
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bonglee Kim | + 2777 word(s) | 2777 | 2020-09-14 10:20:40 | | | |
| 2 | Rita Xu | -651 word(s) | 2126 | 2020-09-16 12:17:45 | | |
Video Upload Options
A natural product is a chemical compound or substance produced by a living organism—that is, found in nature.
Sequela is a result or condition that follows from a disease or illness
Cachexia is a condition that causes extreme weight loss and muscle wasting. It is a symptom of many chronic conditions, such as cancer, chronic renal failure, HIV, and multiple sclerosis.
1. Cancer Therapies and Cachexia
Cancer is a major public health problem worldwide and causes a high mortality rate of 53% [1]. Excluding non-melanoma skin cancer, 18.1 million cases of new cancer diagnosis and 9.6 million deaths were reported and the leading causes of cancer-related deaths in both sexes were lung cancer (18.4%), colorectal cancer (9.2%), stomach cancer (8.2%) and liver cancer (8.2%) [1]. To reduce the cancer morbidity, there have been various approaches to treat cancer. It includes surgery [2][3], chemotherapy [4], radiotherapy [5][6], immunotherapy [7], hormone therapy [8] and so forth.
1.1. Limitation of the Current Cancer Therapies
Some sequela can limit the benefits of these treatments. Surgery is followed by the risk of pain, infection and micro metastasis. Some surgeries also induce deterioration of the function of the organ under surgery. For example, about 75% of sixty-nine patients were reported that usual interstitial pneumonias were diagnosed after 60 lobectomies and 9 segmentectomies compared to the results of preoperative high-resolution computed tomography [9]. Chemotherapy and radiotherapy can damage normal cells and cause secondary cancer. They can also cause fatigue, anorexia, edema, fever for general disorders. There are gastrointestinal, blood, cardiac, nervous system, skin and respiratory disorders, too. These are treatment-related side effects that cannot be avoided in cancer treatment. For example, Cisplatin is known to indicate an anticancer action that suppresses the division of cancer cells by causing DNA damage of cancer cells. However, the patients who were treated with cisplatin suffer from experienced nausea [10][11] or side effects of various neurological symptoms [12] and kidney failure [12][13]. Likewise, there are also the adverse effects of radiotherapy. It is used with anticancer drugs or to reduce the size of preoperative cancer and the possibility of postoperative recurrence but there is also a high risk of side effects that cause brain metastasis [14], as in the case of brain radiation therapy.
Beyond each symptomatic side effect of those treatments, those antineoplastic drugs have been shown to induce oxidative stress which can interfere with cell cycle progression and apoptotic pathways [15]. This can lower the effect of antineoplastic activity from chemotherapy and affect normal cells. Also, radiation therapy produces oxidative stress related to reactive oxygen species (ROS) signaling, deoxyribonucleic acid (DNA) damage response, membrane lipid peroxidation, mitochondrial damage, endoplasmic reticulum stress (ER stress) and autophagy [16]. During this cellular stress, p53 may play a pivotal role and it is supposed to ameliorate radiation-induced oxidative stress [17]. As a result, certain antioxidants appear to prevent some chemotherapy-induced side effects and there are also some plants that can get involved in some mechanisms from radiation-induced oxidative stress.
1.2. Plants-Derived Drugs for Side-Effects of Cancer Therapies and Cachexia
Until now, research has been conducted on how to suppress the cancer therapeutics. However, including sequela of anti-cancer treatments, pharmacological clinical studies of cachexia have not been fully conducted. Considering patients’ treatment rates and quality of life, we should develop a way to handle them with minimal risks in the future. In spite of the complex mechanisms and symptoms related to oxidative stress, the efficacy of suppressing the side effects and cachexia is being studied with natural products including plant extracts. Therefore, those studies regarding antioxidant and anti-inflammation mechanisms of plant-derived drugs were reviewed in this study for the first time which manage side effects of cancer treatments and cachexia.
2. Cachexia and Plant Extracts
2.1. Cachexia
Patients commonly undergo not only treatment-related symptoms but also cancer-induced symptoms which can be categorized as cancer cachexia. In cancer patients, cachexia is characterized by systemic inflammation associating with decreased caloric intake, anorexia, decreased muscle strength and fatigue [18][19]. Cancer cachexia has been regarded as non-curable symptom and has devastating impact on patient’s quality of life and survival. Nevertheless, the severity tends to be underestimated [20][21]. The energy wasting has been attributed to inefficient adenosine triphosphate (ATP) production due to mitochondrial dysfunction. Dysfunctional mitochondria produce high levels of ROS causing oxidative damage to lipids and proteins with enhanced inflammation [22]. It is related to ER stress induced unfolded protein response (UPR) pathways and it causes catabolic conditions [23]. This may lead to myofibrillar protein breakdown, increased lipolysis, insulin resistance, elevated energy expenditure, reduced food intake and psychological factors related to quality of life [24]. These also do not act as a single variable but are related to the whole. This is why clinical research on cachexia treatment has been insufficient.
2.2. Clinical Difficulties in Treatment of Cachexia
Current therapies focus on palliation of symptoms rather than cure. However, therapies such as simple improvement of nutritional intake are not enough considering that mechanisms of cancer cachexia are complex and multifactorial [25]. Numerous cytokines, hormones, metabolism and neurologic factors involve in the etiology of cancer cachexia. TNF-α, IL-6 and IL-1 mimic leptin signaling and suppress orexigenic ghrelin and neuropeptide (NPY) signaling, which result in weight loss and anorexia [26]. Upregulation of myostatin, an extracellular cytokine regulating hypertrophy, was observed in the pathogenesis of muscle wasting during cachexia [27]. Fatigue is induced by serotonin dysregulation, hypothalamic-pituitary-adrenal (HPA) axis disturbance, alteration in circadian rhythms, ATP dysregulation, vagal afferent nerve activation and cytokine dysregulation [28]. Treatment should be developed based on understanding of molecular mechanisms that induce cachectic symptoms. Absence of established guideline and insufficient clinical evidence for cancer cachexia treatment are another difficulty. Appetite stimulants, currently used to supplement calories and improve anorexia, do not always lead to weight gain. Agents affecting cachectic mediators or signaling pathways such as EPA, β-Hydroxy-β-methylbutyrate (HMB), Thalidomide, NSAIDs are also being used but further studies are required to confirm their efficacies [29]. Thus, the further research and clinical practice of cancer cachexia are needed to promote antioxidant and therapeutic effect.
2.3. Plant-Derived Drugs and Cachexia
There have been significant advances in cancer therapies. In spite of these progress, half of all patients with cancer commonly undergo cachexia which is associated with quality of life, poor response to cancer therapy and finally survival [20]. The symptoms include weight loss, anorexia, muscle atrophy, fatigue, anemia and so forth. Some compounds and extracts of plants were observed to decrease these symptoms (Table 1) and their mechanisms were organized in Figure 1. Li, B et al. elucidated that Bicalin, which is isolated from Scutellaria baicalensis, ameliorated the anorexia, weight loss and muscle atrophy [30]. CT26 adenocarcinoma inoculated male BALB/c mice was administered 50, 150 mg/kg of Bicalin by intraperitoneal injection for 15 days. These observing groups were demonstrated that p-p65/GAPDH band intensity was decreased and inhibitor of nuclear factor kappa B (IκBα)/GAPDH expression was elevated. This result suggests that Bicalin inhibits NF-κB activation which leads muscle wasting. Kim et al. observed that water extract of Citrus unshiu Markovich ameliorated weight loss, muscle wasting and Hb loss when its dosage was 150 and 500 mg/kg for 17 days [31]. The treatment not only decreased the levels of p-p38, extracellular signal-regulated kinase (ERK), JNK, IκBα and STAT3 but also mitigated lipopolysaccharide (LPS)-induced NO, iNOS expression and pro-inflammatory cytokine production. Also, it alleviated the CT-26-mediated C2C12 myotube wasting and changed myosin heavy chain (MyH), Akt phosphorylation and p65 phosphorylation. Curcumin and green tea extract, extracted from Curcuma longa and Camellia sinensis respectively, ameliorated weight loss and muscle wasting [32]. This treatment was implemented on C2C12 myotubes for 24 h as its dosage of 10 μg/mL. Such results were shown when TNF-α and proteolysis-inducing factor (PIF) was combined with not only curcumin and green tea extract but eicosapentaenoic acid (EPA). Rikkunshito was demonstrated to ameliorate anorexia and weight loss by upregulating hypothalamic orexigenic neuropeptide Y (NPY) and decreasing thyrotropin-releasing hormone (TRH) in paraventricular nucleus (PVN) [33]. However, Rikkunshito treatment did not affect the elevated plasma ghrelin levels and growth hormone secretagogue receptor (GHS-R) gene expression. Such results showed when 1 g/kg/day of Rikkunshito was orally administered twice daily for 7 days to 85As2 cells inoculated six-week-old male F344/NJcl-rnu/rnu rats. Shen et al. also reported that the oral administration of SiBaoChongCao (1, 2 g/kg for 20 days) which is isolated from Cordyceps sinensis ameliorated weight loss and muscle atrophy with shrinking adipocyte cell [34]. The muscle atrophy was alleviated by decreasing myosin heavy chain (MHC), myogenic differentiation antigen (MyoD), myogenic regulatory factors (MyoG), target of rapamycin kinase complex 1 (TORC1) and peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-α) and upregulating AKT and mammalian target of rapamycin (mTOR) pathway. This result suggests that it could facilitate myoblast protein synthesis in muscle tissue not regardless of blocking protein degradation. Choi et al. observed that Sipjeondaebo-tang ameliorated cancer-induced anemia and anorexia including weight loss and muscle wasting in CT-26 tumor-bearing mice [35]. In this process, Sipjeongdaebo-tang suppressed IL-6 and monocyte chemoattractant protein-1 (MCP-1) but not TNF-α. It also affected anorexia by regulating glucagon like peptide-1 (GLP-1) and peptide YY (PYY) but not ghrelin and leptin. Finally, it increased the levels of RBC, Hb and hematocrits (HCT), which means treating anemia. Such results were shown when its dosage was 6.784, 67.84 and 678.4 mg/kg for 21 days. Kim et al. reported that oral administration of Soshio-tang (50, 100 mg/kg for 18 days) was identified to alleviate weight loss, muscle wasting and improve appetite [36]. As a part of the result, Soshio-tang strongly prevented LPS-induced p38, IκBα, IKKαβ and STAT3. However, the levels of ERK and JNK were not because this treatment inhibited p65 nuclear translocation, which can block NF-κB activation and attenuated muscle atrophy. Zhuang et al. demonstrated that Zhimu and Huangbai herb pair which was isolated from Anemarrhena asphodeloides and Phellodendron amurense ameliorated body weight loss and muscle protein catabolism [37]. This effect was carried out by activating insulin-like growth factor 1 (IGF-1)/Akt signal and increasing the expression of microtubule-associated protein 1A/1B-light chain 3 (LC3B) and sirtuin1 (SIRT1). Such results were shown when 104 mg/kg of Zhimu and Huangbai her pair was administered to colon-26 adenocarcinoma inoculated Male C57BL/6 mice for 18 days.
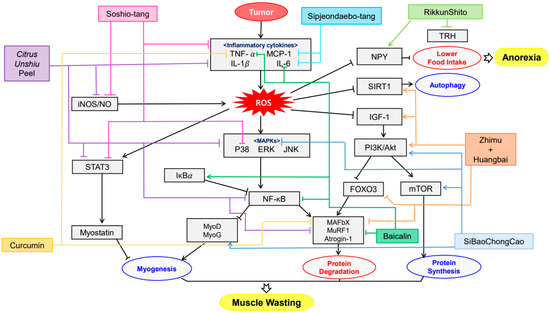
Figure 1. Schematic diagram of anti-inflammatory & antioxidant mechanisms of plant-derived drugs in cachexia.
Table 1. Plant-derived drugs and cachexia.
| Compound/Extract | Source | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Baicalin | Scutellaria baicalensis | CT26 adenocarcinoma inoculated BALB/c mice | 50, 150 mg/kg; 15 days | Amelioration of anorexia, weight loss and muscle atrophy | ↑IκBα ↓NF-κB, TNF-α, IL-6, MURF1, Atrogin-1, p65 |
[30] |
| Citrus unshiu peel water extract | Citrus unshiu Markovich | CT26 adenocarcinoma-induced cancer cachexia BALB/c mice | 250, 500 mg/kg; 17 days | Amelioration of weight loss, muscle wasting and Hb loss | ↑MyH, p-Akt ↓MAFbx, MuRF-1, IL-6, NO, iNOS, IL-1β, TNF-α, p-p38, ERK, JNK, IκBα, STAT3, p-p65, |
[31] |
| Curcumin green tea extract | Curcuma longa, Camellia sinensis |
C2C12 myotubes | 10 μg/mL; 24 h | Amelioration of weight loss and muscle wasting | ↓20S proteasome subunits, p42, MuRF1, MAFbx, PIF, TNF-α | [32] |
| Rikkunshito | Atractylodes lancea, Panax ginseng, Pinellia ternate, Poria cocos, Zizyphus jujuba, Citrus unshiu, Glycyrrhiza uralensis, Zingiber officinale | 85As2 cells inoculated F344/NJcl-rnu/rnu rats |
1 g/kg/day; 7 days | Amelioration of anorexia and weight loss | ↑NPY ↓TRH |
[33] |
| SiBaoChongCao | Cordyceps sinensis | C26 tumor-bearing BALB/c mice | 1, 2 g/kg; 20 days | Amelioration of weight loss, muscle wasting and adipocyte cell reduction | ↑MHC, MyoD, MyoG, p-AKT, p-mTOR, AMPKα, ERK, TORC1, PGC-1α ↓IL-6, TG, AMPK, p38 MAPK, p-HSL, UCP1 |
[34] |
| Sipjeondaebo-tang | Angelica gigas, Astragalus membranaceus, Atractylodes japonica, Cinnamomum cassia, Cnidium officinale, Glycyrrhiza uralensis, Paeonia lactiflora, Panax ginseng, Poria cocos, Rehmannia glutinosa | CT-26 inoculated- BALB/c mice | 6.784, 67.84, 678.4 mg/kg; 21 days | Amelioration of anorexia, weight loss, muscle wasting and anemia | ↓IL-6, MCP-1, PYY, GLP-1 | [35] |
| Soshio-tang | Bupleurum falcatum, Glycyrrhiza uralensis, Panax ginseng, Pinellia ternata, Scutellaria baicalensis, Zingiber officinale, Ziziphus jujuba | J774A.1 macrophage cell line inoculated CT-26-bearing mice | 50, 100 mg/kg; 18 days | Alleviation of weight loss, muscle wasting and appetite loss | ↓NO, iNOS, IL-6, IL-1α, IL-1β, TNF-α, p38, NF-κB, IκBα, IKKαβ, STAT3 | [36] |
| Zhimu and Huangbai herb pair | Anemarrhena asphodeloides, Phellodendron amurense | colon-26 adenocarcinoma inoculated C57BL/6 mice | 104 mg/kg; 18 days | Amelioration of weight loss and muscle wasting | ↑IGF-1, Akt, LC3B, SIRT1 ↓TNF-α, IL-6, atrogin-1, MuRF1, FOXO3 |
[37] |
Common symptoms of cancer cachexia, including anorexia, weight loss and muscle atrophy, were ameliorated by plant-derived drugs through various mechanisms. Bicalin inhibited NF-κB activation [30], Citrus unshiu extract suppressed the production of pro-cachectic cytokines [31] and curcumin and green tea enhanced protein degradation through TNF-α and PIF [32]. Effectiveness of plant-derived drugs was prominent in herbal formulas or herbal pairs. It can be assumed that such tendency is due to complex and multifactorial characteristic of cachexia. Rikkunshito attenuated anorexia and weight loss by upregulating hypothalamic orexigenic NPY and decreasing TRH [33]. SiBaoChongCao facilitated myoblast protein synthesis in muscle tissue, preventing weight loss and muscle atrophy with shrinking adipocyte cells [34]. Sipjeondaebo-tang ameliorated cancer-induced anemia and anorexia [35]. Soshio-tang inhibited p65 nuclear translocation, which can block NF-κB activation [36]. Zhimu and Huangbai herb pair activated IGF-1/Akt signal, therefore preventing body weight loss and muscle protein catabolism [37].
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int. J. Cancer 2019, 144, 1941–1953.
- Wyld, L.; Audisio, R.A.; Poston, G.J. The evolution of cancer surgery and future perspectives. Nat. Rev. Clin. Oncol. 2015, 12, 115–124.
- Yoon, Y.S.; Han, H.S.; Agarwal, A.; Belli, G.; Itano, O.; Gumbs, A.A.; Yoon, D.S.; Kang, C.M.; Lee, S.E.; Wakai, T.; et al. Survey Results of the Expert Meeting on Laparoscopic Surgery for Gallbladder Cancer and a Review of Relevant Literature. Dig. Surg. 2019, 36, 7–12.
- Martin, J.D.; Cabral, H.; Stylianopoulos, T.; Jain, R.K. Improving cancer immunotherapy using nanomedicines: Progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020, 17, 251–266.
- Hwang, W.L.; Pike, L.R.; Royce, T.J.; Mahal, B.A.; Loeffler, J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 2018, 15, 477–494.
- Sharma, R.A.; Plummer, R.; Stock, J.K.; Greenhalgh, T.A.; Ataman, O.; Kelly, S.; Clay, R.; Adams, R.A.; Baird, R.D.; Billingham, L. Clinical development of new drug–radiotherapy combinations. Nat. Rev. Clin. Oncol. 2016, 13, 627–642.
- Kaiser, J.; Couzin-Frankel, J. Cancer immunotherapy sweeps Nobel for medicine. Science 2018, 362, 13, doi:10.1126/science.362.6410.13.
- Whitmore, W.F., Jr. Hormone therapy in prostatic cancer. Am. J. Med. 1956, 21, 697–713.
- Ito, H.; Nakayama, H.; Yokose, T.; Nagashima, T.; Morohoshi, T.; Tajiri, M.; Maehara, T.; Watanabe, K.; Arai, H.; Yamamoto, T.; et al. A prophylaxis study of acute exacerbation of interstitial pneumonia after lung cancer surgery. Jpn. J. Clin. Oncol. 2020, 50, 198–205.
- Hashimoto, H.; Abe, M.; Yanai, T.; Yamaguchi, T.; Zenda, S.; Uchitomi, Y.; Fukuda, H.; Mori, M.; Iwasa, S.; Yamamoto, N.; et al. Study protocol for j-support 1604 (j-force): A randomized, double blind, placebo-controlled phase iii study evaluating olanzapine (5 mg) plus standard triple antiemetic therapy for prevention of chemotherapy induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy. Jpn. J. Clin. Oncol. 2018, 48, 950–952.
- Ohnishi, S.; Watari, H.; Kanno, M.; Ohba, Y.; Takeuchi, S.; Miyaji, T.; Oyamada, S.; Nomura, E.; Kato, H.; Sugiyama, T.; et al. Additive effect of rikkunshito, an herbal medicine, on chemotherapy-induced nausea, vomiting and anorexia in uterine cervical or corpus cancer patients treated with cisplatin and paclitaxel: Results of a randomized phase ii study (jortc kmp-02). J. Gynecol. Oncol. 2017, 28, e44.
- Stone, J.B.; DeAngelis, L.M. Cancer-treatment-induced neurotoxicity—Focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105.
- Mi, X.-J.; Hou, J.-G.; Wang, Z.; Han, Y.; Ren, S.; Hu, J.-N.; Chen, C.; Li, W. The protective effects of maltol on cisplatin-induced nephrotoxicity through the ampk-mediated pi3k/akt and p53 signaling pathways. Sci. Rep. 2018, 8, 15922, doi:10.1038/s41598-018-34156-6.
- Tsao, M.N.; Xu, W.; Wong, R.K.; Lloyd, N.; Laperriere, N.; Sahgal, A.; Rakovitch, E.; Chow, E. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 2018, 1, CD003869.
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer 2004, 3, 294–300.
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular stress responses in radiotherapy. Cells 2019, 8, 1105, doi:10.3390/cells8091105.
- Lee, C.-L.; Blum, J.M.; Kirsch, D.G. Role of p53 in regulating tissue response to radiation by mechanisms independent of apoptosis. Transl. Cancer Res. 2013, 2, 412–421.
- Strasser, F. Diagnostic criteria of cachexia and their assessment: Decreased muscle strength and fatigue. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 417–421.
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495.
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29.
- Marceca, G.P.; Londhe, P.; Calore, F. Management of cancer cachexia: Attempting to develop new pharmacological agents for new effective therapeutic options. Front. Oncol. 2020, 10, 298, doi:10.3389/fonc.2020.00298.
- Roy, A.; Kumar, A. Er stress and unfolded protein response in cancer cachexia. Cancers 2019, 11, 1929, doi:10.3390/cancers11121929.
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194.
- Mallinson, J.E.; Marimuthu, K.; Murton, A.; Selby, A.; Smith, K.; Constantin-Teodosiu, D.; Rennie, M.J.; Greenhaff, P.L. Statin myalgia is not associated with reduced muscle strength, mass or protein turnover in older male volunteers but is allied with a slowing of time to peak power output, insulin resistance and differential muscle mrna expression. J. Physiol. 2015, 593, 1239–1257.
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling and metabolic pathways. Cell Metab. 2012, 16, 153–166.
- Suzuki, H.; Asakawa, A.; Amitani, H.; Nakamura, N.; Inui, A. Cancer cachexia—Pathophysiology and management. J. Gastroenterol. 2013, 48, 574–594.
- Elkina, Y.; von Haehling, S.; Anker, S.D.; Springer, J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle 2011, 2, 143–151.
- Ryan, J.L.; Carroll, J.K.; Ryan, E.P.; Mustian, K.M.; Fiscella, K.; Morrow, G.R. Mechanisms of cancer-related fatigue. Oncologist 2007, 12 (Suppl. 1), 22–34.
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410.
- Li, B.; Wan, L.; Li, Y.; Yu, Q.; Chen, P.; Gan, R.; Yang, Q.; Han, Y.; Guo, C. Baicalin, a component of scutellaria baicalensis, alleviates anorexia and inhibits skeletal muscle atrophy in experimental cancer cachexia. Tumor Biol. 2014, 35, 12415–12425.
- Kim, A.; Im, M.; Gu, M.J.; Ma, J.Y. Citrus unshiu peel extract alleviates cancer-induced weight loss in mice bearing ct-26 adenocarcinoma. Sci. Rep. 2016, 6, 24214, doi:10.1038/srep24214.
- Mirza, K.A.; Luo, M.; Pereira, S.; Voss, A.; Das, T.; Tisdale, M.J. In vitro assessment of the combined effect of eicosapentaenoic acid, green tea extract and curcumin c3 on protein loss in c2c12 myotubes. In Vitro Cell. Dev. Biol.-Anim. 2016, 52, 838–845.
- Terawaki, K.; Kashiwase, Y.; Sawada, Y.; Hashimoto, H.; Yoshimura, M.; Ohbuchi, K.; Sudo, Y.; Suzuki, M.; Miyano, K.; Shiraishi, S.; et al. Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85as2 cells and the palliative effects of the kampo medicine rikkunshito on the model. PLoS ONE 2017, 12, e0173113, doi:10.1371/journal.pone.0173113.
- Shen, Q.; Miao, C.-X.; Zhang, W.-L.; Li, Y.-W.; Chen, Q.-Q.; Li, X.-X.; Liu, X.; Zhang, X.-W. Sibaochongcao exhibited anti-fatigue activities and ameliorated cancer cachexia in mice. RSC Adv. 2019, 9, 17440–17456.
- Choi, Y.K.; Jung, K.Y.; Woo, S.M.; Yun, Y.J.; Jun, C.Y.; Park, J.H.; Shin, Y.C.; Cho, S.G.; Ko, S.G. Effect of sipjeondaebo-tang on cancer-induced anorexia and cachexia in ct-26 tumor-bearing mice. Mediat. Inflamm. 2014, 2014, 736563.
- Kim, A.; Im, M.; Ma, J.Y. Sosihotang ameliorates cachexiarelated symptoms in mice bearing colon 26 adenocarcinoma by reducing systemic inflammation and muscle loss. Oncol. Rep. 2016, 35, 1841–1850.
- Zhuang, P.; Zhang, J.; Wang, Y.; Zhang, M.; Song, L.; Lu, Z.; Zhang, L.; Zhang, F.; Wang, J.; Zhang, Y.; et al. Reversal of muscle atrophy by zhimu and huangbai herb pair via activation of igf-1/akt and autophagy signal in cancer cachexia. Support. Care Cancer 2016, 24, 1189–1198.




