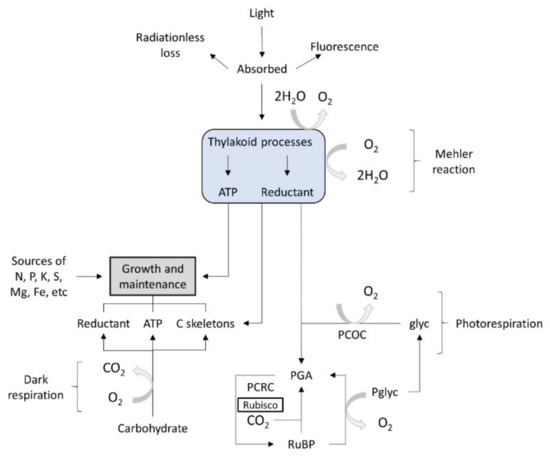
Figure 3. Algal metabolism [23]. Glyc, glycolate; PCOC, photorespiratory carbon-fixation cycle (or its equivalent); PCRC, photosynthetic carbon reduction cycle; PGA, 3-phosphoglycerate; Pglyc, phosphoglycolate; RuBP, ribulose bisphosphate; Rubisco, ribulose bisphosphate carboxylase–oxygenase.).
4. Fungal Metabolism
A series of specific reactions characterize fungal metabolism. The catabolic processes consist in the biosynthesis of a large number of compounds, usually divided into primary and secondary metabolites [25]. The primary fungal metabolites, such as alcohols, organic acids (citric and lactic acid) and amino acids (l-glutamate, l-lysine) are required for growth and reproduction. The secondary metabolites are not essential for cellular life, but have importance from an ecological point of view, because they are involved in versatile metabolic pathways and have the capacity to break down organic matter, which cannot otherwise be recycled [26][27]. These secondary metabolites are natural compounds, such as small peptides, amino acids, pigments and products with a potentially toxic action, such as mycotoxins and antibiotics [28][29][30][31].
The precursors of the primary metabolites of SCP are intermediate molecules involved in anabolic and catabolic pathways that may be used for the synthesis of macromolecular subunits (lipids, amino acids, nucleotides) and/or may be oxidized to generate ATP. Glucose and pyruvate (Figure 4) are used for the biosynthesis of fungal secondary metabolites. They are linked together as result of reactions catalyzed by enzymes such as dimethyl-allyl tryptophan synthetases (DMATSs), polyketide synthases (PKSs), terpene cyclases (TCs) or non-ribosomal peptide synthetases (NRPSs). Oligomers result from these reactions, which are often chemically modified by the action of tailoring enzymes controlled by transcriptional regulation [32]. Macromolecular biosynthesis consists in lipids and amino acids, which are the main nutritional components of biomass.
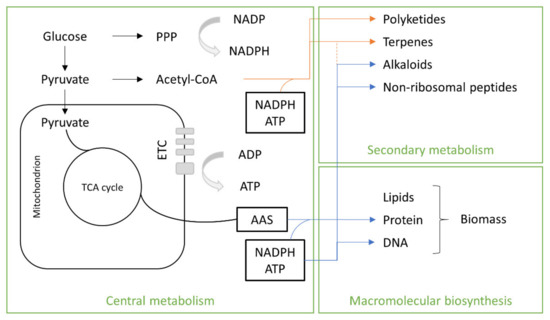
Figure 4. Fungal metabolism. TCA, tricarboxylic acid; ETC, electron transport chain.
5. Yeast Metabolism
In yeasts, the basic metabolic mechanisms are identical for different cells, but the resulting metabolites are different. Complex enzymatic reactions are involved in yeasts’ metabolism. The metabolic process begins when the substrates penetrate the cell. Action-specific factors then produce alterations of their basic structure [33]. The enzymatic reactions catalyzing the metabolic processes are needed for different aims: production and development of other cells, oxidation–reduction reactions which generate carbon dioxide and alcohol. Carbohydrates are the main carbon and energy source for most yeast species. However, there are known yeast species that use other sources of carbon, such as Cryptococcus aureus, Cryptococcus laurentii, Hannaella aff. zeae, Tremella encephala and Trichosporon coremiiforme [34]. These microorganisms have the capacity to form proteins and amino acids from simple nitrogenous sources as presented in Table 4.
The glycolysis process uses simple carbohydrates as substrates, mainly fructose and glucose. If other, more complex carbohydrates (maltotriose or maltose) are involved, they are hydrolyzed to glucose by α-glucosidase. During the first step of the yeast’s metabolic process, glucose is degraded to pyruvate, through glycolysis. The pyruvate metabolic pathway leads to the release of energy. Fermentation generates small amounts of energy, while respiration produces a larger amount (Figure 5). The carbon substrate is totally oxidated through respiration. Oxidative phosphorylation and the Krebs cycle are the mechanisms involved in this process. The energy produced and accumulated in ATP contributes to the generation of intermediary metabolites. In presence of oxygen, pyruvate enters into mitochondria where, under the action of the pyruvate dehydrogenase multi-enzyme system, it is the subject of the oxidative decarboxylation to acetyl-CoA. When yeasts are grown on two carbon sources (e.g., ethanol or acetate), the glyoxylate cycle, which may be considered a shortcut for the Krebs cycle, is often the supplier of energetic compounds. The alcoholic fermentation of carbohydrates involves a process whereby yeasts re-oxidize NADH to NAD. It is a two-step reaction: (1) pyruvate decarboxylase catalyzes the process of pyruvate decarboxylation, (2) alcohol dehydrogenase catalyzes the subsequent reaction of the reduction of acetaldehyde at the same time. Simultaneously, dihydroxyacetone phosphate generates glycerol.
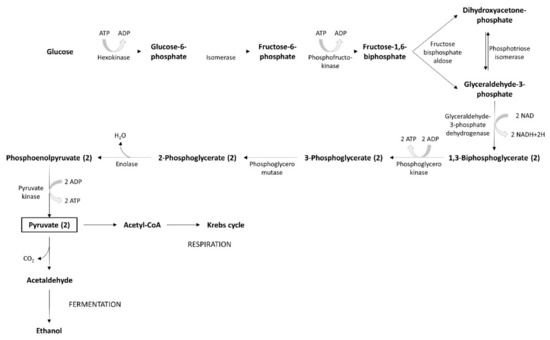
Figure 5. Glycolysis process.
6. Nutritional Benefits of SCP
Wu et al. (2014) indicated that the worldwide human protein diet is 65% plant based and 35% animal based [35]. The average meat intake per capital is projected to rise by 29%, from 40.0 kg in 2013 to 51.5 kg in 2050. Global meat production is therefore expected to increase from 288 million tons in 2013 to 494 million tons by 2050. According to previous research, SCP could be a valuable solution for suppling the global demand of protein, due to its low cost of production, easy process and nutritional quality [8][11][36][37].
As previously stated, the primary goal of SCP production is to use it as a protein (meat) substitute to address food scarcity and hunger in the near future. SCP must meet nutritional requirements to be consumed as human food or animal feed, such as the required protein content, amino acid composition of the protein being generated and digestibility of that protein. According to the researchers, SCP must be produced to the highest standards to be safe and beneficial for food and feed [38].
Finnigan et al. (2017) defined some of the macro and micronutrients provided by SCP as follows: proteins, lipids, carbohydrates, β-carotene, vitamin A precursor, biotin, folic acid, niacin, pantothenic acid, pyridoxine, riboflavin, thiamine, vitamin B12 (cyanocobalamin), vitamin C and vitamin E [39].
Beside its protein content, the nutritional value of SCP depends on its chemical composition (amino acids, nucleic acids minerals, enzymes and vitamins) but it is relatively cheap compared to other plant and animal sources [40]. It was reported that dried cells of Pseudomonas spp. grown on petroleum-based liquid paraffin contain as much as 69% protein. SCP obtained by algae processing is about 40%. Though proteins obtained from microbes contain all essential amino acids, their composition depends on the type of substrate used (carbon or nitrogen) and the type of microorganism grown on a specific medium [41]. Microorganisms like bacteria and yeast have a very short multiplication time as they double their population in just 5–15 min, while algae and mold species double themselves in 2–4 h. The amino acid profile of SCP from bacteria shows a close resemblance to fish protein and protein from yeast resembles soya protein [42]. In addition, SCP is reported to be deficient in sulfur-containing amino acids such as methionine and cysteine, whereas high levels of lysine and other amino acids have been observed. Supplementation of methionine and cysteine is required for the use of SCP as a feed ingredient. Microorganisms normally contain vitamin B12 in significant quantities. Bacteria and algae are reported with high vitamin B12 and vitamin A content, respectively [43]. Most common vitamins present in SCP are riboflavin, thiamine, pyridoxine, niacin choline, folic acid, pantothenic acid, biotin, para-amino benzoic acid, inositol and B12 [43]. Most microorganisms have very fast growth rate that yields a high amount of biomass (algae: 3–6 h, bacteria: 30 min to 2 h, yeast: 40 min to 3 h) [36]. These microorganisms can be used as a whole in contrast to most crop and animal protein sources which cannot be used entirely [44]. SCP produced from different microbes has high protein content (30–70%) as compared to different green plants and animal sources [45]. Moreover, these proteins have an excellent amino acid profile that makes them nutritionally more useful than conventional protein sources [46]. Nevertheless, scientists can add more valuable amino acids to proteins through genetic engineering, obtaining SCP with the desired composition. Some microorganisms during the course of SCP production produce significant amounts of vitamins which cannot be produced by the host individual in appropriate amounts. Production of SCP also requires low water content as compared to plant sources [47]. Unlike plant protein sources, SCP is independent of environmental and climatic variation and can be produced throughout the year as microbes are available round the clock. The shelf life of protein preparation is dependent on the nature of the protein and the storage conditions used, and can vary from a few days to more than a year. Optimal conditions for storage are distinctive to each protein.
Nutritive and food values of SCP vary with the microorganisms used. The microorganisms used for SCP production must be non-pathogenic, toxin-free, easy to handle and separate from the substrate and should also tolerate the scaling-up of the process. Fast-growing microorganisms are required for getting massive output (biomass weight produced per unit time). However, high output produces more quantities of RNA in the cell, and this is not desirable as it acts as anti-nutritional factor in the final product. The method of harvesting, drying and processing affects the nutritive value of the finished product [48][49][50][51][52]. The idea that SCP could help to overcome food shortages in developing countries has garnered interest among scientists and industry. For future success of SCP, food technology problems must be solved to make these foods familiar, while the production should compare favorably with other protein sources.
The raw materials for SCP production based on waste substances are cheap, readily available and their processing contributes to reducing environmental pollution. The origin of the feedstock must be carefully selected. Various types of raw materials are attractive sources for SCP production from a cost and sustainability perspective but may raise safety concerns. In addition to the safety requirements, the use of other unconventional waste-derived protein sources in human nutrition requires efforts to improve public perception and acceptance and increase consumer awareness of the benefits of SCP consumption in human diets.
Yeast SCPs have been used in aquaculture diets as partial replacement for fishmeal, thanks to their excellent nutrient profiles and cost-effective large-scale production [52][53], and have also been applied for the highly unsaturated fatty acid fortification of Artemia and rotofiers [51]. Some yeast strains with probiotic properties, such as Saccharomyces cerevisiae [54] and Debaryomyces hansenii [55], increased larval survival by early maturation of the pancreas and intestine [55]. However, many of these yeast SCPs are deficient in sulfated amino acids, e.g., methionine [54], thus, cannot be used as sole protein sources. Micro-algal SCP may be used for both animal and human consumption, and their nutritional value is similar to, and sometimes higher than, values reported for conventional food/feed supplements. Besides their high protein content, they are a source of nucleic acids (up to 6%), minerals (sodium, magnesium, potassium, iodine) and vitamins (A, B group, D, C and E) and essential amino acids (leucine, valine, lysine, phenylalanine) [56] (Figure 6). Compared to many vegetable foods, micro-algae SCP contains higher amounts of vitamins such as riboflavin, thiamine, folic acid or pro-vitamins as carotene [57]. Micro-algae, as Spirulina and Chlorella, are sources of vitamin B12 (cyanocobalamin), which otherwise has animals as almost exclusive source [57]. Algal SCP has a nutritional value like other SCP sources. The crude protein content (N 6.25) varies between 45 and 73%, while the lipid content is 2–20% and is rich in essential fatty acids, and the mineral content is 5–10%. The protein content of algae is higher than the value for soybean (70% and 40%, respectively), and its amino acid profile shows an adequate balance except, as for any other microbial biomass, for the sulfur-containing amino acids methionine and cysteine [36][57]. Algal SCP is a good source of vitamins A, B group, D, C and E; the content of some vitamins such as thiamine, riboflavin, folic acid and carotene is higher in algae than in many vegetable foodstuffs. Some microalgae, such as Chlorella and Spirulina, contain vitamin B12 (cyanocobalamin), which is found almost exclusively in animal origin foods. The content of nutrients, however, is highly dependent on cultivation and processing conditions [57]. In addition, the search for new insects as a source of protein and the related technology for processing requires further research.
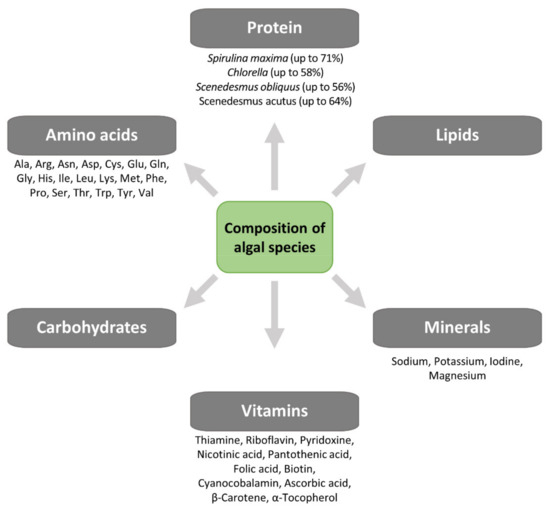
Figure 6. Composition of algal species.
References
- Pihlajaniemi, V.; Ellilä, S.; Poikkimäki, S.; Nappa, M.; Rinne, M.; Lantto, R.; Siika-aho, M. Comparison of pretreatments and cost-optimization of enzymatic hydrolysis for production of single cell protein from grass silage fibre. Bioresour. Technol. Rep. 2020, 9, 100357.
- Verstraete, W.; Clauwaert, P.; Vlaeminck, S.E. Used water and nutrients: Recovery perspectives in a ‘panta rhei’ context. Bioresour. Technol. 2016, 215, 199–208.
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8.
- World Health Organization. The State of Food Security and Nutrition in the World 2020: Transforming Food Systems for Affordable Healthy Diets; Food and Agriculture Organization: Rome, Italy, 2020.
- Food and Agricultural Organization of the United Nations. How to Feed the World in 2050. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 18 June 2021).
- Junaid, F.; Khawaja, L.A.; Ali, S. Single cell proteins as a potential meat substitute: A critical review. World J. Pharm. Res. 2020, 9, 141–161.
- Srividya, A.R.; Vishnuvarthan, V.J.; Murugappan, M.; Dahake, P.G. Single Cell Protein- A Review. Int. J. Pharm. Res. Sch. 2013, 2, 472–485.
- Suman, G.; Nupur, M.; Anuradha, S.; Pradeep, B. Single Cell Protein Production: A Review. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 251–262.
- Zhou, Y.-M.; Chen, Y.-P.; Guo, J.-S.; Shen, Y.; Yan, P.; Yang, J.-X. Recycling of orange waste for single cell protein production and the synergistic and antagonistic effects on production quality. J. Clean. Prod. 2019, 213, 384–392.
- Nagare, B.; Bhambere, S.; Kumar, S.; Kakad, K.; Nagare, N. In Situ Gelling System: Smart Carriers for Ophthalmic Drug Delivery. Int. J. Pharm. Res. Sch. 2015, 4, 10–23.
- Yunus, F.-u.-N.; Nadeem, M.; Rashid, F. Single-cell protein production through microbial conversion of lignocellulosic residue (wheat bran) for animal feed. J. Inst. Brew. 2015, 121, 553–557.
- Matassa, S.; Boon, N.; Pikaar, I.; Verstraete, W. Microbial protein: Future sustainable food supply route with low environmental footprint. Microb. Biotechnol. 2016, 9, 568–575.
- Ali, S.; Mushtaq, J.; Nazir, F.; Sarfraz, H. Production and Processing of Single Cell Protein (SCP)—A review. Eur. J. Pharm. Med. Res. 2017, 4, 86–94.
- Raziq, A.; Lateef, M.; Ullah, A.; Ullah, H.; Khan, M.W. Single cell protein (SCP) production and potential substrates: A comprehensive review. Philipp. Accredit. Bur. 2020, 9.
- Queiroz, M.I.; Lopes, E.J.; Zepka, L.Q.; Bastos, R.G.; Goldbeck, R. The kinetics of the removal of nitrogen and organic matter from parboiled rice effluent by cyanobacteria in a stirred batch reactor. Bioresour. Technol. 2007, 98, 2163–2169.
- Kadim, I.T.; Mahgoub, O.; Baqir, S.; Faye, B.; Purchas, R. Cultured meat from muscle stem cells: A review of challenges and prospects. J. Integr. Agric. 2015, 14, 222–233.
- Zepka, L.Q.; Jacob-Lopes, E.; Goldbeck, R.; Queiroz, M.I. Production and biochemical profile of the microalgae Aphanothece microscopica Nägeli submitted to different drying conditions. Chem. Eng. Process. 2008, 47, 1305–1310.
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193.
- Zha, X.; Tsapekos, P.; Zhu, X.; Khoshnevisan, B.; Lu, X.; Angelidaki, I. Bioconversion of wastewater to single cell protein by methanotrophic bacteria. Bioresour. Technol. 2021, 320, 124351.
- Jurtshuk, P.J. Bacterial Metabolism. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; Chapter 4.
- Vasdekis, A.E.; Stephanopoulos, G. Review of methods to probe single cell metabolism and bioenergetics. Metab. Eng. 2015, 27, 115–135.
- Müller, W.E.G.; Schröder, H.C.; Wang, X. Inorganic Polyphosphates As Storage for and Generator of Metabolic Energy in the Extracellular Matrix. Chem. Rev. 2019, 119, 12337–12374.
- Beardall, J.; Raven, J.A. Algal Metabolism. Encycl. Life Sci. 2012.
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis, 2nd ed.; STU-Student Edition, Ed.; Princeton University Press: Princeton, NJ, USA, 2007.
- Takahashi, J.A.; Barbosa, B.V.R.; Martins, B.d.A.; Guirlanda, C.P.; Moura, M.A.F. Use of the Versatility of Fungal Metabolism to Meet Modern Demands for Healthy Aging, Functional Foods, and Sustainability. J. Fungi 2020, 6, 223.
- Demain, A.L.; Fang, A. The Natural Functions of Secondary Metabolites. In History of Modern Biotechnology I; Fiechter, A., Ed.; Springer: Heidelberg, Germany, 2000; pp. 1–39.
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947.
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61.
- Hoffmeister, D.; Keller, N.P. Natural products of filamentous fungi: Enzymes, genes, and their regulation. Nat. Prod. Rep. 2007, 24, 393–416.
- Kohlhaw, G.B. Leucine biosynthesis in fungi: Entering metabolism through the back door. Microbiol. Mol. Biol. Rev. 2003, 67, 1–15.
- Schardl, C.L.; Young, C.A.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci. PLoS Genet. 2013, 9, e1003323.
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32.
- Klug, L.; Daum, G. Yeast lipid metabolism at a glance. FEMS Yeast Res. 2014, 14, 369–388.
- Sitepu, I.; Selby, T.; Lin, T.; Zhu, S.; Boundy-Mills, K. Carbon source utilization and inhibitor tolerance of 45 oleaginous yeast species. J. Ind. Microbiol. Biotechnol. 2014, 41, 1061–1070.
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and supply of high-quality food protein for human consumption: Sustainability, challenges, and innovations. Ann. N. Y. Acad. Sci. 2014, 1321, 1–19.
- Nasseri, A.T.; Rasoul-Amini, S.; Morowvat, M.H.; Younes, G. Single Cell Protein: Production and Process. Am. J. Food Technol. 2011, 6.
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.M.; Fischer, A.R.H.; van Boekel, M.A.J.S.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The future supply of animal-derived protein for human consumption. Trends Food Sci. Technol. 2013, 29, 62–73.
- Linder, T. Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Secur. 2019, 11, 265–278.
- Finnigan, T.; Needham, L.; Abbott, C. Mycoprotein: A Healthy New Protein With a Low Environmental Impact. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; Chapter 19; pp. 305–325.
- Bogdahn, I. Agriculture-independent, sustainable, fail-safe and efficient food production by autotrophic single-cell protein. PeerJ PrePrints 2015, 3, e1279v1273.
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84.
- Yousufi, M.K. To determine protein content of single cell protein produced by using various combination of fruit wastes and two standard food fungi. Int. J. Adv. Biotechnol. Res. 2012, 3, 533–536.
- Anupama; Ravindra, P. Value-added food:: Single cell protein. Biotechnol. Adv. 2000, 18, 459–479.
- Zhou, Y.-M.; Chen, Y.-P.; Shen, Y. Single cell protein-feed: Taking orange waste as raw material for fermentation. In Advanced Materials and Energy Sustainability; World Scientific: Singapore, 2017; pp. 323–335.
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885.
- Aruna, T.E.; Aworh, O.C.; Raji, A.O.; Olagunju, A.I. Protein enrichment of yam peels by fermentation with Saccharomyces cerevisiae (BY4743). Ann. Agric. Sci. 2017, 62, 33–37.
- Adedayo, M.R.; Ajiboye, E.A.; Akintunde, J.K.; Odaibo, A. Single Cell Proteins: As Nutritional Enhancer. Adv. Appl. Sci. Res. 2011, 2, 396–409.
- Bhalla, T.; Sharma, N.N.; Sharma, M. Production of Metabolites, Industrial enzymes, Amino acid, Organic acids, Antibiotics, Vitamins and Single Cell Proteins. J. Environ. 2007, 6, 34–78.
- Jamal, P.; Alam, M.; Salleh, N. Medai optimization for bioproteins productions from cheaper carbon source. J. Eng. Sci. Technol. 2008, 3, 124–130.
- Mahajan, A.; Dua, S. A perspective on biotechnological potential. J. Food Sci. Technol. 1995, 32, 162–165.
- McEvoy, L.A.; Navarro, J.C.; Hontoria, F.; Amat, F.; Sargent, J.R. Two novel Anemia enrichment diets containing polar lipid. Aquaculture 1996, 144, 339–352.
- Olvera-Novoa, M.A.; MartÍNez-Palacios, C.A.; Olivera-Castillo, L. Utilization of torula yeast (Candida utilis) as a protein source in diets for tilapia (Oreochromis mossambicus Peters) fry. Aquac. Nutr. 2002, 8, 257–264.
- Hardy, R.W.; Patro, B.; Pujol-Baxley, C.; Marx, C.J.; Feinberg, L. Partial replacement of soybean meal with Methylobacterium extorquens single-cell protein in feeds for rainbow trout (Oncorhynchus mykiss Walbaum). Aquac. Res. 2018, 49, 2218–2224.
- Oliva-Teles, A.; Gonçalves, P. Partial replacement of fishmeal by brewers yeast (Saccaromyces cerevisae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2001, 202, 269–278.
- Tovar, D.; Zambonino, J.; Cahu, C.; Gatesoupe, F.J.; Vázquez-Juárez, R.; Lésel, R. Effect of live yeast incorporation in compound diet on digestive enzyme activity in sea bass (Dicentrarchus labrax) larvae. Aquaculture 2002, 204, 113–123.
- Nangul, A.; Bhatia, R. Microorganisms: A marvelous source of single cell proteins. J. Microbiol. Biotechnol. Food Sci. 2013, 3, 15–18.
- García-Garibay, M.; Gómez-Ruiz, L.; Cruz-Guerrero, A.E.; Bárzana, E. Single cell protein|The Algae. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Oxford, UK, 2014; pp. 425–430.
More
Information
Subjects:
Food Science & Technology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
4.0K
Revisions:
2 times
(View History)
Update Date:
30 Aug 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




