
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | piergiorgio messa | + 1916 word(s) | 1916 | 2021-09-01 04:54:23 | | | |
| 2 | Peter Tang | Meta information modification | 1916 | 2021-09-02 02:56:26 | | |
Video Upload Options
Native hypovitaminosis D (n-hVITD) is frequently found from the early stages of chronic kidney disease (CKD) and its prevalence increases with CKD progression.
1. Introduction
2. Native Hypovitaminosis D during Chronic Kidney Disease (CKD): The Size of the Problem
2.1. Vitamin D Metabolism and Status Categorization in CKD Patients
2.2. Native Hypovitaminosis D Epidemiology in CKD Patients
2.3. Native Vitamin D Supplementation: Dose, Interval,Toxicity
3. Native Hypovitaminosis D in CKD: Experimental and Clinical Evidence
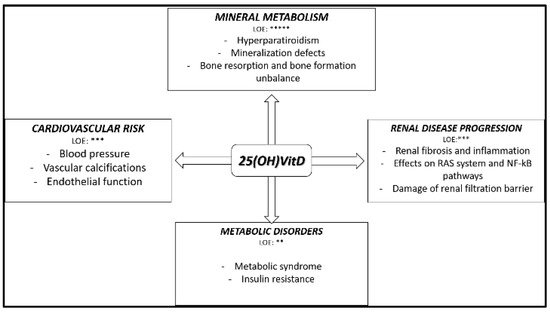
4. Native Vitamin D Supplementation in CKD Patients: Evidence from Randomized Interventional Studies
|
Type of Patients |
Number of Patients |
Type of Intervention |
Results |
Reference |
|---|---|---|---|---|
|
CKD stages 2–4 |
87 |
GrA: Cholecalciferol 5000 IU/wk; GrB: Cholecalciferol 20,000 IU/wk |
In GrB vs. GrA: significant increase of 25OHVitD, 1,25OHD2 and reduction PTH |
Oksa et al., 2008 |
|
CKD stages 1–5 |
25 |
GrA: Cholecalciferol 40,000 IU/wk; GrB: placebo |
In GrA vs. GrB: significant increase of 25OHVitD, 1,25(OH)D2, FGF23 and reduction PTH |
Markmann et al., 2012 |
|
CKD 3–4 |
38 |
GrA: Ergocalciferol 50,000 IU/wk for one month followed by 50,000 IU/mth for 5 mths; GrB: Placebo |
In GrA vs. GrB: significant increase of 25(OH)VitD, endothelium dependent microcirculatory vasodilatation and reduction of pulse pressure. No effects on PWV and LVMI |
Dreyer et al., 2014 |
|
CKD stages 3–4 |
429 |
Study 1 (n = 213): GrA: ER Calcifediol 12 wk at 30 g/daily + 14 wks 30 or 60 g/daily; GrB 26 wks placebo Study 2 (n = 216): GrA: ER Calcifediol 12 wks 30 g/daily + 14 wks 30 or 60 g/daily; GrB: 26 wks placebo |
In GrA vs. GrB in both studies: significant increase of 25OHVitD and reduction PTH |
Sprague et al., 2016 |
|
CKD 3–5 |
44 |
GrA:Cholecalciferol 50.000 IU/wk; GrB: Ergocalciferol 50.000 IU/wk |
In GrA vs. GrB: significantly higher increase of 25(OH)VitD. No differences in 1,25(OH)D2 and PTH |
Weltmore et al., 2016 |
|
CKD stages 3–4 |
128 |
GrA: Cholecalciferol 2000 IU/day; GrB: Calcitriol 0.5 g/day |
In GrA vs. GrB: significant increase of 25OHVitD in GrA vs. GrB. No effects on FMD |
Kendrick et al., 2017 |
|
CKD stages 3–4 |
120 |
GrA: Cholecalciferol 300,000 UI mth; GrB: Placebo |
In GrA vs. GrB: significant increase of 25OHVitD, 1,25OHD2 and reduction PTH. Increase of FMD and PWV |
Kumar et al., 2017 |
|
CKD stages 3–4 |
119 |
GrA: Placebo; GrB: Calcitriol 0.5 g 3×/wk; GrC: Calcifediol 5.000 IU 3×/wk |
In GrC vs. others: significant decrease of PWV. Patients in the highest 25(OH)VitD tertile at trial end had significant decreases in PWV |
Levin et al., 2017 |
Footnotes: GrA: group A; GrB: Group B; GrC: Group C; wk: week; wks: weeks; mth: month; mths: months; PWV: pulse wave velocity; FMD: flow mediated dilation; LVMI: left ventricular mass index. PTH: Parathormone.
References
- González, E.A.; Sachdeva, A.; Oliver, D.A.; Martin, K.J. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am. J. Nephrol. 2004, 24, 503–510.
- Cozzolino, M.; Ciceri, P.; Volpi, E.M.; Olivi, L.; Messa, P.G. Pathophysiology of calcium and phosphate metabolism impairment in chronic kidney disease. Blood Purif. 2009, 27, 338–344.
- Messa, P.; Regalia, A.; Alfieri, C. Nutritional vitamin D in renal transplant patients: Speculations and reality. Nutrients 2017, 9, 550.
- Holick, M.F.; MacLaughlin, J.A.; Clark, M.B.; Holick, S.A.; Potts, J.T.; Anderson, R.R.; Elias, P. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 1980, 210, 203–205.
- Nykjaer, A.; Dragun, D.; Walther, D.; Vorum, H.; Jacobsen, C.; Herz, J.; Willnow, T.E. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 1999, 96, 507–515.
- Jones, G.; Prosser, D.E.; Kaufmann, M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch. Biochem. Biophys. 2012, 523, 9–18.
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281.
- Ginde, A.A.; Liu, M.C.; Camargo, C.A. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch. Intern. Med. 2009, 169, 626.
- Kuro-o, M.; Moe, O.W. FGF23-αKlotho as a paradigm for a kidney-bone network. Bone 2017, 100, 4–18.
- Takemoto, F.; Shinki, T.; Yokoyama, K.; Inokami, T.; Hara, S.; Yamada, A.; Uchida, S. Gene expression of vitamin D hydroxylase and megalin in the remnant kidney of nephrectomized rats. Kidney Int. 2003, 64, 414–420.
- Matsui, I.; Hamano, T.; Tomida, K.; Inoue, K.; Takabatake, Y.; Nagasawa, Y.; Isaka, Y. Active vitamin D and its analogue, 22-oxacalcitriol, ameliorate puromycin aminonucleoside-induced nephrosis in rats. Nephrol. Dial. Transpl. 2009, 24, 2354–2361.
- Leheste, J.R.; Rolinski, B.; Vorum, H.; Hilpert, J.; Nykjaer, A.; Jacobsen, C.; Willnow, T.E. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am. J. Pathol. 1999, 155, 1361–1370.
- Bohnert, B.N.; Daniel, C.; Amann, K.; Voelkl, J.; Alesutan, I.; Lang, F.; Artunc, F. Impact of phosphorus restriction and vitamin D-substitution on secondary hyperparathyroidism in a proteinuric mouse Model. Kidney Blood Press. Res. 2015, 40, 153–165.
- Messa, P.; Alfieri, C.; Rastaldi, M.P. Recent insights into vitamin D and its receptor. J. Nephrol. 2011, 24 (Suppl. 18), 30–37.
- Cupisti, A.; Vigo, V.; Baronti, M.E.; D’Alessandro, C.; Ghiadoni, L.; Egidi, M.F. Vitamin D status and cholecalciferol supplementation in chronic kidney disease patients: An Italian cohort report. Int. J. Nephrol. Renov. Dis. 2015, 8, 151–157.
- Jhee, J.H.; Kim, H.; Park, S.; Yun, H.R.; Jung, S.Y.; Kee, Y.K.; Yoo, T.H. Vitamin D deficiency is significantly associated with depression in patients with chronic kidney disease. PLoS ONE 2017, 12, e0171009.
- Kidney Disease: Improving global outcomes (KDIGO) CKD-MBD update work group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int. 2017, 7 (Suppl. 1), 1–59.
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Lanham-New, S. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364.
- Vieth, R. Vitamin D toxicity, policy, and science. J. Bone Miner. Res. 2007, 22 (Suppl. S2), V64–V68.
- Sprague, S.M.; Crawford, P.W.; Melnick, J.Z.; Strugnell, S.A.; Ali, S.; Mangoo-Karim, R.; Bishop, C.W. Use of extended-release calcifediol to treat secondary hyperparathyroidism in stages 3 and 4 chronic kidney disease. Am. J. Nephrol. 2016, 44, 316–325.
- Alfieri, C.; Regalia, A.; Zanoni, F.; Vettoretti, S.; Cozzolino, M.; Messa, P. The importance of adherence in the treatment of secondary hyperparathyroidism. Blood Purif. 2019, 47, 37–44.
- Morrone, L.F.; Bolasco, P.; Camerini, C.; Cianciolo, G.; Cupisti, A.; Galassi, A.; Cozzolino, M. Vitamin D in patients with chronic kidney disease: A position statement of the Working Group; Trace elements and mineral metabolism. J. Nephrol. 2016, 29, 305–328.
- Townsend, K.; Evans, K.N.; Campbell, M.J.; Colston, K.W.; Adams, J.S.; Hewison, M. Biological actions of extra-renal 25-hydroxyvitamin D-1α-hydroxylase and implications for chemoprevention and treatment. J. Steroid. Biochem. Mol. Biol. 2005, 97, 103–109.
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin D3 -1α-Hydroxylase 1. J. Clin. Endocrinol. Metab. 2001, 86, 888–894.
- Holick, M.F. Vitamin D in health and disease: Vitamin D for health and in chronic kidney disease. Semin. Dial. 2005, 18, 266–275.
- Jean, G.; Souberbielle, J.; Chazot, C. Vitamin D in chronic kidney disease and dialysis patients. Nutrients 2017, 9, 328.
- Shroff, R.; Wan, M.; Gullett, A.; Ledermann, S.; Shute, R.; Knott, C.; Rees, L. Ergocalciferol supplementation in children with CKD delays the onset of secondary hyperparathyroidism: A randomized trial. Clin. J. Am. Soc. Nephrol. 2012, 7, 216–223.




