
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Inwoong Um | + 3413 word(s) | 3413 | 2021-05-10 12:15:45 | | | |
| 2 | Inwoong Um | Meta information modification | 3413 | 2021-05-26 09:42:32 | | |
Video Upload Options
: Studies on allogeneic demineralized dentin matrix (Allo-DDM) implantation in the 1960s and 1970s provided the most reliable preclinical evidence of bone formation and antigenicity in an extraosseous site. Recently, applications of Allo-DDM at skeletal sites were studied, and have provided reliable evidence of bone-forming capacity and negligible antigenicity. However, the osteoinductivity and antigenicity properties of Allo-DDM in extraskeletal sites have not yet been investigated due to the lack of follow-up studies after the initial research. This review aims to provide a foundation on the preclinical studies of Allo-DDM from 1960 to 2019, which could enable future researches on its osteogenic capability and antigenicity. In conclusion, Allo-DDM showed great potential for osteoinductivity in extraskeletal sites with low antigenicity, which neither adversely affected osteogenic capability nor provoked immunologic reactions.
1. Introduction
2. Osteoinductivity
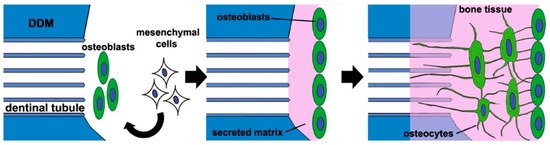
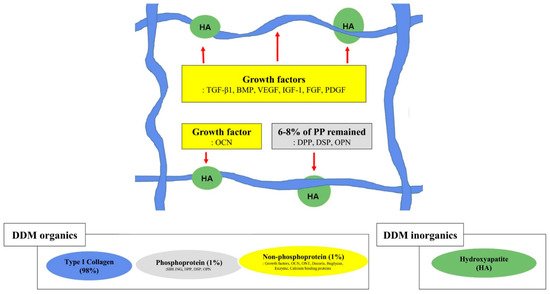
Figure 2. Graphical illustration of the structural relationships among the components of the extracellular matrix on demineralized dentin matrix [1][7][46]. Collagen and acid-insoluble non-collagenous protein networking. Type I collagen (in blue), hydroxyapatite (in green), non-phosphoprotein (in yellow), and phosphoprotein (in gray). The red arrow indicates hydroxyapatite binding; the red dotted arrow indicates collagen binding. SIBLING—small integrin-binding ligand, N-linked glycoprotein; DPP—dentin phosphoprotein; DSP—dentin sialoprotein; OPN—osteopontin; VEGF—vascular endothelial growth factor; BMP—bone morphogenetic protein; OCN—osteocalcin; IGF-1—insulin-like growth factor 1; FGF—fibroblast growth factor; PDGF—platelet-derived growth factor.
3. Antigenicity
4. Demineralization of Dentin Matrix
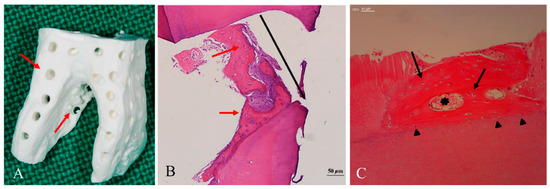
5. Geometry of Allo-DDM
References
- Avery, S.J.; Sadaghiani, L.; Sloan, A.J.; Waddington, R.J. Analysing the bioactive makeup of demineralised dentine matrix on bone marrow mesenchymal stem cells for enhanced bone repair. Eur. Cell Mater. 2017, 34, 1–14.
- Urist, M.R.; Silverman, B.F.; Büring, K.; Dubuc, F.L.; Rosenberg, J.M. The bone induction principle. Clin. Orthop. Relat. Res. 1967, 53, 243–283.
- Murata, M.; Okubo, N.; Shakya, M.; Kabir, M.; Yokozeki, K.; Zhu, B.; Ishikawa, M.; Kitamura, R.; Akazawa, T. Dentin Materials as Biological Scaffolds for Tissue Engineering. In Biomaterial-Supported Tissue Reconstruction or Regeneration; IntechOpen: London, UK, 2019; pp. 1–12.
- Kim, Y.-K.; Um, I.-W.; Murata, M. Tooth Bank System for Bone Regeneration—Safety Report. J. Hard Tissue Biol. 2014, 23, 371–376.
- Murata, M. Bone Engineering Using Human Demineralized Dentin Matrix and Recombinant Human BMP-2. J. Hard Tissue Biol. 2005, 14, 80–81.
- Kim, Y.-K.; Um, I.-W.; An, H.-J.; Kim, K.-W.; Hong, K.-S.; Murata, M. Effects of Demineralized Dentin Matrix Used as an rhBMP-2 Carrier for Bone Regeneration. J. Hard Tissue Biol. 2014, 23, 415–422.
- Butler, W.T.; Mikulski, A.; Urist, M.R.; Bridges, G.; Uyeno, S. Noncollagenous proteins of a rat dentin matrix possessing bone morphogenetic activity. J. Dent. Res. 1977, 56, 228–232.
- Kawai, T.; Urist, M.R. Bovine tooth-derived bone morphogenetic protein. J. Dent. Res. 1989, 68, 1069–1074.
- Bessho, K.; Tagawa, T.; Murata, M. Purification of rabbit bone morphogenetic protein derived from bone, dentin, and wound tissue after tooth extraction. J. Oral. Maxillofac. Surg. 1990, 48, 162–169.
- Bessho, K.; Tanaka, N.; Matsumoto, J.; Tagawa, T.; Murata, M. Human dentin-matrix-derived bone morphogenetic protein. J. Dent. Res. 1991, 70, 171–175.
- Murata, M. Autogenous demineralized dentin matrix for maxillary sinus augmentation in humans: The first clinical report. J. Dent. Res. 2003, 82, B243.
- Gomes, M.F.; Abreu, P.P.; Morosolli, A.R.; Araújo, M.M.; Goulart, M. Densitometric analysis of the autogenous demineralized dentin matrix on the dental socket wound healing process in humans. Braz. Oral. Res. 2006, 20, 324–330.
- Kim, Y.K.; Kim, S.G.; Byeon, J.H.; Lee, H.J.; Um, I.U.; Lim, S.C.; Kim, S.Y. Development of a novel bone grafting material using autogenous teeth. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2010, 109, 496–503.
- Minetti, E.; Giacometti, E.; Gambardella, U.; Contessi, M.; Ballini, A.; Marenzi, G.; Celko, M.; Mastrangelo, F. Alveolar Socket Preservation with Different Autologous Graft Materials: Preliminary Results of a Multicenter Pilot Study in Human. Materials 2020, 13, 1153.
- Li, P.; Zhu, H.; Huang, D. Autogenous DDM versus Bio-Oss granules in GBR for immediate implantation in periodontal postextraction sites: A prospective clinical study. Clin. Implant. Dent. Relat. Res. 2018, 20, 923–928.
- Kim, Y.K.; Lee, J.H.; Um, I.W.; Cho, W.J. Guided Bone Regeneration Using Demineralized Dentin Matrix: Long-Term Follow-Up. J. Oral. Maxillofac. Surg. 2016, 74, 515.e1–515.e9.
- Minetti, E.; Palermo, A.; Contessi, M.; Gambardella, U.; Schmitz, J.; Giacometti, E.; Celko, M.; Trisi, P. Autologous tooth graft for maxillary sinus augmentation: A multicenter clinical study. Int. J. Growth Factors Stem. Cells Dent. 2019, 2, 45–51.
- Del Canto-Díaz, A.; de Elío-Oliveros, J.; Del Canto-Díaz, M.; Alobera-Gracia, M.A.; Del Canto-Pingarrón, M.; Martínez-González, J.M. Use of autologous tooth-derived graft material in the post-extraction dental socket. Pilot study. Med. Oral. Patol. Oral. Y Cir. Bucal 2019, 24, e53–e60.
- Tanoue, R.; Ohta, K.; Miyazono, Y.; Iwanaga, J.; Koba, A.; Natori, T.; Iwamoto, O.; Nakamura, K.I.; Kusukawa, J. Three-dimensional ultrastructural analysis of the interface between an implanted demineralised dentin matrix and the surrounding newly formed bone. Sci. Rep. 2018, 8, 2858.
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899.
- Zhang, M.; Powers, R.M., Jr.; Wolfinbarger, L., Jr. Effect(s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J. Periodontol. 1997, 68, 1085–1092.
- Glowacki, J. A review of osteoinductive testing methods and sterilization processes for demineralized bone. Cell Tissue Bank 2005, 6, 3–12.
- Bang, G.; Urist, M.R. Bone induction in excavation chambers in matrix of decalcified dentin. Arch. Surg. 1967, 94, 781–789.
- Yeomans, J.D.; Urist, M.R. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch. Oral. Biol. 1967, 12, 999–1008.
- Urist, M.R.; Dowell, T.A.; Hay, P.H.; Strates, B.S. Inductive substrates for bone formation. Clin. Orthop. Relat. Res. 1968, 59, 59–96.
- Huggins, C.B.; Urist, M.R. Dentin matrix transformation: Rapid induction of alkaline phosphatase and cartilage. Science 1970, 167, 896–898.
- Huggins, C.; Wiseman, S.; Reddi, A.H. Transformation of fibroblasts by allogeneic and xenogeneic transplants of demineralized tooth and bone. J. Exp. Med. 1970, 132, 1250–1258.
- Bang, G. Induction of heterotopic bone formation by demineralized dentin in guinea pigs: Antigenicity of the dentin matrix. J. Oral. Pathol. 1972, 1, 172–185.
- Reddi, A.H.; Huggins, C.B. Influence of geometry of transplanted tooth and bone on transformation of fibroblasts. Proc. Soc. Exp. Biol. Med. 1973, 143, 634–637.
- Bang, G. Induction of heterotopic bone formation by demineralized dentin: An experimental model in guinea pigs. Scand. J. Dent. Res. 1973, 81, 240–250.
- Linden, G.J. Bone induction in implants of decalcified bone and dentine. J. Anat. 1975, 119, 359–367.
- Nilsen, R. Electron microscopy of induced heterotopic bone formation in guinea pigs. Arch. Oral. Biol. 1977, 22, 485–493.
- Inoue, T.; Deporter, D.A.; Melcher, A.H. Induction of chondrogenesis in muscle, skin, bone marrow, and periodontal ligament by demineralized dentin and bone matrix in vivo and in vitro. J. Dent. Res. 1986, 65, 12–22.
- Pinholt, E.M.; Bang, G.; Haanaes, H.R. Alveolar ridge augmentation by osteoinduction in rats. Scand. J. Dent. Res. 1990, 98, 434–441.
- Bang, G.; Nordenram, Å.; Anneroth, G. Allogenic demineralized dentin implants in jaw defects of Java monkeys. Int. J. Oral. Surg. 1972, 1, 126–136.
- Bakhshalian, N.; Hooshmand, S.; Campbell, S.C.; Kim, J.S.; Brummel-Smith, K.; Arjmandi, B.H. Biocompatibility and microstructural analysis of osteopromotive property of allogenic demineralized dentin matrix. Int. J. Oral. Maxillofac. Implant. 2013, 28, 1655–1662.
- Bakhshalian, N.; Jalayer, T.; Shahoon, H.; Arjmandi, B.H.; Azimi, H.R. Osteopromotive property of allogenic demineralized dentin matrix: A pilot study. J. West. Soc. Periodontol. Periodontal Abstr. 2013, 61, 35–38.
- Um, I.-W.; Kim, Y.-K.; Jun, S.-H.; Kim, M.-Y.; Cui, N. Demineralized Dentin Matrix as a Carrier of Recombinant Human Bone Morphogenetic Proteins: In vivo Study. J. Hard Tissue Biol. 2018, 27, 219–226.
- Um, I.W.; Ku, J.K.; Kim, Y.K.; Lee, B.K.; Leem, D.H. Histological Review of Demineralized Dentin Matrix as a Carrier of rhBMP-2. Tissue Eng. Part. B Rev. 2020, 26, 284–293.
- Dubuc, F.L.; Urist, M.R. The accessibility of the bone induction principle in surface-decalcified bone implants. Clin. Orthop. Relat. Res. 1967, 55, 217–223.
- Carvalho, V.A.; Tosello Dde, O.; Salgado, M.A.; Gomes, M.F. Histomorphometric analysis of homogenous demineralized dentin matrix as osteopromotive material in rabbit mandibles. Int. J. Oral. Maxillofac. Implant. 2004, 19, 679–686.
- Gomes, M.F.; Banzi, E.C.; Destro, M.F.; Lavinicki, V.; Goulart, M. Homogenous demineralized dentin matrix for application in cranioplasty of rabbits with alloxan-induced diabetes: Histomorphometric analysis. Int. J. Oral. Maxillofac. Implant. 2007, 22, 939–947.
- Gomes, M.F.; Destro, M.F.; Banzi, E.C.; Vieira, E.M.; Morosolli, A.R.; Goulart, M. Optical density of bone repair after implantation of homogenous demineralized dentin matrix in diabetic rabbits. Braz. Oral. Res. 2008, 22, 275–280.
- Gomes, M.F.; Valva, V.N.; Vieira, E.M.; Giannasi, L.C.; Salgado, M.A.; Vilela-Goulart, M.G. Homogenous demineralized dentin matrix and platelet-rich plasma for bone tissue engineering in cranioplasty of diabetic rabbits: Biochemical, radiographic, and histological analysis. Int. J. Oral. Maxillofac. Surg. 2016, 45, 255–266.
- Urist, M.R.; Iwata, H.; Strates, B.S. Bone morphogenetic protein and proteinase in the guinea pig. Clin. Orthop. Relat. Res. 1972, 85, 275–290.
- Masaru, M. Collagen biology for bone regenerative surgery. J. Korean Assoc. Oral. Maxillofac. Surg. 2012, 38, 321–325.
- Fuentes, G.C.; Newgren, J. Physiology and clinical pathology of laboratory new zealand white rabbits housed individually and in groups. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 35–38.
- Urist, M.R.; Mikulski, A.; Boyd, S.D. A chemosterilized antigen-extracted autodigested alloimplant for bone banks. Arch. Surg. 1975, 110, 416–428.
- Horowitz, M.C.; Friedlaender, G.E. Induction of specific T-cell responsiveness to allogeneic bone. J. Bone Jt. Surg. Am. 1991, 73, 1157–1168.
- Mikulski, A.J.; Urist, M.R. An antigenic antimorphogenetic bone hydrophobic glycopeptide (AHG). Prep. Biochem. 1975, 5, 21–37.
- Russell, J.L.; Block, J.E. Clinical utility of demineralized bone matrix for osseous defects, arthrodesis, and reconstruction: Impact of processing techniques and study methodology. Orthopedics 1999, 22, 524–531, quiz 532–533.
- Um, I.W.; Ku, J.K.; Lee, B.K.; Yun, P.Y.; Lee, J.K.; Nam, J.H. Postulated release profile of recombinant human bone morphogenetic protein-2 (rhBMP-2) from demineralized dentin matrix. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 123–128.
- Murata, M.; Akazawa, T.; Mitsugi, M.; Um, I.W.; Kim, K.W.; Kim, Y.K. Human dentin as novel biomaterial for bone regeneration. In Pignatello R, 1st ed.; InTech: New York, NY, USA, 2011; pp. 127–140.
- Ike, M.; Urist, M.R. Recycled dentin root matrix for a carrier of recombinant human bone morphogenetic protein. J. Oral. Implant. 1998, 24, 124–132.
- Koga, T.; Minamizato, T.; Kawai, Y.; Miura, K.-I.; I, T.; Nakatani, Y.; Sumita, Y.; Asahina, I. Bone Regeneration Using Dentin Matrix Depends on the Degree of Demineralization and Particle Size. PLoS ONE 2016, 11, e0147235.
- Um, I.W.; Kim, Y.K.; Mitsugi, M. Demineralized dentin matrix scaffolds for alveolar bone engineering. J. Indian Prosthodont. Soc. 2017, 17, 120–127.
- Bormann, K.H.; Suarez-Cunqueiro, M.M.; Sinikovic, B.; Kampmann, A.; von See, C.; Tavassol, F.; Binger, T.; Winkler, M.; Gellrich, N.C.; Rücker, M. Dentin as a suitable bone substitute comparable to ß-TCP—An experimental study in mice. Microvasc. Res. 2012, 84, 116–122.
- Al-Namnam, N.; Shanmuhasuntharam, P.; Ha, K.O.; Siar, C.H. Processed allogenic dentine as a scaffold for bone healing: An in vivo study. Aust. J. Basic Appl. Sci. 2010, 4, 5932–5940.
- Rijal, G.; Shin, H.I. Human tooth-derived biomaterial as a graft substitute for hard tissue regeneration. Regen. Med. 2017, 12, 263–273.
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987, 20, 263–272.
- Owen, M. Marrow stromal stem cells. J. Cell Sci. Suppl. 1988, 10, 63–76.
- Folkman, J.; Greenspan, H.P. Influence of geometry on control of cell growth. Biochim. Et Biophys. Acta (BBA) Rev. Cancer 1975, 417, 211–236.
- Kim, Y.K.; Pang, K.M.; Yun, P.Y.; Leem, D.H.; Um, I.W. Long-term follow-up of autogenous tooth bone graft blocks with dental implants. Clin. Case Rep. 2017, 5, 108–118.
- Moon, Y.S.; Sohn, D.S.; Kim, G.; Park, I. Comparative Histomorphometric Evaluation of Bone Regeneration with Different Preparations of Xenogeneic Tooth Block Bone. Int. J. Oral. Maxillofac. Implant. 2019, 34, 1413–1422.
- Kabir, M.A.; Murata, M.; Akazawa, T.; Kusano, K.; Yamada, K.; Ito, M. Evaluation of perforated demineralized dentin scaffold on bone regeneration in critical-size sheep iliac defects. Clin. Oral. Implant. Res. 2017, 28, e227–e235.
- Glowacki, J.; Altobelli, D.; Mulliken, J.B. Fate of mineralized and demineralized osseous implants in cranial defects. Calcif. Tissue Int. 1981, 33, 71–76.
- Dozza, B.; Lesci, I.G.; Duchi, S.; Della Bella, E.; Martini, L.; Salamanna, F.; Falconi, M.; Cinotti, S.; Fini, M.; Lucarelli, E.; et al. When size matters: Differences in demineralized bone matrix particles affect collagen structure, mesenchymal stem cell behavior, and osteogenic potential. J. Biomed. Mater. Res. A 2017, 105, 1019–1033.
- Nam, J.W.; Kim, M.Y.; Han, S.J. Cranial bone regeneration according to different particle sizes and densities of demineralized dentin matrix in the rabbit model. Maxillofac. Plast Reconstr. Surg. 2016, 38, 27.
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 2011, 32, 4525–4538.
- Kim, Y.K.; Kim, S.G.; Yun, P.Y.; Yeo, I.S.; Jin, S.C.; Oh, J.S.; Kim, H.J.; Yu, S.K.; Lee, S.Y.; Kim, J.S.; et al. Autogenous teeth used for bone grafting: A comparison with traditional grafting materials. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2014, 117, e39–e45.




