Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Debasis Chakrabarty | + 2920 word(s) | 2920 | 2021-05-09 13:24:23 | | | |
| 2 | Lily Guo | Meta information modification | 2920 | 2021-05-28 03:34:20 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chakrabarty, D. Cr and Plants. Encyclopedia. Available online: https://encyclopedia.pub/entry/10181 (accessed on 08 February 2026).
Chakrabarty D. Cr and Plants. Encyclopedia. Available at: https://encyclopedia.pub/entry/10181. Accessed February 08, 2026.
Chakrabarty, Debasis. "Cr and Plants" Encyclopedia, https://encyclopedia.pub/entry/10181 (accessed February 08, 2026).
Chakrabarty, D. (2021, May 27). Cr and Plants. In Encyclopedia. https://encyclopedia.pub/entry/10181
Chakrabarty, Debasis. "Cr and Plants." Encyclopedia. Web. 27 May, 2021.
Copy Citation
Extensive industrial activities resulted in an increase in chromium (Cr) contamination in the environment. The toxicity of Cr severely affects plant growth and development. Cr is also recognized as a human carcinogen that enters the human body via inhalation or by consuming Cr-contaminated food products.
chromium (Cr)
1. Introduction
Heavy metal contamination is becoming a serious environmental issue worldwide for the past few decades due to their increased concentration beyond the permissible limit. Chromium (Cr) is a naturally occurring heavy metal and the 17th most abundant element in the earth’s mantle [1]. Although Cr is required in trace amounts in plants and animals, at higher concentrations it serves as a major contaminant to the environment. Natural sources, as well as various anthropogenic activities, are responsible for the release of Cr in the soil, air, and water which have ultimately led to Cr pollution globally [2]. Regarding the consequences of the contamination of food lands and the drinking system, Cr easily enters into the food chain and affects the health of all life forms directly or indirectly [3][4][5].
In plants, the toxic effects of Cr are also evident, showing symptoms such as delay in seed germination, damaged roots and reduction of root growth, reduced biomass, reduction in plant height, photosynthetic impairment, membrane damage, leaf chlorosis, necrosis, low grain yield and eventually plant death [6]. Cr is a fairly active metal and reacts easily with environmental oxygen. Different oxidation states of Cr have been reported ranging from 0 to +6. The trivalent Cr(III) and hexavalent Cr(VI) are the most stable forms of Cr in nature. Also, Cr(VI) shows higher toxicity than Cr(III) due to its higher solubility and mobility in the water system [1]. Both valence states of Cr i.e., Cr(III) and Cr(VI) are taken up by plants [7]. Cr(VI) is actively taken up into the plant cells by sulfate carriers [8]. On the other hand, Cr(III) enters passively by the cation exchange sites of the plant cell walls [9]. Moreover, the carboxylic acids present in the root exudates facilitate the Cr solubilization, and thus its uptake into the plants [10].
Phytoremediation is a rapidly growing field of research for heavy metal contaminated regions. There are various processes of phytoremediation such as phytovolatilization, phytoextraction, phytostabilization, and hyperaccumulation [11][12]. Numerous research studies have shown that many plant species are capable of effectively removing Cr from contaminated regions which could be useful for the phytoremediation process [13]. Cr hyperaccumulators, with their associated microflora, have been used around the industrial effluent sites to remove the excess toxic Cr, as well as organic matters. Plant-microbe interaction is also one of the efficient strategies for Cr detoxification due to its high efficiency, low cost, and eco-friendly nature [14][15]. At the molecular level, various pathways involved in Cr detoxification have been deciphered to provide tolerance in response to Cr toxicity. Cr stress activates Reactive Oxygen Species (ROS) signaling, antioxidant responses, defense proteins such as phytochelatins (PCs), metallothionine (MTs), and glutathione-S-transferases (GSTs) followed by phytosequestration and compartmentalization thereby accelerating the bio-accumulating potential of the plants [16][17][18]. One approach could be developing transgenics by upregulating genes responsible for Cr uptake, transport, and sequestration to enhance the tolerance and accumulation rate of the plant.
Taking all into consideration, this review addresses Cr sources, effects, uptake, translocation, and subcellular distribution in plants. We also discussed Cr detoxification remedies in plants through phytoremediation and biotechnological approaches. Also, the molecular events underlying Cr toxicity and the defense signal transduction have been discussed. Overall, the review summarizes the recent development of sustainable approaches for Cr detoxification in the environment.
2. Cr Occurrence and Sources
The distribution of Cr(III) and Cr(VI) containing compounds in the environment depends on the presence of oxidizing or reducing compounds, redox potential, the formation of Cr(III) complexes or insoluble Cr(III) salts, the kinetics of the redox reactions, pH, and the total chromium concentration [2]. In the environment, Cr(VI) occurs mostly as chromate ions (Cr2O4 and Cr2O7) or chromic acid (H2CrO4), whereas Cr(III) occurs in the form of oxides, hydroxides(Cr(OH)n(3−n)+), and sulfates [2][9]. The chemical structure of various existing forms of Cr(VI) and Cr(III) in the environment has been given in Figure 1. Cr is also found in the air where it occurs in the form of aerosols. Cr(VI) has been reported to be 0.01–30% of the total Cr present in the air. Tobacco smokes result in a total Cr level of around 1000 ng/m3 in indoor air, i.e., 10–400 times higher than outdoor levels [2]. The average level of Cr in surface water, seawater, and rainwater is around 0.001–0.010 mg/L, 0.00004–0.0005 mg/L, and 0.0002–0.001 mg/L, respectively [2][5].
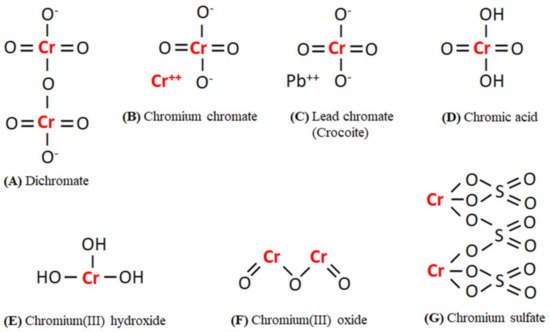
Figure 1. The chemical structures of existing forms of Cr(VI) (A–D) and Cr(III) (E–G) in the environment. Abbreviations: Cr, Chromium; O, Oxygen; H, Hydrogen; Pb, Lead; S, Sulphur.
Both natural, as well as anthropogenic sources, contribute to total Cr toxicity in the environment. Mineral leaching accounts for the natural origin of Cr in groundwater that is dominated by Cr(VI). However, above 70% of total Cr in the environment is due to the anthropogenic pollutants from effluent streams from paper and pulp mills, non-ferrous base metal smelters, leather tanning industries, refineries, releases from thermal generating stations, and urban stormwater runoff [2]. Among all sources, tanning industries play a vital role as they use Cr2(SO4)3 as a tanning agent, of which 30–40% is unused and discharged into the environment via tannery effluent [19]. This inefficient usage of Cr brings about water defilement, carrying 500–1000 mg/L Cr from the current high-exhaust chrome tanning approaches and as high as 1500–3000 mg/L Cr from the conventionalmethodologies [20].
In nature, Cr(III) predominates in soil and occurs in small amounts in rocks. Worldwide, the average concentration of Cr in the soil is dependent on the bedrock and range between 10–100 mg/kg with an average concentration of 60 mg/kg [21][22]. The estimated acceptable level of Cr in the soil for environmental safety and human health is 64 mg/kg that is difficult to maintain because of the discharge of the anthropogenic pollutants [23]. Cr(III) is present in the form of chromite [FeCr(III)2O4], bentorite Ca6(Cr,Al)2(SO4)3, and vauquelinite (CuPb2CrO4PO4OH) in rocks from where it can be released into groundwater via weathering and erosion [1]. However, Cr(VI) seldom occurs naturally and is released from anthropogenic sources. The only natural source of Cr(VI) is an uncommon mineral, crocoite (PbCrO4). High Cr(VI) concentration in California (0.006–0.036 mg/L in aquifer) [24], Italy (0.005–0.073 mg/L in groundwater) [25], Mexico (270–4120 mg/kg in rocks) [26], Zimbabwe (310–8600 mg/kg in soil) [27], Brazil (0.0025–0.11 mg/L in groundwater) [28], Indonesia (50–90 mg/kg in river sediment) and Japan (510–1420 mg/kg in river sediment) [29] were reported owing to the contact of water with ultramafic rocks and soil such as ophiolites, dunites, and serpentinite. Cr emitted into the air through sources such as Cr-based automotive catalytic convertors, tobacco smoke, and coal combustion also end up in soils [9].
Food is also a source of Cr exposure to the living population, containing a total Cr level spanning between <0.0005 to 1.3 mg/kg. However, fresh food is reported to be very low in Cr i.e., 0.02–0.05 µg/kg. As the food is recognized as a reducing medium, the total Cr found in it can be classed as Cr(III). Food items such as meat, seafood, fish, cereal products, black pepper, tea, cheese, some fruits, vegetables, and wheat germ have high Cr content (>0.1 mg/kg). Beer, spirits, and wine have total Cr concentrations of around 0.45, 0.135, and 0.30 mg/L respectively. Utensils, especially made up of stainless steel, can also add to total Cr concentrations in food [2].
3. The Effects of Cr on Plants
Oxidation state is the deciding factor of the toxicologic or physiologic effects of Cr. Higher concentrations of Cr(III) might be inhibitory to plant growth and development. On the contrary, Cr(VI) is highly toxic for plants and inhibits various morphological, physiological, and metabolic activities in plants, and may even lead to their complete damage [30][31].
3.1. Effects on Seed Germination
Seed germination in a Cr enriched environment depends on the plant’s ability to withstand Cr toxicity. Excess Cr limits the seed germination rate of Cucumis melo L. (>10 mg/L Cr(III) in culture medium) [32], Hibiscus esculentus (>50 mg/kg Cr(VI) in soil) [6], Triticum aestivum (>25 mg/L Cr(VI) in the nutrient solution) [33], and Echinochola colona (2.5 mg/L Cr(VI) in the nutrient solution) [34]. Two freshwater plants, Lemna minor, and Pistia stratiotes when grown in nutrient solution containing 1, 5, or 10 mg/L of Cr(VI) resulted in retardation of the growth [35]. The seed germination of Salvia sclarea L. was inhibited when treated with different concentrations of Cr(VI) ranging from 1–10 mg/L [36].
3.2. Effects on Shoot Growth
Cr exposure affects the shoot length and biomass of the plant [37]. With the increase in Cr(VI) concentration, the shoot length of Helianthus annus L. was found to be decreased [38]. In Allium cepa, Cr(III) concentration higher than 100 mg/L, reduced the shoot growth [39]. In Citrus aurantium L., an increase in Cr(III) concentration in the soil resulted in 39.3% and 90.4% reduced shoot length at 50 mg/kg and 200 mg/kg of Cr, respectively [40]. The small stem with a slow growth rate was observed in Camellia sinensis on exposure to 600 mg/kgCr(III) [41]. It was reported that a lower Cr(VI) concentration (0.05 mg/L) increases the shoot length, while a higher Cr(VI) concentration (1 mg/L) retarded the shoot length and weight of Myriophyllum spicatum [30].
3.3. Effects on Root Growth
Being a primary organ for nutrient uptake, the roots are directly associated with Cr uptake and thus serve as a principal site of Cr toxicity in plants. In a greenhouse experiment, root growth of sour orange seedlings was analyzed under 50–200 mg/kg Cr(III) concentrations and the significant reduction in the root length was found at 200 mg/kg Cr(III) [40]. In Pistia stratiotes, Cr at low concentration (0.25 mg/L) promotes root length, laminal length, and breadth as compared to control but at higher concentration (2.5 mg/L), the root length was found to be decreased [42]. C. sinensis roots were severely affected on high Cr concentration (600 mg/kg) leading to a reduction in roots dry weight [41]. Cr(VI) at a concentration of 6, 12, 18,and 24 mg/kg caused a reduction in root length and root dry weight in T. aestivum [43]. Sundaramoorty et al. [44] reported decreased root growth of field grown Oryza sativa L. treated with 200 mg/L Cr(VI) in distilled water. Also, the thin and brittle roots of Pisum sativum were observed under high Cr(VI) concentration (>1000 mg/L) [45].
3.4. Effects on Total Leaf Area
Plant takes up Cr via the root and it gets transported to the upper plant parts via various transporters. The leaf is one of the important organs of a plant that performs photosynthesis. The total leaf area is one of the determining factors for photosynthesis. The Cr(VI) toxicity affects the total leaf area and also showed a 50% reduction in leaf number per plant in O. sativa [44]. Brassica oleracea showed a decrease in leaf size, wilting, and chlorosis when grown in refined sand with complete nutritional media under 0.5 mM Cr(III) toxicity [46]. In Lolium perenne L., a noticeable wilting has been observed under 0.50mM Cr(VI) prepared in nutrient media [47]. Leaf chlorosis and leaf necrosis have been reported in Saccharum officinarum on Cr(VI) exposure of 40 mg/kg and 80 mg/kg, respectively [48]. Phaseolus vulgaris showed a reduction in leaf biomass under 0.01 mM Cr(III) treatment in nutrient solution [49]. The decreased number of leaves was reported in Prosopis laevigatar under 3.4 mM Cr(VI) toxicity in nutrient media [50].
3.5. Effects on Grain Yield
The yield and productivity of crops are affected by Cr exposure as it exerts an adverse impact on biochemical and physiological processes. The reduction in yield of Hordeum vulgare and Zea mays was observed at a concentration of 100 or 300 mg/kg of Cr [51]. In Daucus carota, no harvestable yield was obtained on 270 or 810 kg/ha of Cr(VI) application [52]. In O. sativa, Cr(VI) application under 200 mg/L caused a decrease in grain weight and 80% loss [44].
3.6. Anatomical Changes
Cr induces structural and ultrastructural alterations in plant organs. In Vigna radiata, Cr exposure led to change in the epidermis, cortex, and stele in its stem [53]. The addition of 0.60 mM Cr(VI) in nutrient solution media caused less wax deposition and wide stomatal opening in the leaves of Phyllanthus amarus [54]. The number of palisade and spongy parenchyma cells were found to be decreased in fronds of Pteris vittata at 500 mg/kg of Cr(VI) and 1000 mg/kg of Cr(III) [55]. The roots of Scirpus lacustris L. showed an increase in pith and cortical tissue layer proportion under two different concentrations of Cr(IV) i.e., 4 and 8 mg/L [56]. Mentha aquatica roots showed structural changes such as damaged root cap, loss of root hairs, inhibition in lateral roots formation on exposure of Cr(VI) at a concentration of 20 or 40 mg/L [57].
3.7. Physiological Changes
Exposure of the elevated concentration of heavy metals leads to degradation of photosynthetic pigments that are responsible for deficiency in light-harvesting capacity. The 0.05 mg/L of Cr(VI) exposure resulted in a reduction in photosynthetic rate in M. spicatum [58]. Some species like Citrus aurantium [40], Najas indica, Vallisneria spiralis, and Alternanthera sessilis showed decrease in chlorophyll (Chl) content under Cr toxicity [30]. In T. aestivum, net photosynthetic rate (Pn) was reduced with a gradual increase in Cr exposure time. Less chlorophyll content was found in Cr(VI) treated wheat in comparison to control plants. Interestingly, it was due to the more reduction in Chl b than Chl a content [59]. However, in the case of Chlorella pyrenoidosa, both Chl a and b content were decreased under 0.1–50 mg/L of Cr(VI) [60]. The light-harvesting complex of the photosystem II was extensively affected in T. aestivum after treatment with 0.10 mM, 0.20 mM, and 0.30 mM of Cr(VI) [61].
In higher plants, Cr inhibits mitochondrial electron transport leading to higher ROS generation that causes oxidative stress, pigment, and chloroplast alterations [62]. Chromosomal impairment by Cr(VI) treatments (0.01 mM and 0.1 mM) has been reported in Amaranthus viridis plant tissues, where it regulated the activity of calmodulin that was further responsible for the activation of many key enzymes such as phospholipase and nicotinamide adenine dinucleotide kinase, involved in the chromosomal movement [63]. Furthermore, Cr has also been observed to affect the concentrations of free polyamines in Avena sativa, Brassica napus, and H. vulgare seedlings after treatment with 100 mg/L of Cr(III) for 1–14 days [62][64].
Wilting caused by Cr has been reported in various crops and plant species, but the effect of Cr on water relations of higher plants is less studied. Chatterjee and Chatterjee reported a decrease in water potential, transcription rate, and relative water content in B. oleracea leaves, grown in refined sand in a glass house with 0.5 mM of Cr(VI) toxicity [46]. In Spinacea oleracea leaves, the decrease in water potential and increase in diffusive resistance led to a decrease in physiological availability of water under 0.10 and 0.40 mM of Cr(VI) toxicity [65]. Essential nutrients uptake was reduced in Spartina argentinensis on Cr(III) exposure at 1500 mg/kg [66]. Longitudinal water movement was found to be reduced in beans due to a decrease in tracheary vessel diameter on high Cr(VI) level (0.096 mM) [67].
3.8. Effects on Nutrient Balance
The soil-plant transfer index of Cr(VI) is comparatively higher than Cr(III) due to its better adsorption and high solubility in the cells [68]. Cr(VI) modulates the intracellular concentration of essential nutrients viz phosphorus (P), calcium (Ca), manganese (Mn), magnesium (Mg), potassium (K), and iron (Fe) in plants [69]. It has also been reported that mycorrhizal fungi as well as organic acid such as citric acid increases the uptake of Cr(VI) in plants [70]. The field-grown O. sativa irrigated with different concentrations of Cr(VI), (50–500 mg/kg) showed a gradual decrease in uptake of macronutrients (K, P, and nitrogen (N)) and micronutrients manganese (Mn), zinc (Zn), copper (Cu) with increased Cr concentration [44][71]. In contrast, Mg concentration increased in nucleus and mitochondria with an increase in Cr(VI) concentration in O. sativa, showing a positive correlation [72]. Dube et al. [73] demonstrated that Cr(VI) exposure increased the accumulation of P, Mn, and Zn while decreased sulfur (S) and Cu content in Citrullus vulgaris. Zea mays roots showed a decrease in Cu absorption on Cr(VI) exposure [74]. In B. oleracea, a high concentration of Cr(VI) (0.5 mM) affected the Fe concentration and translocation of Zn, Cu, S, P, Mn from the roots to other plant parts [46].
3.9. Molecular Changes
The molecular changes after the Cr stress were explored through comparative transcriptome analyses in several plants to get insight of the underlying mechanism during Cr stress. Previous reports showed that the application of Cr modulates several biological processes to lessen the phytotoxicity of Cr stress. A recent microarray analysis on Cr(III) and Cr(VI) treated O. sativa seedlings showed that Cr stress induces the transcript levels of different antioxidant enzymes ascorbate peroxidase (APX), superoxide dismutase (SOD), peroxidase (POD), and glutathione peroxidase (GPX) coding genes in treated plants in comparison to control plants [75]. Likewise, Dubey et al. [17] observed the differential expression pattern of numerous genes (1138 up-regulated and 1610 down-regulated) in O. sativa roots exposed with 0.10 mM Cr(VI). Among all the up-regulated genes, most of the genes were related to secondary metabolites biosynthesis, transporters, and xenobiotics biodegradation. Interestingly, this study also showed the modulation of the sucrose degradation pathway which could be salvage machinery in response to Cr stress. On the other hand, Cr stress also stimulates the expression of microRNAs. Some Cr(VI)-responsive miRNAs have been identified in Raphanus sativus and O. sativa [76][77].
References
- Bhalerao, S.A.; Sharma, A.S. Chromium: As an environmental pollutant. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 732–746.
- World Health Organization. Chromium in Drinking-water (No. WHO/HEP/ECH/WSH/2020.3); World Health Organization: Geneva, Switzerland, 2020.
- McNeill, L.; McLean, J.; Edwards, M.; Parks, J. State of the science of hexavalent chromium in drinking water. Water Res. 2012, 44, 5.
- Shrivastava, R.; Upreti, R.K.; Seth, P.K.; Chaturvedi, U.C. Effects of chromium on the immune sys-tem. FEMS Immunol. Med. Microbiol. 2002, 34, 1–7.
- Batayneh, A.T. Toxic (aluminum, beryllium, boron, chromium and zinc) in groundwater: Health risk assessment. Int. J. Environ. Sci. Technol. 2012, 9, 153–162.
- Amin, H.; Arain, B.A.; Amin, F.; Surhio, M.A. Phytotoxicity of chromium on germination, growth and biochemical at-tributes of Hibiscus esculentus L. Am. J. Plant Sci. 2013, 4, 41293.
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rashid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533.
- Xu, Z.-R.; Cai, M.-L.; Chen, S.-H.; Huang, X.-Y.; Zhao, F.-J.; Wang, P. High-Affinity Sulfate Transporter Sultr1;2 Is a Major Transporter for Cr(VI) Uptake in Plants. Environ. Sci. Technol. 2021, 55, 1576–1584.
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254.
- Srivastava, S.; Nigam, R.; Prakash, S.; Srivastava, M.M. Mobilization of Trivalent Chromium in Presence of Organic Acids: A Hydroponic Study of Wheat Plant (Triticum vulgare). Bull. Environ. Contam. Toxicol. 1999, 63, 524–530.
- Hakeem, K.; Sabir, M.; Ozturk, M.; Mermut, A.R. (Eds.) Soil Remediation and Plants: Prospects and Challenges; Academic Press: Cambridge, MA, USA, 2014.
- Yan, X.; Wang, J.; Song, H.; Peng, Y.; Zuo, S.; Gao, T.; Duan, X.; Qin, D.; Dong, J. Evaluation of the phytoremediation potential of dominant plant species growing in a chromium salt–producing factory wasteland, China. Environ. Sci. Pollut. Res. 2020, 27, 7657–7671.
- Banach, A.M.; Banach, K.; Stepniewska, Z. Phytoremediation as a promising technology for water and soil purification: Azollacaroliniana Willd as a case study. Acta Agrophys. 2012, 19, 241–252.
- Wu, S.; Hu, Y.; Zhang, X.; Sun, Y.; Wu, Z.; Li, T.; Lv, J.; Li, J.; Zhang, J.; Zheng, L.; et al. Chromium detoxification in arbuscular mycorrhizal symbiosis mediated by sulfur uptake and metabolism. Environ. Exp. Bot. 2018, 147, 43–52.
- Sharma, P.; Tripathi, S.; Chaturvedi, P.; Chaurasia, D.; Chandra, R. Newly isolated Bacillus sp. PS-6 assisted phytoreme-diation of heavy metals using Phragmites communis: Potential application in wastewater treatment. Bioresour. Technol. 2021, 320, 124353.
- Shanker, A.; Djanaguiraman, M.; Sudhagar, R.; Chandrashekar, C.; Pathmanabhan, G. Differential antioxidative response of ascorbate glutathione pathway enzymes and metabolites to chromium speciation stress in green gram ((L.) R.Wilczek. cv CO4) roots. Plant Sci. 2004, 166, 1035–1043.
- Dubey, S.; Misra, P.; Dwivedi, S.; Chatterjee, S.; Bag, S.K.; Mantri, S.; Asif, M.H.; Rai, A.; Kumar, S.; Shri, M.; et al. Transcriptomic and metabolomic shifts in rice roots in response to Cr (VI) stress. BMC Genom. 2010, 11, 648.
- Yu, X.-Z.; Lin, Y.-J.; Zhang, Q. Metallothioneins enhance chromium detoxification through scavenging ROS and stimulating metal chelation in Oryza sativa. Chemosphere 2019, 220, 300–313.
- Cassano, A.; Della-Pietra, L.; Drioli, E. Integrated membrane process for the recovery of chromium salts from tannery effluents. Ind. Eng. Chem. Res. 2007, 46, 6825–6830.
- Aravindhan, R.; Madhan, B.; Rao, J.R.; Nair, B.U.; Ramasami, T. Bioaccumulation of Chromium from Tannery Wastewater: An Approach for Chrome Recovery and Reuse. Environ. Sci. Technol. 2004, 38, 300–306.
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2010.
- Alloway, B.J. (Ed.) Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012.
- CEPA. Canadian Soil Quality Guidelines for the Protection of Environmental and Human Health; CEPA: Strasbourg, France, 2007.
- Gonzalez, A.R.; Ndung, U.K.; Flegal, A.R. Natural Occurrence of Hexavalent Chromium in the Aromas Red Sands Aquifer, California. Environ. Sci. Technol. 2005, 39, 5505–5511.
- Fantoni, D.; Brozzo, G.; Canepa, M.; Cipolli, F.; Marini, L.; Ottonello, G.; Zuccolini, M.V. Natural hexavalent chromium in groundwaters interacting with ophiolitic rocks. Environ. Earth Sci. 2002, 42, 871–882.
- Robles-Camacho, J.; Armienta, M. Natural chromium contamination of groundwater at León Valley, México. J. Geochem. Explor. 2000, 68, 167–181.
- Cooper, G. Oxidation and toxicity of chromium in ultramafic soils in Zimbabwe. Appl. Geochem. 2002, 17, 981–986.
- Bourotte, C.; Bertolo, R.; Almodovar, M.; Hirata, R. Natural occurrence of hexavalent chromium in a sedimentary aquifer in Urânia, State of São Paulo, Brazil. An. Acad. Bras. Ciências 2009, 81, 227–242.
- Saputro, S.; Yoshimura, K.; Matsuoka, S.; Takehara, K.; Narsito, K.; Aizawa, J.; Tennichi, Y. Speciation of dissolved chromium and the mechanisms controlling its concentration in natural water. Chem. Geol. 2014, 364, 33–41.
- Chandra, P.; Kulshreshtha, K. Chromium Accumulation and Toxicity in Aquatic Vascular Plants. Bot. Rev. 2004, 70, 313–327.
- Shanker, A.K.; Cervantes, C.; Loza-Tavera, H.; Avudainayagam, S. Chromium toxicity in plants. Environ. Int. 2005, 31, 739–753.
- Akinci, I.E.; Akinci, S. Effect of chromium toxicity on germination and early seedling growth in melon (Cucumis melo L.). Afr. J. Biotechnol. 2010, 9, 4589–4594.
- Riaz, M.; Yasmeen, T.; Arif, M.S.; Ashraf, M.A.; Hussain, Q.; Shahzad, S.M.; Rizwan, M.; Mehmood, M.W.; Zia, A.; Mian, I.A.; et al. Variations in morphological and physiological traits of wheat regulated by chromium species in long-term tannery effluent irrigated soils. Chemosphere 2019, 222, 891–903.
- Rout, G.R.; Samantaray, S.; Das, P. Effects of chromium and nickel on germination and growth in tolerant and non-tolerant populations of Echinochloacolona (L.) Link. Chemosphere 2000, 40, 855–859.
- Bassi, M.; Corradi, M.G.; Realini, M. Effects of chromium (VI) on two freshwater plants, Lemna minor and Pistia stratiotes. 1. Morphological observations. Cytobiosis 1990, 62, 27–38.
- Corradi, M.; Bianchi, A.; Albasini, A. Chromium toxicity in Salvia sclarea—I. Effects of hexavalent chromium on seed germination and seedling development. Environ. Exp. Bot. 1993, 33, 405–413.
- Ding, G.; Jin, Z.; Han, Y.; Sun, P.; Li, G.; Li, W. Mitigation of chromium toxicity in Arabidopsis thaliana by sulfur supple-mentation. Ecotoxicol. Environ. Saf. 2019, 182, 109379.
- Fozia, A.; Muhammad, A.Z.; Muhammad, A.; Zafar, M.K. Effect of chromium on growth attributes in sunflower (Helianthus annuus L.). J. Environ. Sci. 2008, 20, 1475–1480.
- Nematshahi, N.; Lahouti, M.; Ganjeali, A. Accumulation of chromium and its effect on growth of (Allium cepa cv. Hybrid). Eur. J. Exp. Biol. 2012, 2, 969–974.
- Shiyab, S. Morphophysiological Effects of Chromium in Sour Orange (Citrus aurantium L.). HortScience 2019, 54, 829–834.
- Tang, J.; Xu, J.; Wu, Y.; Li, Y.; Tang, Q. Effects of high concentration of chromium stress on physiological and bio-chemical characters and accumulation of chromium in tea plant (Camellia sinensis L.). Afr. J. Biotechnol. 2012, 11, 2248–2255.
- Kakkalameli, S.B.; Daphedar, A.; Hulakoti, N.; Patil, B.N.; Taranath, T.C. Azollafiliculoides lam as a phytotool for re-mediation of heavy metals from sewage. Int. J. Pharm. 2018, 8, 282–287.
- Ghani, A.; Khan, I.; Umer, S.; Ahmed, I.; Mustafa, I.; Mohammad, N. Response of wheat (Triticumaestivum) to exog-enously applied chromium: Effect on growth, chlorophyll and mineral composition. J. Environ. Anal. Toxicol. 2015, 5.
- Sundaramoorthy, P.; Chidambaram, A.; Ganesh, K.S.; Unnikannan, P.; Baskaran, L. Chromium stress in paddy: (i) Nutrient status of paddy under chromium stress; (ii) Phytoremediation of chromium by aquatic and terrestrial weeds. C. R. Biol. 2010, 333, 597–607.
- Rodriguez, E.; Azevedo, R.; Fernandes, P.; Santos, C.A. Cr (VI) induces DNA damage, cell cycle arrest and polyploidization: A flow cytometric and comet assay study in Pisum sativum. Chem. Res. Toxicol. 2011, 24, 1040–1047.
- Chatterjee, J.; Chatterjee, C. Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ. Pollut. 2000, 109, 69–74.
- Vernay, P.; Gauthier-Moussard, C.; Hitmi, A. Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Loliumperenne L. Chemosphere 2007, 68, 1563–1575.
- Radha, J.; Srivastava, S.; Madan, V. Influence of chromium on growth and cell division of sugarcane. Indian J. Plant Physiol. 2000, 5, 228–231.
- Poschenrieder, C.; Gunsé, B.; Barceló, J. Chromium-induced inhibition of ethylene evolution in bean (Phaseolus vulgaris) leaves. Physiol. Plant. 1993, 89, 404–408.
- Buendía-González, L.; Orozco-Villafuerte, J.; Cruz-Sosa, F.; Barrera-Díaz, C.; Vernon-Carter, E. Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresour. Technol. 2010, 101, 5862–5867.
- Golovatyj, S.; Bogatyreva, E. Effect of levels of chromium content in a soil on its distribution in organs of corn plants. In Soil Research And Use of Fertilizers; BRISSA: Minsk, Belarus, 1999; pp. 197–204.
- Biacs, P.A.; Daood, H.G.; Kadar, I. Effect of Mo, Se, Zn, and Cr treatments on the yield, element concentration, and carotenoid content of carrot. J. Agric. Food Chem. 1995, 43, 589–591.
- Ratheesh Chandra, P.; Abdussalam, A.; Nabeesa, S. Distribution of Bio-accumulated Cd and Cr in two Vigna species and the Associated Histological Variations. J. Stress Physiol. Biochem. 2010, 6, 4–12.
- Rai, V.; Mehrotra, S. Chromium-induced changes in ultramorphology and secondary metabolites of PhyllanthusamarusSchum&Thonn—An hepatoprotective plant. Environ. Monit. Assess. 2008, 147, 307–315.
- Su, Y.; Han, F.X.; Sridhar, B.M.; Monts, D.L. Phytotoxicity and phytoaccumulation of trivalent and hexavalent chromium in brake fern. Environ. Toxicol. Chem. 2005, 24, 2019–2026.
- Suseela, M.R.; Sinha, S.; Singh, S.; Saxena, R. Accumulation of Chromium and Scanning Electron Microscopic Studies in Scirpuslacustris L. Treated with Metal and Tannery Effluent. Bull. Environ. Contam. Toxicol. 2002, 68, 540–548.
- Bianchi, A.; Corradi, M.G.; Tirillini, B.; Albasini, A. Effects of Hexavalent Chromium on Mentha aquatica L. J. Herbs Spices Med. Plants 1998, 5, 3–12.
- Guilizzoni, P.; Adams, M.S.; MacGaffey, N. The effect of chromium on growth and photosynthesis of a submersed macrophyte, Myriophyllumspicatum. In Ecological Bulletins, Proceedings of the Third Oikos Conference, Copenhagen, Denmark, 30 November–2 December 1982; Publishing House of the Swedish Research Councils: Stockholm, Sweden, 1984; pp. 90–96.
- Subrahmanyam, D. Effects of chromium toxicity on leaf photosynthetic characteristics and oxidative changes in wheat (Triticum aestivum L.). Photosynthetica 2008, 46, 339–345.
- Horcsik, Z.T.; Kovacs, L.; Laposi, R.; Meszaros, I.; Lakatos, G.; Garab, G. Effect of chromium on photosystem 2 in the unicellular green alga, Chlorella pyrenoidosa. Photosynthetica 2007, 45, 65–69.
- Mathur, S.; Kalaji, H.M.; Jajoo, A. Investigation of deleterious effects of chromium phytotoxicity and photosynthesis in wheat plant. Photosynthetica 2016, 54, 185–192.
- Hauschild, M. Putrescine (1,4-Diaminobutane) as an Indicator of Pollution-Induced Stress in Higher Plants: Barley and Rape Stressed with Cr(III) or Cr(VI). Ecotoxicol. Environ. Saf. 1993, 26, 228–247.
- Zou, J.H.; Wang, M.; Jiang, W.S.; Liu, D.H. Effects of hexavalent chromium (VI) on root growth and cell division in root tip cells of Amaranthusviridis L. Pak. J. Bot. 2006, 38, 673.
- Wettlaufer, S.; Osmeloski, J.; Weinstein, L. Response of polyamines to heavy metal stress in oat seedlings. Environ. Toxicol. Chem. 1991, 10, 1083–1088.
- Gopal, R.; Rizvi, A.H.; Nautiyal, N. Chromium Alters Iron Nutrition and Water Relations of Spinach. J. Plant Nutr. 2009, 32, 1551–1559.
- Redondo-Gómez, S.; Mateos-Naranjo, E.; Vecino-Bueno, I.; Feldman, S.R. Accumulation and tolerance characteristics of chromium in a cordgrass Cr-hyperaccumulator, Spartinaargentinensis. J. Hazard. Mater. 2011, 185, 862–869.
- Vázquez, M.D.; Poschenrieder, C.; Barceló, J. Chromium VI Induced Structural and Ultrastructural Changes in Bush Bean Plants (Phaseolus vulgaris L.). Ann. Bot. 1987, 59, 427–438.
- Han, F.X.; Su, Y.; Sridhar, B.B.M.; Monts, D.L. Distribution, transformation and bioavailability of trivalent and hexavalent chromium in contaminated soil. Plant Soil 2004, 265, 243–252.
- Gardea-Torresdey, J.; Peralta-Videa, J.; Montes, M.; de la Rosa, G.; Corral-Diaz, B. Bioaccumulation of cadmium, chromium and copper by Convolvulus arvensis L.: Impact on plant growth and uptake of nutritional elements. Bioresour. Technol. 2004, 92, 229–235.
- Davies, F.T.; Puryear, J.D.; Newton, R.J.; Egilla, J.N.; SaraivaGrossi, J.A. Mycorrhizal fungi increase chromium uptake by sunflower plants: Influence on tissue mineral concentration, growth, and gas exchange. J. Plant Nutr. 2002, 25, 2389–2407.
- Ahmad, M.; Wahid, A.; Ahmad, S.S.; Butt, Z.A.; Tariq, M. Ecophysiological responses of rice (Oryza sativa L.) to hexavalent chromium. Pak. J. Bot. 2011, 43, 2853–2859.
- Zeng, F.; Ali, S.; Qiu, B.; Wu, F.; Zhang, G. Effects of chromium stress on the subcellular distribution and chemical form of Ca, Mg, Fe, and Zn in two rice genotypes. J. Plant Nutr. Soil Sci. 2010, 173, 135–148.
- Dube, B.; Tewari, K.; Chatterjee, J. Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere 2003, 53, 1147–1153.
- Mallick, S.; Sinam, G.; Mishra, R.K.; Sinha, S. Interactive effects of Cr and Fe treatments on plants growth, nutrition and oxidative status in Zea mays L. Ecotoxicol. Environ. Saf. 2010, 73, 987–995.
- Fan, W.-J.; Feng, Y.-X.; Li, Y.-H.; Lin, Y.-J.; Yu, X.-Z. Unraveling genes promoting ROS metabolism in subcellular organelles of Oryza sativa in response to trivalent and hexavalent chromium. Sci. Total. Environ. 2020, 744, 140951.
- Liu, W.; Xu, L.; Wang, Y.; Shen, H.; Zhu, X.; Zhang, K.; Chen, Y.; Yu, R.; Limera, C.; Liu, L. Transcriptome-wide analysis of chromium-stress responsive microRNAs to explore miRNA-mediated regulatory networks in radish (Raphanus sativus L.). Sci. Rep. 2015, 5, 1–17.
- Dubey, S.; Saxena, S.; Chauhan, A.S.; Mathur, P.; Rani, V.; Chakrabaroty, D. Identification and expression analysis of conserved microRNAs during short and prolonged chromium stress in rice (Oryza sativa). Environ. Sci. Pollut. Res. 2019, 27, 380–390.
More
Information
Subjects:
Environmental Sciences
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
28 May 2021
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




