
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Patricia Lainetti | + 2449 word(s) | 2449 | 2021-01-25 04:06:24 | | | |
| 2 | Peter Tang | -5 word(s) | 2444 | 2021-01-30 11:14:57 | | |
Video Upload Options
Breast cancer (BC) is one of the most important cancers worldwide, and usually, chemotherapy can be used in an integrative approach. Usually, chemotherapy treatment is performed in association with surgery, radiation or hormone therapy, providing an increased outcome to patients. However, tumors can develop resistance to different drugs, progressing for a more aggressive phenotype.
1. Introduction
Breast Cancer (BC) is one of the most important cancers in women worldwide, according to the last global cancer statistics, and it was the second-leading cause of cancer-related deaths in 2018 [1]. Chemotherapy is seldom used for treating BC, but in specific cases, it may be recommended [2]. Usually, BC is classified into molecular subtypes, and for some of them, chemotherapy is an option. Among the molecular subtypes, triple-negative BC is considered one of the most aggressive, and its chemotherapy response rate is considered higher when compared to the others. However, despite adjuvant chemotherapy, the overall survival of these patients is still poor [3].
Since chemotherapy is usually used for triple-negative, inflammatory and advanced-stage BC, new strategies and molecular predictive markers are required to increase the patient's prognosis [4]. New predictive and prognostic markers can provide valuable information regarding the identification of patients that could benefit from chemotherapy. Besides that, different strategies can be used to increase drug delivery into tumor cells, including nanoparticles. Different systems of nanostructured carriers can be effective in cancer chemotherapy and overcome drug resistance.
2. Breast Cancer
Cancer is a common disease, globally distributed and with over 60% growth rate expected for the next 20 years [1]. Among the most common types of cancer, BC is the second-most frequently diagnosed, only after lung tumors [1]. In women, it is the most common type of cancer, representing about 30% of new cases [5]. In 2018, more than two million women were affected by this disease, with a mortality rate of 6.6%, resulting in more than 500 thousand deaths of women [1]. There has been a 40% decline in mortality from breast tumors with the evolution of diagnostic tools and treatments, but since 2010, the reduction in the mortality rate has slowed [5].
The definitive diagnosis is made by histopathology, and tumors should be classified according to Lakhani et al. [6]. For the staging system, the patient's tumor size, nodes and metastasis occurrence are evaluated according to Giuliano et al. [7]. The most prevalent subtypes of breast cancer are invasive carcinoma (70–75%) and lobular carcinoma (12–15%) [8]. Each histological subtype has a specific prognosis and treatment protocol [7][8]. BC can also be classified into molecular subtypes according to their expression of estrogen receptors (ER), progesterone receptors (PR) and human epidermal growth factor (HER2). Moreover, Ki67 is a marker used to estimate tumor proliferation and chemosensitivity and, also, have some prognostic value for certain molecular subtypes [9][10]. Thus, the patient's diagnosis and prognosis are made based on a multidisciplinary approach.
Tumors can be divided into a luminal A-like subtype (ER+ and/or PR+ and HER2-, low Ki67 index); luminal B-like subtype HER2+ (ER+ and/or PR+ and HER2+, high Ki67 index) or luminal B-like HER2- (ER+ and/or PR+ and HER2-, high Ki67 index); HER2 subtype (ER-, PR- and HER2 +, high Ki67 index) and triple-negative (ER-, PR- and HER2-, high Ki67 index) [9][10][11]. Luminal A tumors are the most common molecular subtype of breast carcinoma (30–40%), followed by luminal b (20–30%), HER2 overexpressed (12–20%) and triple-negative (10–15%) [11][12].
Tumors classified as luminal A are associated with a favorable prognosis and, usually, low-grade luminal B with an unfavorable prognosis, higher tumor grades and proliferative activity; HER2 are overexpressed with a higher incidence of local or regional recurrences and triple-negative with a poor prognosis, higher rates of recurrence, distant metastases and mortality [11][12]. Triple-negative tumors are considered the most challenging molecular subtype, once treatment options are scarce, because they have more aggressive and metastatic behavior, resulting in a shorter disease-free interval and survival time for patients [13]. In addition, malignant breast neoplasms are the major condition leading to the high mortality of women. For these reasons, prevention and new therapies are essential for a better prognosis [14].
3. Neoadjuvant Chemotherapy for Breast Cancer
In general, BC treatments focus on the cure of the disease, higher disease-free survival (DFS) and overall survival (OS) time and quality of life. Different types of modalities can be associated, such as local, systemic and support treatments. Local treatments include surgery and radiotherapy, and systemic therapies can be divided into chemotherapy, immunotherapy and target and hormone therapy [8][15]. The choice of the treatment must consider the tumor location and size, lymph node commitment, histopathology, molecular subtype and presence of metastases. Moreover, the patient's health condition, age, hormonal status and preferences should be also discussed [8].
In patients with nonmetastatic tumors, it is recommended to perform local therapy, with surgical removal of the tumor and, in some cases, of the axillary lymph nodes with subsequent radiotherapy. Radiation therapy is recommended for most patients after local surgery with breast conservation. In addition, systemic therapy can be used as a neoadjuvant and/or adjuvant tool for surgery [16][17]. In luminal tumors A and B, tumors ER+ and HER2-, the standard treatment is with adjuvant endocrine therapy. Some patients with this type of tumor can have some benefits adding chemotherapy. Women with stage III tumors and patients with commitments of four or more lymph nodes, even if it is a lobular carcinoma and/or grade 1 tumor or luminal A, should receive chemotherapy [16]. HER2+ tumors should be treated with chemotherapy and targeted monoclonal antibody therapy, and triple-negative tumors are treated mainly with chemotherapy [16][17].
Alkylator and taxane-based regimens with anthracycline are chemotherapy drugs recommended for Luminal A and B BCs. HER2+ tumors, in stage 2 or 3, should be treated with anthracycline-, alkylator- and taxane-based chemotherapy in combination with trastuzumab or pertuzumab and stage 1 with paclitaxel and trastuzumab [16]. The most used treatments for triple-negative breast cancer in women are doxorubicin in combination with cyclophosphamide, doxorubicin with cyclophosphamide and paclitaxel, and in cases of recurrence and resistance to doxorubicin, docetaxel can be used together with cyclophosphamide [17][18][19]. In patients with triple-negative tumors and metastasis at the time of diagnosis, the first line of treatment is chemotherapy with taxanes (paclitaxel and docetaxel), platinum compounds (cisplatin) or anthracyclines (doxorubicin) as a monotherapy [17][18][20].
Triple-negative BC treatment is based on the molecular characteristics of the tumor, and some proposed treatments are chemotherapy with anthracyclines, taxanes, alkylators and platinum-based, PARP1 inhibition when the tumor has an absence of or reduced BRCA1 function, antibody treatment when the tumor overexpresses Epidermal Growth Factor Receptor (EGFR), c-KIT tyrosine kinase inhibitor when it overexpresses c-KIT and multikinase inhibitors when overexpressing EGFR [4][16][19]. Metastatic breast tumors are treated like nonmetastatic breast tumors; however, the focus is on prolonging the patient's survival using palliative therapy [17].
Triple-negative tumors do not have specific targets for therapies, nor do they have estrogen and progesterone receptors for hormone therapy. Thus, treatment options are more restricted. Therefore, even in cases without metastasis to distant organs or lymph nodes, chemotherapy is always used. In the case of triple-negative breast tumors with metastases, other treatment options can be used, in conjunction with chemotherapy, such as immunotherapy and PARP inhibitors and cisplatin or carboplatin [13].
4. Breast Cancer Molecular Mechanisms of Chemoresistance
Chemoresistance or drug resistance is described as the low efficiency and efficacy of a drug to produce a beneficial response in the treatment and, also, is one of the main factors associated with chemotherapy failure [21][22][23]. Tumor chemoresistance can be associated with different mechanisms, including interactions among cancer cells and the tumor microenvironment (Figure 1), cancer cell heterogeneity, cancer stem cells, cancer-associated macrophages and immune cells modulation, can modify the tumor microenvironment during chemotherapy, leading to chemoresistance. Chemoresistance can have intrinsic factors, like tumor heterogeneity, cancer stem cells and epigenetics and/or extrinsic/acquired factors (Figure 2), that includes pH, hypoxia and paracrine signaling and other tumor cells [22][24]. Cell resistance mechanisms in breast cancer involve cell membrane drug absorption, transport and efflux, transporter proteins, cancer-related genes (oncogenes and tumor-suppressor genes), DNA repair, cancer steam cells, the tumor microenvironment and epithelial-mesenchymal transitions (EMT) [22][25].
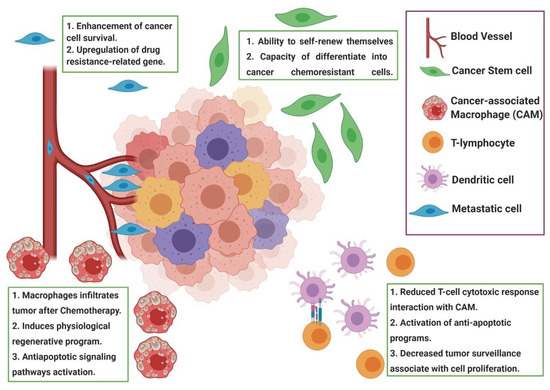
Figure 1. Representation of the tumor microenvironment factors associated with chemoresistance. During chemotherapy, the tumor microenvironment shows cancer-associated macrophages (CAM), cancer stem cells and other immune cells that induce paracrine signaling, leading to tumor resistance induced by drugs. Usually, the modulation of the tumor microenvironment after chemotherapy induces the recruitment of CAM and a reduced cytotoxic response of T cells, leading to a resistance to apoptosis. Figure created in BioRender (https://biorender.com/).
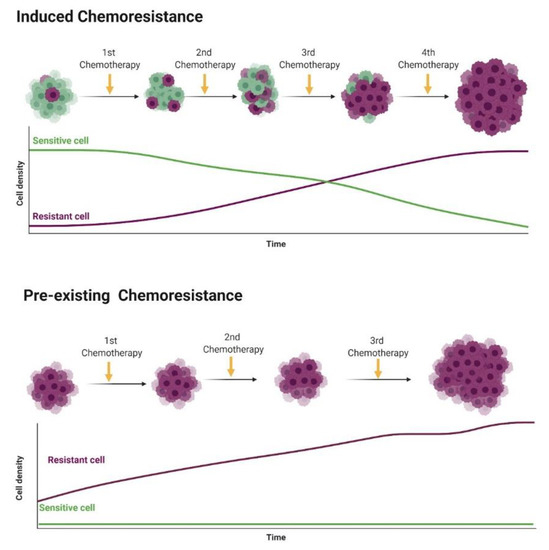
Figure 2. Representation of intrinsic chemoresistance in breast cancer (BC). Usually, tumor cells show individual genetic and epigenetic alterations that lead to chemoresistance (purple cells). In the induced chemoresistance, tumor cells are sensitive to chemotherapy, and during treatment different factors (such as hypoxia, oxidative stress and DNA damage) induce a selection of chemoresistant cells that grow after the apoptosis of sensitive cells by chemotherapy. Then, in a late stage, only chemoresistant cells are growing in the tumor microenvironment. On the other hand, in the pre-existing chemoresistance, cancer cells at the beginning of the therapy show chemoresistance and no response to therapy (cells naturally chemoresistant). Figure created in BioRender (https://biorender.com/).
Chemotherapy resistance is an obstacle for the treatment of neoplasms and compromises the choice of chemotherapy for cases of recurrence. Therefore, it is increasingly important to choose an accurate and effective treatment, in a more personalized way, for each patient. Polychemotherapy or adjuvant therapies to conventional chemotherapy have been used more frequently, due to the heterogeneity of neoplasms and their genomic map [26]. When tumors become resistant to conventional therapy, new types of treatments are necessary.
5. Epigenetics and Breast Cancer Chemoresistance
Endocrine therapy is one of the first-line treatments for BC, since two-thirds of the cases express an estrogen receptor (ER) [27]. It has been successfully used for luminal BC patients, with significant increased overall survival [28]. However, cancer cell resistance to endocrine therapy can develop, and some of the patients may experience tumor recurrence [28]. Interestingly, the mechanisms involved in resistance to endocrine therapy usually involve the epigenetic machinery. In the ER-positive cell line, ESR1-promoter hypermethylation was previously associated with ER downregulation and resistance to endocrine therapy [29][30]. Besides that, the transcriptional ER regulation may impact directly on the PR expression, and the parts of the tumor that are positive for ER also express PR [31]. The PR gene presents a CpG island in its first exon, and around 40% of RP-negative BC are associated with RP-promoter hypermethylation [32]. Recently, using a BC cell line model, it was demonstrated that, in a hormone-free cancer cell, when a decreased PR expression is induced, ESR1 also showed a decreased expression associated with a hypermethylation of the ESR1 promoter [33]. Thus, the ER and PR seem to be regulated by methylation in human BC cell lines, and also, methylation seems to promote a gene-cross regulation. Thus, new therapeutical strategies focused on epigenetic modifications can be associated with patients in hormone-negative BC patients.
Fibroblast growth factor receptor 1 (FGFR1) has been linked to the progression, proliferation, migration and survival of breast tumor cells [34]. It can be considered a predictive factor for decreasing patient survival in HR+ tumors and confers a resistance to estrogen. FGFR1 amplification is related to a resistance to ER, PI3K and CDK4/6 inhibitors, providing resistance to different drug therapies [34]. A high FGFR1 expression has also been linked to a worse prognosis and overall survival in cases of triple-negative tumors and is a significant gene for tumor cell survival, with inhibition of the FGFR pathway being an adjunct therapy to chemotherapy and, also, an alternative to cancers refractory to treatment [35][36].
Another member of the fibroblast growth factor family, FGF-2, is related to chemoresistance in triple-negative breast tumors. Its super-expression helps to regulate the cell cycle and survival, in addition to assisting resistance to radiotherapy and metastasis formation [37]. FGF-2 overexpression has been reported in subpopulations of chemoresistant cells, leading to the formation of metastases and a worse prognosis [37]. In addition, when targeted for treatment and downregulated, the cell apoptosis and chemotherapeutic sensitivity of chemically resistant cells increases [38].
The MAPK8 gene, also known as JNK1, is related to tumor cell survival by controlling autophagy by phosphorylation of the antiapoptotic protein BCL-2 [39]. JNK1 is associated with the chemoresistance of tumor cells, and its expression stimulates the overexpression of P-glycoprotein, conferring resistance to doxorubicin. Blocking the JNK or MAPK pathway decreases the expression of the ABCG2 transporter and leads to tumor cell autophagy [39][40].
Besides gene methylation, microRNA dysregulation has been implied in BC chemoresistance [41][42]. When a microRNA targets a tumor-suppressor gene, its overexpression is expected and induces gene downregulation [35]. In BC patients, miR-27b is associated with tumor invasiveness and migration [43], and miR-200a [44] and miR-155 [45] were previously related to apoptosis inhibition. Since chemotherapy induces cellular apoptosis, miR-200a and miR-155 overexpression can lead with BC chemoresistance. Interestingly, microRNA overexpression can be a therapeutic target, and a combination of drug delivery systems with a microRNA inhibitor can increase drug delivery and enhance the antitumor response. Wang et al. (56) identified several microRNAs associated with BC resistance using bioinformatics tools and extracted transcriptome data from drug-resistant BC patients (N = 5) compared to drug-sensitive BC patients (N = 5). In this analysis, 22 dysregulated microRNAs were identified, 12 downregulated and 10 upregulated (Table 1). Among the dysregulated microRNAs, hsa-miR-195a-5p showed the highest fold change (FC: 5.44) and hsa-miR-4472 the lowest fold change.
Table 1. Dysregulated microRNAs associated with chemoresistant breast cancer patients evaluated by transcriptome analysis.
|
MicroRNA |
Expression |
Fold Change |
|
hsa-miR-195a-5p |
upregulated |
5.44 |
|
hsa-miR-4266 |
upregulated |
3.45 |
|
hsa-miR-200b-3p |
upregulated |
3.13 |
|
hsa-miR-214-3p |
upregulated |
3.00 |
|
hsa-miR-107 |
upregulated |
2.96 |
|
hsa-miR-4454 |
upregulated |
2.88 |
|
hsa-miR-5100 |
upregulated |
2.41 |
|
hsa-miR-23a-3p |
upregulated |
2.30 |
|
hsa-miR-23b-3p |
upregulated |
2.29 |
|
hsa-miR-16-5p |
upregulated |
2.09 |
|
hsa-miR-4707-5p |
downregulated |
0.49 |
|
hsa-miR-3656 |
downregulated |
0.46 |
|
hsa-miR-1233-1-5p |
downregulated |
0.46 |
|
hsa-miR-3621 |
downregulated |
0.44 |
|
hsa-miR-3141 |
downregulated |
0.44 |
|
hsa-miR-489 |
downregulated |
0.41 |
|
hsa-miR-1227-5p |
downregulated |
0.41 |
|
hsa-miR-1275 |
downregulated |
0.39 |
|
hsa-miR-1268b |
downregulated |
0.36 |
|
hsa-miR-572 |
downregulated |
0.30 |
|
hsa-miR-4467 |
downregulated |
0.29 |
|
hsa-miR-4472 |
downregulated |
0.18 |
Since small-interfering RNAs (siRNAs) can be associated with chemoresistance, it is important to identify the target genes regulated by these long noncoding RNAs and, also, identify the interactions among the target genes. For this reason, we performed a search on the TargetScanHuman database (http://www.targetscan.org/vert_72/) to identify the hsa-miR-195a-5p target genes, and we identified 127 genes. Then, we performed a protein-to-protein interaction (PPI) prediction using the STRING database (https://string-db.org/), and we identified two major PPI networks. We identified two major networks, and to increase the visibility, we excluded the disconnect nodes and presented the two networks in Figure 3. Among the genes, we identified FGF2, FGFR1 and MAPK8. These genes are regulated by hsa-miR-195a-5p, and they may associate with tumor chemoresistance and can be used as future targets to new drugs.
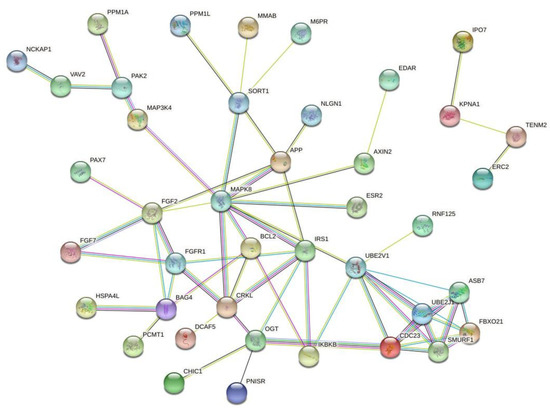
Figure 3. Protein-to-protein interaction (PPI) network based on the prediction of genes regulated by hsa-miR-195a-5p. Since this microRNA was associated with cancer chemoresistance, these genes may represent important drug targets. Figure generated with the online tool STRING (https://string-db.org/).
References
- Erratum: Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2020, 70, 313, doi:10.3322/caac.21609.
- Loboda, A.; Sr, I.S.; Orel, V.E.; Syvak, L.; Golovko, T.; Dosenko, I.; Lyashenko, A.; Smolanka, J.I.; Dasyukevich, O.; Tarasenko, T.; et al. Efficacy of combination neoadjuvant chemotherapy and regional inductive moderate hyperthermia in the treatment of patients with locally advanced breast cancer. Technol. Cancer Res. Treat. 2020, 19, 1–10, doi:10.1177/1533033820963599.
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9, S73–S81, doi:10.3816/cbc.2009.s.008.
- Cleator, S.; Heller, W.; Coombes, R.C. Triple-negative breast cancer: Therapeutic options. Lancet Oncol. 2007, 8, 235–244, doi:10.1016/s1470-2045(07)70074-8.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30, doi:10.3322/caac.21590.
- Tan, P.H.; Ellis, I.; Allison, K.; Brogi, E.; Fox, S.B.; Lakhani, S.; Lazar, A.J.; Morris, E.A.; Sahin, A.; Salgado, R.; et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology 2020, 77, 181–185, doi:10.1111/his.14091.
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast cancer-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA A Cancer J. Clin. 2017, 67, 290–303, doi:10.3322/caac.21393.
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.; Zackrisson, S.; Senkus, E. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220, doi:10.1093/annonc/mdz173.
- Ruddy, K.J.; Ganz, P.A. Treatment of nonmetastatic breast cancer. JAMA 2019, 321, 1716–1717, doi:10.1001/jama.2019.3927.
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 1–31, doi:10.1038/s41572-019-0111-2.
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular subtypes and local-regional control of breast cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120, doi:10.1016/j.soc.2017.08.005.
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; Van Leeuwen, F.E.; Wesseling, J.; Cheang, M.C.; Gelmon, K.; Nielsen, T.O.; Blomqvist, C.; et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010, 7, e1000279, doi:10.1371/journal.pmed.1000279.
- American Cancer Society. Treatment of Triple Negative Breast Cancer. Treating Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/treatment-of-triple-negative.html (accessed on 14 September 2020).
- Abdelmegeed, S.M.; Mohammed, S. Canine mammary tumors as a model for human disease (Review). Oncol. Lett. 2018, 15, 8195–8205, doi:10.3892/ol.2018.8411.
- National Cancer Institute (NCI). Breast Cancer Treatment. Breast Cancer. Available online: https://www.cancer.gov/types/breast/hp/breast-treatment-pdq (accessed on 15 September 2020).
- Burstein, H.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer. Ann. Oncol. 2019, 30, 1541–1557, doi:10.1093/annonc/mdz235.
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300, doi:10.1001/jama.2018.19323.
- Curigliano, G.; Burstein, H.; Winer, E.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. De-escalating and escalating treatments for early-stage breast cancer: The St. Gallen International Expert Consensus Conference on the primary therapy of early breast cancer. Ann. Oncol. 2017, 28, 1700–1712, doi:10.1093/annonc/mdx308.
- Balic, M.; Thomssen, C.; Würstlein, R.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2019: A brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care 2019, 14, 103–110, doi:10.1159/000499931.
- Rangarao, R.; Smruti, B.K.; Singh, K.; Gupta, A.; Batra, S.; Choudhary, R.K.; Sahani, S.; Kabra, V.; Parikh, P.M.; Aggarwal, S. Practical consensus recommendations on management of triple-negative metastatic breast cancer. South Asian J. Cancer 2018, 7, 127–131, doi:10.4103/sajc.sajc_118_18.
- Gottesman, M.M. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002, 53, 615–627, doi:10.1146/annurev.med.53.082901.103929.
- Nikolaou, M.; Pavlopoulou, A.; Cetin, Z.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318, doi:10.1007/s10585-018-9903-0.
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nat. Cell Biol. 2019, 575, 299–309, doi:10.1038/s41586-019-1730-1.
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339–348, doi:10.15171/apb.2017.041.
- Min-Ji, W.; Lu, Y.; Tian, H.; Meng, X.; Wei, M.; Cho, W.C. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed. Pharmacother. 2019, 114, 108800, doi:10.1016/j.biopha.2019.108800.
- Hernandez-Aya, L.F.; Gonzalez-Angulo, A.M. Adjuvant systemic therapies in breast cancer. Surg. Clin. N. Am. 2013, 93, 473–491, doi:10.1016/j.suc.2012.12.002.
- Magnani, L.; Brunelle, M.; Gévry, N.; Lupien, M. Chromatin landscape and endocrine response in breast cancer. Epigenomics 2012, 4, 675–683, doi:10.2217/epi.12.64.
- Amorim, M.; Salta, S.; De Sousa, S.P.; Henrique, R. Predicting resistance to endocrine therapy in breast cancer: It’s time for epigenetic biomarkers (Review). Oncol. Rep. 2019, 41, 1431–1438, doi:10.3892/or.2019.6967.
- Stone, A.; Zotenko, E.; Locke, W.J.; Korbie, D.; Millar, E.K.A.; Pidsley, R.; Stirzaker, C.; Graham, P.; Trau, M.; Musgrove, E.A.; et al. DNA methylation of oestrogen-regulated enhancers defines endocrine sensitivity in breast cancer. Nat. Commun. 2015, 6, 7758, doi:10.1038/ncomms8758.
- Achinger-Kawecka, J.; Valdés-Mora, F.; Luu, P.-L.; Giles, K.A.; Caldon, C.E.; Qu, W.; Nair, S.; Soto, S.; Locke, W.J.; Yeo-Teh, N.S.; et al. Epigenetic reprogramming at estrogen-receptor binding sites alters 3D chromatin landscape in endocrine-resistant breast cancer. Nat. Commun. 2020, 11, 1–17, doi:10.1038/s41467-019-14098-x.
- Lustberg, M.; Ramaswamy, B. Epigenetic targeting in breast cancer: Therapeutic impact and future direction. Drug News Perspect. 2009, 22, 369–381, doi:10.1358/dnp.2009.22.7.1405072.
- Lapidus, R.G.; Ferguson, A.T.; Ottaviano, Y.L.; Parl, F.F.; Smith, H.S.; Weitzman, S.A.; Baylin, S.B.; Issa, J.P.; Davidson, N.E. Methylation of estrogen and progesterone receptor gene 5′ CpG islands correlates with lack of estrogen and progesterone receptor gene expression in breast tumors. Clin. Cancer Res. 1996, 2, 805–810.
- Verde, G.; Cucalon, L.I.D.L.; Wright, R.H.G.; Quilez, J.; Peiró, S.; LeDily, F.; Beato, M. Unliganded progesterone receptor governs estrogen receptor gene expression by regulating DNA methylation in breast cancer cells. Cancers 2018, 10, 371, doi:10.3390/cancers10100371.
- Drago, J.Z.; Formisano, L.; Juric, D.; Niemierko, A.; Servetto, A.; Wander, S.A.; Spring, L.M.; Vidula, N.; Younger, J.; Peppercorn, J.; et al. FGFR1 amplification mediates endocrine resistance but retains TORC sensitivity in metastatic hormone receptor–positive (HR+) breast cancer. Clin. Cancer Res. 2019, 25, 6443–6451, doi:10.1158/1078-0432.ccr-19-0138.
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690, doi:10.1038/nrclinonc.2016.66.
- Jang, H.S.; Woo, S.R.; Song, K.-H.; Cho, H.; Chay, D.B.; Hong, S.-O.; Lee, H.-J.; Oh, S.J.; Chung, J.-Y.; Kim, J.-H.; et al. API5 induces cisplatin resistance through FGFR signaling in human cancer cells. Exp. Mol. Med. 2017, 49, e374, doi:10.1038/emm.2017.130.
- Li, S.; Payne, S.; Wang, F.; Claus, P.; Su, Z.; Groth, J.; Geradts, J.; De Ridder, G.; Álvarez, R.; Marcom, P.K.; et al. Nuclear basic fibroblast growth factor regulates triple-negative breast cancer chemo-resistance. Breast Cancer Res. 2015, 17, 91, doi:10.1186/s13058-015-0590-3.
- Hu, Y.; Qiu, Y.; Yagüe, E.; Ji, W.; Liu, J.; Zhang, J. miRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer. Cell Death Dis. 2016, 7, e2291, doi:10.1038/cddis.2016.194.
- Wu, Q.; Wu, W.; Fu, B.; Shi, L.; Wang, X.; Kuca, K. JNK signaling in cancer cell survival. Med. Res. Rev. 2019, 39, 2082–2104, doi:10.1002/med.21574.
- Xiang, H.; Zhang, J.; Lin, C.; Zhang, L.; Liu, B.; Ouyang, L. Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm. Sin. B 2020, 10, 569–581, doi:10.1016/j.apsb.2019.10.003.
- Wang, Y.-W.; Zhang, W.; Ma, R. Bioinformatic identification of chemoresistance-associated microRNAs in breast cancer based on microarray data. Oncol. Rep. 2018, 39, 1003–1010, doi:10.3892/or.2018.6205.
- Yang, Z.; Liu, Z. The emerging role of microRNAs in breast cancer. J. Oncol. 2020, 2020, 1–7, doi:10.1155/2020/9160905.
- Wang, Y.; Rathinam, R.; Walch, A.; Alahari, S.K. ST14 (suppression of tumorigenicity 14) gene is a target for miR-27b, and the inhibitory effect of ST14 on cell growth is independent of miR-27b regulation. J. Biol. Chem. 2009, 284, 23094–23106, doi:10.1074/jbc.m109.012617.
- Yu, S.-J.; Hu, J.-Y.; Kuang, X.-Y.; Luo, J.-M.; Hou, Y.-F.; Di, G.-H.; Wu, J.; Shen, Z.-Z.; Song, H.-Y.; Shao, Z.-M. MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clin. Cancer Res. 2013, 19, 1389–1399, doi:10.1158/1078-0432.ccr-12-1959.
- Zhang, C.-M.; Zhao, Y.; Deng, H.-Y. MiR-155 promotes proliferation of human breast cancer MCF-7 cells through targeting tumor protein 53-induced nuclear protein J. Biomed. Sci. 2013, 20, 79, doi:10.1186/1423-0127-20-79.




