
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | ANNALISA DEL PRETE | + 1483 word(s) | 1483 | 2020-06-04 08:46:36 | | | |
| 2 | Nicole Yin | -10 word(s) | 1473 | 2020-11-05 10:00:37 | | |
Video Upload Options
Dendritic cells (DCs) constitute a complex network of cell subsets with common functions but also with many divergent aspects. All dendritic cell subsets share the ability to prime T cell response and to undergo a complex trafficking program related to their stage of maturation and function. For these reasons, dendritic cells are implicated in a large variety of both protective and detrimental immune responses, including a crucial role in promoting anti-tumor responses. Although cDC1s are the most potent subset in tumor antigen cross-presentation, they are not sufficient to induce full-strength anti-tumor cytotoxic T cell response and need close interaction and cooperativity with the other dendritic cell subsets, namely cDC2s and pDCs. Here, the functional role of dendritic cell subsets in suppressing tumor growth is discussed. Understanding the specificity of dendritic cell subsets will allow to gain insights on role of these cells in pathological conditions and to design new selective promising therapeutic approaches.
1. Introduction
An efficient T cell-mediated antitumor immune response requires cross-presentation of tumor-associated antigens by Dendritic Cells (DCs)[1]. cDC1s represent a subset specialized in cross-presenting tumor antigens on MHC-I molecules to CD8+ T lymphocytes for the generation of efficient cytotoxic T cell (CTL)-mediated immunity (for a review,[2][3]). The critical role of cDC1s in antitumor immune defense was demonstrated by the genetic model of Batf3 deficient mice, where cDC1 depletion led to the inability to reject transplantable immunogenic tumors[4][5][6] and to sustain immunotherapies based on adoptive T cell transfer or immune checkpoint inhibition[5][7][8]. Several molecules involved in membrane trafficking are required for efficient tumor antigen cross-presentation, such as the Soluble NSE Attachment Protein Receptor (SNARE) member Sec22b and the regulator of vesicular trafficking WDFY4. These molecules are also required for the control of tumor growth and for the efficacy of anti-PD1-based immunotherapies[9][10]. In addition to cross-presentation, other cDC1-associated molecules are necessary to promote anti-tumor immunity and tumor rejection[11]. For the initial priming of CD8+ CTLs tumor antigens must be delivered to tumor-draining lymph nodes by migratory CD103+ cDC1s in a CCR7-dependent manner[12]. Although resident CD8α+ cDC1s may also be involved, migratory CD103+ cDC1s have unique abilities in tumor-antigen cross presentation[7][12]. The expression of XCR1 is crucial for cDC1 functions, since it favors their localization in response to the ligand (XCL1) produced by CTLs and NK cells and the XCR1/XCL1 axis appears indispensable in the development of efficient cytotoxic immunity[13][14]. cDC1s in turn orchestrate local anti-tumor immunity, being the main producer of CXCL9 and CXCL10, two chemokines active on CXCR3+ effector T and NK cells[8][15]. Both chemokines are considered to be crucial also in the positioning of memory CD8+ T cells in cDC1-rich areas in order to promote local T cell restimulation[16][17]. Moreover, by locally producing high amounts of IL-12, cDC1s promote CTL and NK cell cytotoxicity and IFN-γ production[5][18][19][20]. As a positive feedback loop, IFN-γ boosts IL-12 production by cDC1s and potentiates cross-presentation[18][21]. By producing CCL5, NK cells can recruit circulating cDC1s to neighboring tissues and tumors[22]. Intratumor cDC1s represent a crucial source of Flt3L a factor that sustains the viability and functions of cDC1s within the TME and promotes their local differentiation from precursor cells[23]. cDC1s not only promote CTL expansion by MHC-I-mediated Ag presentation but also promote the generation of CD4+ Th1 cells through the presentation of antigens on MHC class II[24]. The antitumor functions of cDC1s may also be supported by pDCs [25]. pDCs are a major source of type I IFN, a potent activator of antigen cross-presentation and CD8+ T cell antitumor response[26][27]. T cell-mediated anti-tumor response may also be induced by cytosolic DNA from dying tumor cells through the activation of cGAS/STING-mediated type I IFN production[28]. In summary, the interaction of cDC1s with components of both innate and adaptive immunity represents an efficient and versatile system for CTL activation and antitumor functions.
2. cDC2s
The role of cDC2s in cancer immunology is apparently more limited compared to that of cDC1s. This is possibly due to the lack of selective membrane markers that allow the clear identification of these cells in pathological contexts and the availability of few preclinical studies. Even if cDC2s are in many aspects less efficient than cDC1s, such as in taking up tumor antigens, trafficking to draining lymph nodes, producing IL-12, and stimulating CD8+ T cells[5][7][8][12], these cells are very efficient in the presentation of MHC-II-associated tumor antigens to CD4+ T cells[29][30][31][32][33]. Activated CD4+ T cells contribute to antitumor immunity not only by concurring in CTL activation, but also through the production of IFN-γ that activates NK cells and macrophages, inhibits angiogenesis, regulates the generation of tumor stroma, and promotes direct cytolytic effects[34].
The cross-talk between T cells and DC subsets plays a crucial role at different levels. Maximal induction of the cytotoxic CD8+ CTL response requires not only cDC1s, but also involves cDC2, as shown by differential localization and spatiotemporal interactions of the two DC subsets in draining lymph nodes during viral infection[24]. A similar type of collaboration is also conceivable to happen in tumors[35][36]. During tumor growth, cDC2s were shown to be suppressed in their ability to induce differentiation of antitumor CD4+ T cells. Depletion of T regulatory (Treg) cells was shown to enhance their migration and ability to prime proinflammatory CD4+ T cells for IFN-γ production and tumor rejection[37]. Moreover, a role for tumor cDC2s in inducing activation of CD4+ T cells towards IL-17-producing T lymphocytes has also been described[29]. Th17 cells are apparently crucial for the efficacy of cDC2 vaccination because of their capacity to reprogram pro-tumoral macrophages and to reduce suppressive myeloid cells[29].
Human studies show some overlapping functions between cDC1s and cDC2s, such as IL-12 production and requirement of Flt3L for their development[38][39]. Similar to cDC1s, the number of circulating cDC2s is usually decreased in tumor patients[40]. Nevertheless, cDC2s were shown to be part of an immune signature in early lung adenocarcinoma lesions[41]. In breast cancer lesions, the expression of costimulatory molecules by cDC2s were differentially regulated in relation to cancer subtype, being higher in triple negative than in luminal breast cancers[42]. cDC2s do not express a unique gene signature. Indeed, cDC2 share a common signature with monocytes, with only a few genes selectively expressed, such as CCL22, a gene that encodes for a chemokine active on CCR4+ T cells[42]. In a different study, the gene CD207 (that encodes for langerin) was identified as a specific marker for tumor-associated cDC2s, both in mouse and human lung cancers[43].
PDCs may favor antitumor immunity mostly through the production of IFN-α, an inhibitor of tumor cell proliferation, angiogenesis, and metastasis[44]. PDCs possess direct cytotoxic activity through the expression of TRAIL and Granzyme B[45][46]; this function was reported both in in vitro and in vivo experimental models[47][48]. TLR7-mediated production of type IFN I is essential for the regulation of TRAIL and Granzyme B secretion by pDCs via IFNAR1 signaling[46][47][48] and inhibition of this pathway by an anti-BDCA-2 moAb resulted in decreased TRAIL-mediated cytotoxic functions[45]. PDCs can exert also indirect antitumor effects through the CCR5-mediated recruitment of NK cells and the OX40L-mediated induction of IFN-γ[49]. In head and neck squamous carcinoma, a morphologically, functionally, and transcriptionally unique pDC subset expressing high levels of OX40 was described for being able to synergize with cDCs in generating potent tumor antigen specific CD8+ T cell responses[50].
Because of the high degree of overlap with other myeloid cells, the relevance of moDCs in human tumors is unclear. MoDCs may have an important role in anti-tumor response promoting the proliferation of naïve CD8+ T cells[51]. In preclinical studies, moDCs were found to play a crucial role in mediating immune responses during chemotherapy, T cell adoptive therapy, and cell vaccination[52][53][54]. The main mechanisms exploited by DC subsets to perform efficient anti-tumor immune responses are summarized in Figure 1.
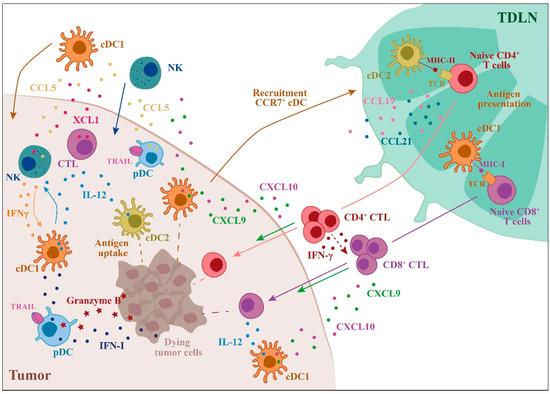
Figure 1. Role of dendritic cells (DC) subsets in the regulation of the anti-tumor immune response. The main events that involve DC subsets and contribute to a robust anti-tumor response are illustrated. The anti-tumor action of DC subsets starts with the uptake of tumor antigens followed by DC recruitment to the draining lymph nodes, where antigen presentation to T cells occurs. cDC1s are specialized in tumor antigen cross-presentation to CD8+ T cells, leading to tumor-specific cytotoxic T cell (CTL) differentiation, whereas cDC2s are the most efficient CD4+ T cell activators. In the tumor microenvironment (TME), DCs induce the recruitment and activation of NK cells and CTLs through the production of IL-12 and other chemokines/cytokines. Plasmacytoid DCs (PDCs) can kill tumor cells through the expression of TRAIL and Granzyme B (TDLN = tumor draining lymph node).[55]
References
- Vu Manh, T.P.; Bertho, N.; Hosmalin, A.; Schwartz-Cornil, I.; Dalod, M. Investigating Evolutionary Conservation of Dendritic Cell Subset Identity and Functions. Front. Immunol. 2015, 6, 260.
- Cancel, J.C.; Crozat, K.; Dalod, M.; Mattiuz, R. Are Conventional Type 1 Dendritic Cells Critical for Protective Antitumor Immunity and How? Front. Immunol. 2019, 10, 9.
- Bottcher, J.P.; Reis, E.S.C. The Role of Type 1 Conventional Dendritic Cells in Cancer Immunity. Trends Cancer 2018, 4, 784–792.
- Hildner, K.; Edelson, B.T.; Purtha, W.E.; Diamond, M.; Matsushita, H.; Kohyama, M.; Calderon, B.; Schraml, B.U.; Unanue, E.R.; Diamond, M.S.; et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science 2008, 322, 1097–1100.
- Broz, M.L.; Binnewies, M.; Boldajipour, B.; Nelson, A.E.; Pollack, J.L.; Erle, D.J.; Barczak, A.; Rosenblum, M.D.; Daud, A.; Barber, D.L.; et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 2014, 26, 638–652.
- Sanchez-Paulete, A.R.; Cueto, F.J.; Martinez-Lopez, M.; Labiano, S.; Morales-Kastresana, A.; Rodriguez-Ruiz, M.E.; Jure-Kunkel, M.; Azpilikueta, A.; Aznar, M.A.; Quetglas, J.I.; et al. Cancer Immunotherapy with Immunomodulatory Anti-CD137 and Anti-PD-1 Monoclonal Antibodies Requires BATF3-Dependent Dendritic Cells. Cancer Discov. 2016, 6, 71–79.
- Salmon, H.; Idoyaga, J.; Rahman, A.; Leboeuf, M.; Remark, R.; Jordan, S.; Casanova-Acebes, M.; Khudoynazarova, M.; Agudo, J.; Tung, N.; et al. Expansion and Activation of CD103(+) Dendritic Cell Progenitors at the Tumor Site Enhances Tumor Responses to Therapeutic PD-L1 and BRAF Inhibition. Immunity 2016, 44, 924–938.
- Spranger, S.; Dai, D.; Horton, B.; Gajewski, T.F. Tumor-Residing Batf3 Dendritic Cells Are Required for Effector T Cell Trafficking and Adoptive T Cell Therapy. Cancer Cell 2017, 31, 711–723.e4.
- Theisen, D.J.; Davidson, J.T.T.; Briseno, C.G.; Gargaro, M.; Lauron, E.J.; Wang, Q.; Desai, P.; Durai, V.; Bagadia, P.; Brickner, J.R.; et al. WDFY4 is required for cross-presentation in response to viral and tumor antigens. Science 2018, 362, 694–699.
- Alloatti, A.; Rookhuizen, D.C.; Joannas, L.; Carpier, J.M.; Iborra, S.; Magalhaes, J.G.; Yatim, N.; Kozik, P.; Sancho, D.; Albert, M.L.; et al. Critical role for Sec22b-dependent antigen cross-presentation in antitumor immunity. J. Exp. Med. 2017, 214, 2231–2241.
- Derek J. Theisen; Stephen T. Ferris; Carlos G Briseño; Nicole Kretzer; Arifumi Iwata; Kenneth M. Murphy; Theresa L. Murphy; Batf3-Dependent Genes Control Tumor Rejection Induced by Dendritic Cells Independently of Cross-Presentation.. Cancer Immunology Research 2018, 7, 29-39, 10.1158/2326-6066.CIR-18-0138.
- Edward W. Roberts; Miranda L. Broz; Mikhail Binnewies; Mark B. Headley; Amanda E. Nelson; Denise M. Wolf; Tsuneyasu Kaisho; Dusan Bogunovic; Nina Bhardwaj; Matthew F. Krummel; et al. Critical Role for CD103 + /CD141 + Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell 2016, 30, 324-336, 10.1016/j.ccell.2016.06.003.
- Kroczek, R.A.; Henn, V. The Role of XCR1 and its Ligand XCL1 in Antigen Cross-Presentation by Murine and Human Dendritic Cells. Front. Immunol. 2012, 3, 14.
- Matsuo, K.; Kitahata, K.; Kawabata, F.; Kamei, M.; Hara, Y.; Takamura, S.; Oiso, N.; Kawada, A.; Yoshie, O.; Nakayama, T. A Highly Active Form of XCL1/Lymphotactin Functions as an Effective Adjuvant to Recruit Cross-Presenting Dendritic Cells for Induction of Effector and Memory CD8(+) T Cells. Front. Immunol. 2018, 9, 2775.
- Mikucki, M.E.; Fisher, D.T.; Matsuzaki, J.; Skitzki, J.J.; Gaulin, N.B.; Muhitch, J.B.; Ku, A.W.; Frelinger, J.G.; Odunsi, K.; Gajewski, T.F.; et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat. Commun. 2015, 6, 7458.
- Kastenmuller, W.; Brandes, M.; Wang, Z.; Herz, J.; Egen, J.G.; Germain, R.N. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity 2013, 38, 502–513.
- Michel Enamorado; Salvador Iborra; Elena Priego; Francisco J. Cueto; Juan A. Quintana; Sarai Martínez-Cano; Ernesto Mejías-Pérez; Mariano Esteban; Ignacio Melero; Andrés Hidalgo; et al.David Sancho Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8+ T cells. Nature Communications 2017, 8, 16073, 10.1038/ncomms16073.
- Mittal, D.; Vijayan, D.; Putz, E.M.; Aguilera, A.R.; Markey, K.A.; Straube, J.; Kazakoff, S.; Nutt, S.L.; Takeda, K.; Hill, G.R.; et al. Interleukin-12 from CD103(+) Batf3-Dependent Dendritic Cells Required for NK-Cell Suppression of Metastasis. Cancer Immunol. Res. 2017, 5, 1098–1108.
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014, 26, 623–637.
- Greyer, M.; Whitney, P.G.; Stock, A.T.; Davey, G.M.; Tebartz, C.; Bachem, A.; Mintern, J.D.; Strugnell, R.A.; Turner, S.J.; Gebhardt, T.; et al. T Cell Help Amplifies Innate Signals in CD8(+) DCs for Optimal CD8(+) T Cell Priming. Cell Rep. 2016, 14, 586–597.
- Florence Deauvieau; Vincent Ollion; Anne-Claire Doffin; Carole Achard; Jean-François Fonteneau; Estelle Verronese; Isabelle Durand; Raffaella Ghittoni; Jacqueline Marvel; Colette Dezutter-Dambuyant; et al.Thierry WalzerHenri VieIvan PerrotNadège GoutagnyChristophe CauxJenny Valladeau-Guilemond Human natural killer cells promote cross-presentation of tumor cell-derived antigens by dendritic cells. International Journal of Cancer 2014, 136, 1085-1094, 10.1002/ijc.29087.
- Jan P. Böttcher; Eduardo Bonavita; Probir Chakravarty; Hanna Blees; Mar Cabeza-Cabrerizo; Stefano Sammicheli; Neil C. Rogers; Erik Sahai; Santiago Zelenay; Caetano Reis E Sousa; et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022-1037.e14, 10.1016/j.cell.2018.01.004.
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191.
- Hor, J.L.; Whitney, P.G.; Zaid, A.; Brooks, A.G.; Heath, W.R.; Mueller, S.N. Spatiotemporally Distinct Interactions with Dendritic Cell Subsets Facilitates CD4+ and CD8+ T Cell Activation to Localized Viral Infection. Immunity 2015, 43, 554–565.
- Brewitz, A.; Eickhoff, S.; Dahling, S.; Quast, T.; Bedoui, S.; Kroczek, R.A.; Kurts, C.; Garbi, N.; Barchet, W.; Iannacone, M.; et al. CD8(+) T Cells Orchestrate pDC-XCR1(+) Dendritic Cell Spatial and Functional Cooperativity to Optimize Priming. Immunity 2017, 46, 205–219.
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 2011, 208, 1989–2003.
- Fuertes, M.B.; Kacha, A.K.; Kline, J.; Woo, S.R.; Kranz, D.M.; Murphy, K.M.; Gajewski, T.F. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 2011, 208, 2005–2016.
- Marcus, A.; Mao, A.J.; Lensink-Vasan, M.; Wang, L.; Vance, R.E.; Raulet, D.H. Tumor-Derived cGAMP Triggers a STING-Mediated Interferon Response in Non-tumor Cells to Activate the NK Cell Response. Immunity 2018, 49, 754–763.e4.
- Laoui, D.; Keirsse, J.; Morias, Y.; Van Overmeire, E.; Geeraerts, X.; Elkrim, Y.; Kiss, M.; Bolli, E.; Lahmar, Q.; Sichien, D.; et al. The tumour microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumour immunity. Nat. Commun. 2016, 7, 13720.
- Qin, Z.; Blankenstein, T. CD4+ T cell--mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity 2000, 12, 677–686.
- Quezada, S.A.; Simpson, T.R.; Peggs, K.S.; Merghoub, T.; Vider, J.; Fan, X.; Blasberg, R.; Yagita, H.; Muranski, P.; Antony, P.A.; et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010, 207, 637–650.
- Kim, H.J.; Cantor, H. CD4 T-cell subsets and tumor immunity: the helpful and the not-so-helpful. Cancer Immunol. Res. 2014, 2, 91–98. ]
- Kennedy, R.; Celis, E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol. Rev. 2008, 222, 129–144.
- Jannie Borst; Tomasz Ahrends; Nikolina Bąbała; Cornelis J. M. Melief; Wolfgang Kastenmüller; CD4+ T cell help in cancer immunology and immunotherapy. Nature Reviews Immunology 2018, 18, 635-647, 10.1038/s41577-018-0044-0.
- Amigorena, S. Helping the Help for CD8+ T Cell Responses. Cell 2015, 162, 1210–1212.
- Janssen, E.M.; Lemmens, E.E.; Wolfe, T.; Christen, U.; von Herrath, M.G.; Schoenberger, S.P. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003, 421, 852–856.
- Mikhail Binnewies; Adriana M. Mujal; Joshua L. Pollack; Alexis J. Combes; Emily A. Hardison; Kevin C. Barry; Jessica Tsui; Megan K. Ruhland; Kelly Kersten; Marwan A. Abushawish; et al.Marko SpasicJonathan P. GiurintanoVincent ChanAdil I. DaudPatrick HaChun J. YeEdward W. RobertsMatthew F. Krummel Unleashing Type-2 Dendritic Cells to Drive Protective Antitumor CD4+ T Cell Immunity. Cell 2019, 177, 556-571.e16, 10.1016/j.cell.2019.02.005.
- Leal Rojas, I.M.; Mok, W.H.; Pearson, F.E.; Minoda, Y.; Kenna, T.J.; Barnard, R.T.; Radford, K.J. Human Blood CD1c(+) Dendritic Cells Promote Th1 and Th17 Effector Function in Memory CD4(+) T Cells. Front. Immunol. 2017, 8, 971.
- Anandasabapathy, N.; Breton, G.; Hurley, A.; Caskey, M.; Trumpfheller, C.; Sarma, P.; Pring, J.; Pack, M.; Buckley, N.; Matei, I.; et al. Efficacy and safety of CDX-301, recombinant human Flt3L, at expanding dendritic cells and hematopoietic stem cells in healthy human volunteers. Bone Marrow Transplant. 2015, 50, 924–930.
- Pinzon-Charry, A.; Ho, C.S.; Maxwell, T.; McGuckin, M.A.; Schmidt, C.; Furnival, C.; Pyke, C.M.; Lopez, J.A. Numerical and functional defects of blood dendritic cells in early- and late-stage breast cancer. Br. J. Cancer 2007, 97, 1251–1259.
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e17.
- Michea, P.; Noel, F.; Zakine, E.; Czerwinska, U.; Sirven, P.; Abouzid, O.; Goudot, C.; Scholer-Dahirel, A.; Vincent-Salomon, A.; Reyal, F.; et al. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat. Immunol. 2018, 19, 885–897.
- Zilionis, R.; Engblom, C.; Pfirschke, C.; Savova, V.; Zemmour, D.; Saatcioglu, H.D.; Krishnan, I.; Maroni, G.; Meyerovitz, C.V.; Kerwin, C.M.; et al. Single-Cell Transcriptomics of Human and Mouse Lung Cancers Reveals Conserved Myeloid Populations across Individuals and Species. Immunity 2019, 50, 1317–1334.e10.
- Koucky, V.; Boucek, J.; Fialova, A. Immunology of Plasmacytoid Dendritic Cells in Solid Tumors: A Brief Review. Cancers (Basel) 2019, 11, 470.
- Riboldi, E.; Daniele, R.; Cassatella, M.A.; Sozzani, S.; Bosisio, D. Engagement of BDCA-2 blocks TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. Immunobiology 2009, 214, 868–876.
- Salvi, V.; Vermi, W.; Cavani, A.; Lonardi, S.; Carbone, T.; Facchetti, F.; Bosisio, D.; Sozzani, S. IL-21 May Promote Granzyme B-Dependent NK/Plasmacytoid Dendritic Cell Functional Interaction in Cutaneous Lupus Erythematosus. J. Investig. Dermatol. 2017, 137, 1493–1500.
- Drobits, B.; Holcmann, M.; Amberg, N.; Swiecki, M.; Grundtner, R.; Hammer, M.; Colonna, M.; Sibilia, M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J. Clin. Investig. 2012, 122, 575–585.
- Jing Wu; Shuang Li; Yang Yang; Shan Zhu; Mingyou Zhang; Yuan Qiao; Yong-Jun Liu; Jingtao Chen; TLR-activated plasmacytoid dendritic cells inhibit breast cancer cell growth in vitro and in vivo. Oncotarget 2016, 8, 11708-11718, 10.18632/oncotarget.14315.
- Chengwen Liu; Yanyan Lou; Gregory Lizée; Hong Qin; Shujuan Liu; Brian Rabinovich; Grace J. Kim; Yi-Hong Wang; Yang Ye; Andrew G. Sikora; et al.Willem W. OverwijkYong-Jun LiuGang WangPatrick Hwu Plasmacytoid dendritic cells induce NK cell–dependent, tumor antigen–specific T cell cross-priming and tumor regression in mice. Journal of Clinical Investigation 2008, 118, 1165-1175, 10.1172/jci33583.
- Kate O. Poropatich; Donye Dominguez; Wen-Ching Chan; Jorge Andrade; Yuanyuan Zha; Brian D. Wray; Jason Miska; Lei Qin; Lisa E. Cole; Sydney Coates; et al.Urjeet A. PatelSandeep SamantBin Zhang OX40+ plasmacytoid dendritic cells in the tumor microenvironment promote antitumor immunity. Journal of Clinical Investigation 2020, 130, 3528-3542, 10.1172/jci131992.
- Kuhn, S.; Yang, J.; Ronchese, F. Monocyte-Derived Dendritic Cells Are Essential for CD8(+) T Cell Activation and Antitumor Responses After Local Immunotherapy. Front. Immunol. 2015, 6, 584.
- Ma, Y.; Adjemian, S.; Mattarollo, S.R.; Yamazaki, T.; Aymeric, L.; Yang, H.; Portela Catani, J.P.; Hannani, D.; Duret, H.; Steegh, K.; et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013, 38, 729–741.
- Pfirschke, C.; Engblom, C.; Rickelt, S.; Cortez-Retamozo, V.; Garris, C.; Pucci, F.; Yamazaki, T.; Poirier-Colame, V.; Newton, A.; Redouane, Y.; et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity 2016, 44, 343–354.
- Diao, J.; Gu, H.; Tang, M.; Zhao, J.; Cattral, M.S. Tumor Dendritic Cells (DCs) Derived from Precursors of Conventional DCs Are Dispensable for Intratumor CTL Responses. J. Immunol. 2018, 201, 1306–1314.
- Annalisa Del Prete; Francesca Sozio; Ilaria Barbazza; Valentina Salvi; Laura Tiberio; Mattia Laffranchi; Angela Gismondi; Daniela Bosisio; Tiziana Schioppa; Silvano Sozzani; et al. Functional Role of Dendritic Cell Subsets in Cancer Progression and Clinical Implications. International Journal of Molecular Sciences 2020, 21, 3930, 10.3390/ijms21113930.




