
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Soon Hee Kim | + 3243 word(s) | 3243 | 2020-11-24 10:15:12 | | | |
| 2 | Peter Tang | -213 word(s) | 3030 | 2020-12-03 14:25:25 | | |
Video Upload Options
Three-dimensional (3D) printing technology holds great potential to fabricate complex constructs in the field of regenerative medicine. Researchers in the surgical fields have used 3D printing techniques and their associated biomaterials for education, training, consultation, organ transplantation, plastic surgery, surgical planning, dentures, and more. In addition, the universal utilization of 3D printing techniques enables researchers to exploit different types of hardware and software in, for example, the surgical fields. To realize the 3D-printed structures to implant them in the body and tissue regeneration, it is important to understand 3D printing technology and its enabling technologies.
1. Introduction
Recently, three-dimensional (3D) printing techniques have been evolving rapidly. 3D printing is based on the principle of building objects by adding materials layer by layer [1]. Since the invention of a stereolithography apparatus (SLA) in 1984 by Charles W. Hullin, novel 3D printing methods and materials have been introduced [2][3]. 3D printing refers to the volumetric presswork of digital data converted from an imaginary model, whereas two-dimensional (2D) printing is typically achieved on paper [4]. These techniques have rapidly been adopted across many fields [5]. The ubiquitous presence of Internet computing and the availability of 3D printing enable the personalization of products tailored to individual needs. This progress enables engineers to develop various types of hardware and software that are beneficial in diverse areas [6]. 3D printing involves the use of various materials, such as filament, powder, resin, and paper [7].
Researchers in the surgical fields utilize these techniques due to their great potential [1][5][6][7][8][9]. 3D printing has significant implications in various surgical fields, such as education, training, consultation, surgical planning, organ transplantation, plastic surgery, and prosthesis [10][11][12][13][14]. Commercial manufacturers are developing low-priced, small-scale, customized products for patients [10]. For surgeons, a virtual 3D architecture from 2D presurgical images is limited by the difficult intuitive comprehension of interspatial relationships [15]. However, information retrieved from 3D-printed products can help surgeons augment sensory perception using tactile feedback. Additionally, different materials and devices are being tested for novel surgical applications. Recently, the number of reports has increased regarding the utilization of 3D printing techniques to solve the medical dilemma of the need for artificial organs owing to the shortage of transplantable organs caused by accelerated low birth rates and the aging population [5][6][16][17][18][19].
In addition, 3D bioprinting provides precise spatiotemporal control of bioactive substances including cells, proteins, DNA, drugs, and growth factors, making them more effective in tissue formation for patient-specific treatment [20]. 3D bioprinting has the potential to fabricate large structures; however, it is difficult to image the resulting structures with superficial imaging modalities. The imaging of engineered structures fabricated with a thickness of millimeter (mm) or more will be important for surgically implanting 3D printed structures. The 3D imaging of engineered tissues is of great importance in obtaining information on structures as well as cells seeded in structures by printing. After transplantation, the implant is subjected to a number of fluctuations in vivo environments such as temperature, fluid flow, and transplant depth. Therefore, it is recommended to monitor the 3D structure in a non-invasive manner in real time. Different imaging modalities have been used to monitor non-invasively implants in vivo. Near-infrared (NIR) fluorescence that presents minimal background autofluorescence, reduced light scattering, and low tissue absorption can be a safe and cost-effective imaging source with a high resolution and high sensitivity [21].
2. Hardware in 3D Printing Techniques
3D printer hardware can be categorized into seven types according to their discriminative process, although innovative products are under development [3]. These types are extrusion, direct energy deposition, sheet lamination, light polymerization, jetting, sintering, and binder jetting. Representative 3D printers include fused deposition modeling (FDM) for extrusion, direct metal deposition (DMD) for direct energy deposition, laminated object manufacturing (LOM) for lamination, SLA and digital light processing (DLP) for light polymerization, color jet 3D printing (CJP) for binder jetting, polyjet for jetting, and selective laser sintering (SLS) and selective laser melting (SLM) for sintering (Figure 1) [22].
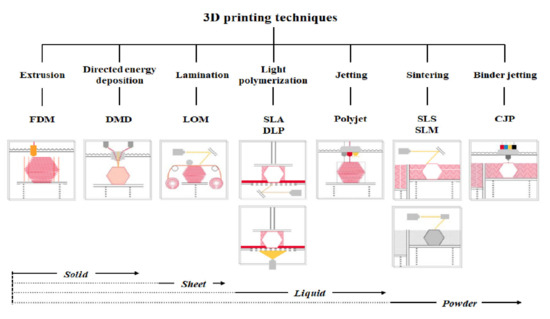
Figure 1. Three-dimensional printers classified according to type and material.
FDM as a universal modeling system in surgery involves building an extruded viscous fluid layer by layer after a heated nozzle melts a solid material [9][23][24]. FDM is cheaper and easier compared with other types. Various materials can be printed using FDM. However, the printout surface is rough despite the low output speed. Therefore, FDM necessitates a burdensome post-printing process. The extruder is the salient part of FDM for printing thermoplastic filaments (Figure 2a). This extruder comprises cold and hot ends (Figure 2b). The cold end feeds the filament to the hot end, which extrudes the filament melted by the extruder nozzle. The union of cold and hot ends moves together in a direct extruder, which is the primary and original extruder in FDM (Figure 2c). In a direct extruder, wheels can push a filament continuously through a nozzle because of the short distance between the cold and hot ends. Furthermore, the filament can easily be replaced. However, this heavy structure moves unevenly, which adversely affects the output. Additionally, undesired effects can occur owing to a change in direction. Therefore, FDM should be equipped with a heavy direct extruder and allow the end structures to move slowly. Hence, the Bowden extruder, which separates a filament feeder from a nozzle, was developed. In this extruder, a filament is supplied through a tube (Figure 2d). The Bowden extruder affords vibration control and fast printing because only the hot end moves. Nonetheless, the long distance between the feeder and nozzle renders it difficult to supply the filament. While the filament passes through the tube from feeder to the nozzle, the elasticity of the tube hampers the desired advancement of the filament. Hence, flexible polylactic acid (PLA) is not used in the Bowden extruder, as it can cause the breakage or deformation of the extruder in the long distance between the feeder and nozzle.
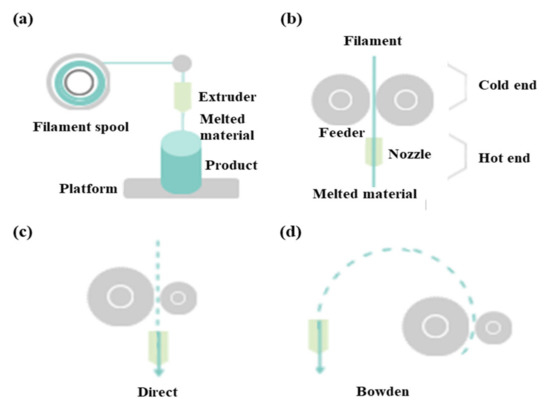
Figure 2. Differences between direct extruder and Bowden extruder in fused deposition modeling: (a) printing filament through an extruder, (b) distinction between cold and hot ends, (c) direct extruder, (d) Bowden extruder three-dimensional printers classified according to type and material.
In DMD, heat from the laser, plasma arc, and electron beam melts materials simultaneously as the material (typically wire or metallic powder) is deposited. Furthermore, a vacuum environment is necessary to prevent electrons from interacting with molecules in the air. If this process is repeated, layers are solidified and yield an object. Additionally, an LOM technique cuts resin film or paper into certain shapes and then glues them together [24][25]. Post-processing required for this technique includes mechanical grinding, drilling, and machining.
The SLA of vat photopolymerization has been used for the longest in 3D printing. SLAs comprise a vat containing resin with a platform at the bottom. Ultraviolet (UV) laser selectively cures the resin layer by layer. The completely cured layer ascends (or descends) and is covered uniformly by resin in the vat. After printing is completed, a secondary curing may be required depending on the resin [9][24]. A photocurable liquid resin is also used in DLP, but the related process differs slightly from that of SLAs. In DLP, an entire layer of resin is used using a UV lamp and a dimming device similar to an image projector, whereas the UV laser moves linearly in SLAs [26]. Compared with SLAs, DLP is faster and cheaper. The photopolymerization performed in these techniques enables exquisite designs and rapid printing with low noise. The support structure can be removed easily during post-processing. Although a limited amount of material is used in photopolymerization, the material and bulky equipment required are expensive. More importantly, the resin should be handled with care. Although the cured printout is not harmful, masks and gloves are necessary under the circumstances of patent air ventilation because liquid resin on a printout surface is toxic [9].
The CJP of binder jetting spurts colored liquid adhesives and hardens fine powder [26]. Although CJP occurs quickly, the printout is brittle, and its strength depends on the adhesives used. The bulky equipment used in CJP is expensive and not suitable for personal purposes. Meanwhile, a polyjet combines the techniques of SLAs and CJP, where a UV lamp immediately cures the resin sprayed layer by layer from the head. Subsequently, those layers are laminated for a complete output. Various materials and colors are used more exquisitely in polyjets than in CJP, although supports are more difficult to remove. The material and equipment required in this technique are also expensive.
In SLS, a ruler spreads powder evenly on a platform [9]. Subsequently, a laser melts and fuses the powder selectively. These processes are repeated layer by layer in SLS. SLS does not require support for printout because the unsintered power can provide support. Therefore, the surface quality is excellent, although post-processing is required. Metal materials exhibit merits in strength and speed, which can be linked to mass production. However, SLS is bulky and expensive. Additionally, protective masks and post-processing booths are recommended for SLS. Finally, SLM melts and fuses metallic powder, whereas the laser selectively sinters powdered material in SLS [9]. In SLM, the material property is improved, although more energy is consumed.
There are difference price ranges on the market, depending on the types of 3D printer hardware. There are also a wide range of prices in the same type, depending on whether they are for consumer or industrial use. Based on the available market information as of 2020, the usual price of 3D printer hardware is as follows: FDM (USD 5000), DMD (USD 500,000), LOM (USD 15,000), SLA (USD 3500), DLP (USD 10,000), polyjet (USD 43,000), SLS (USD 10,000), SLM (USD 80,000), and CJP (USD 100,000).
3. Software in 3D Printing Techniques
3D printing requires prior digital conversion in software. The overall processes are composed of modeling output sculptures, slicing geometric code (G-code), and transferring G-code [27]. G-code is a programming language recognized by 3D printers. A model source refers to files containing model information of 3D shape as the output of 3D printers. The file extension of the basic model source in 3D printers is stereolithography (STL) [23]. The STL format encodes the surface geometry of 3D objects. This format represents the surface of a model as triangular meshes [26]. A basic triangular unit is referred to as a polygon or mesh [19]. The STL format utilizes triangles because they are the least-constituent polygon, and all 3D surfaces can be divided into triangles of the simplest polygon. The smaller the triangular size and the larger the triangular number, the more exquisite the object becomes, and accordingly, the capacity increases exponentially. Slicer is a software that converts 3D data into 2D data of layers that are stacked sequentially for a certain shape. A 3D printer operates based on the G-code created in the slicer [27]. Additionally, the slicer determines the approach to fill up the empty inner space of the model source, which contains only surface information. After completing all settings including temperature and output speed, slicer creates a G-code to operate the 3D printer [28].
In surgeries, digital imaging and communication in medicine (DICOM) from computed tomography or magnetic resonance imaging of patients is preferentially converted to STL for 3D modeling (Figure 3a) [9][18][29][30]. Therefore, 3D design software is essential for 3D modeling (Figure 3b). An STL file can be modified adjunctively in 3D design software if necessary. Subsequently, the slicer transfers 3D data to a 3D printer for the object output, which can recognize only G-code (Figure 3c) [9][27][31]. Slicer can form crosslinks, adjust sizes, and configure various conditions, including speed, temperature, thickness, and infill density, according to the 3D printer type and material [17][23][32]. Finally, post-processing fabrication is performed to yield the final object (Figure 3d).
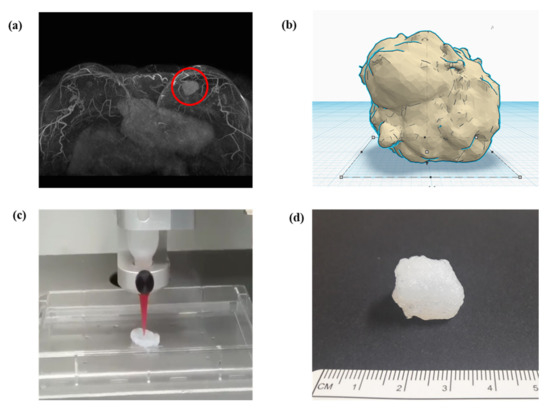
Figure 3. Schematic of converting a structure to (a) a 3D model through software and the final printout: a magnetic resonance imaging converted to stereolithography file, (b) modification in 3D model of stereolithography, (c) G-code generation in slicing, which is then delivered to a 3D printer, and(d) final printout after processing.
The results are significantly affected by the set layer thickness. In thin layers, the output surface becomes elaborate and thin projection is post-processed readily. However, thick layers exacerbate the staircase effect on rough surfaces. The diameter of a nozzle should be set accurately for filament extrusion [23][33]. The shell means the outer wall of a 3D model. The thickness of the shell refers to the thickness of the outer wall, which combines the outermost and a few inner layers [23]. This number must be an integer multiple of the nozzle diameter. A larger thickness yields sturdier objects. The bottom and top layers are laid down before or after infill printing. The thicknesses of the bottom and top layers should be a multiple of the layer height to cover the infill adequately. Additionally, a higher infill density enables a denser and stronger lattice [23][34]. Support can be important depending on the 3D printer type. A number of variables should be considered for support, including protrusion sustenance, ease of support removal, and quality of output surface. Each slicer provides a different type of support. Moreover, before printing the first layer of the 3D model in FDM, for the adherence of the output to the platform, the software devises diverse methods such as skirt, raft, and brim. The skirt refers to borders created at regular intervals around the first layer. Raft means a layered wide plate on the platform, which places an interface between layers, and is the most reliable method to fix a printout. However, the raft requires more time and material for the printout. It can be difficult to separate this structure from the output. The brim forms a wide contact area on the platform, in which borders are repeatedly created along the border of the first layer. Compared to the raft, the brim requires a shorter time and can be easily removed.
Prontface and Cura can be used as open source host software to control 3D printers. Prontface can activate and monitor 3D printers; Cura can manipulate 3D printers diversely and create a G-code for printout. In addition, Marlin and Repetier-firmware are open source firmware software mounted on the control panel. Marlin is a popular 3D printer driver and enables the full control of the process. Repetier-firmware is more complex owing to the minute setting, which is highly compatible with its own host software.
4. Materials in 3D Printing Techniques
Different materials are used according to the type of 3D printer. Extrusion and direct energy deposition are based on solid materials (Figure 1). Hence, acrylonitrile butadiene styrene (ABS) and PLA are representative materials in FDM [23][26]. An ABS filament as a light synthetic material is capable of acetone fumigation in post-processing, although it tends to contract severely. In contrast, a PLA filament as a heavy eco-friendly material (corn starch) shrinks less, although it is incapable of acetone fumigation [23]. Liquid materials such as photocurable liquid resin and casting wax are utilized for light polymerization and jetting, whereas powder materials are essential for sintering and binder jetting. Therefore, synthetic resin, metal, and clay are used as representative materials in SLS [2][10][35]. Furthermore, thin-layered materials, such as paper, polymer, or metal foil, are used for sheet lamination [6].
Silicone as a polymer material is widely applied in the surgical fields [4]. Recently, silicone has been used widely from homes to industries owing to its desirable properties, such as non-ionicity, non-polarity, hydrophobicity, water repellency, thermostability, oxidative stability, frigostability, gas permeability, chemical inertness, environmental friendliness, and non-toxicity [29][36]. The safety profile of silicone enables its practical applications in medicine, pharmaceuticals, cosmetics, and food production, where FDA approval is necessary. Medical silicone appears in the poppets of artificial heart valves, breast implants, plastic prostheses, wound dressing, and catheters [36]. However, external pressures can cause defects in silicone products, which have deficient tear strength and inferior resistance to fatigue. The development of silicone that is more suitable for the human body is in progress through the diversification of various silicone formulations [16][19][37][38]. Because researchers have reported breast-implant-associated anaplastic large-cell lymphoma in certain products, additional research should be conducted regarding this issue [39].
Biomaterials in surgeries are designed for in vivo implants or interactions with the body without exhibiting biologic, pharmacological, or histological reactions. Therefore, biomaterials can complement or replace functions of damaged tissues or organs [40]. Biomaterials for the treatment of human diseases imply implant materials that exhibit biocompatibility, i.e., materials that do not cause adverse reactions or toxic responses when exposed to a patient’s tissue, blood, or body fluid [25][28][41]. Neither toxicity nor carcinogenicity should occur during the implantation or degradation of biomaterials in the human body [42]. Additionally, biomaterials should exhibit appropriate mechanical properties without deterioration in physical properties for replacing human tissues [5][43][44].
It is important to fabricate a supporting structure comprising regeneration tissues or organs [45]. The supporting structure should facilitate cellular adhesion, proliferation, and differentiation, which can provide an extracellular matrix environment similar to the human body [41]. This structure should exhibit mechanical properties that are suitable for the implant site. Porosity in the supporting structure induces tissue regeneration by allowing cells to infiltrate and nutrients (or oxygen) to flow. Researchers have continued to apply various chemical, physical, and mechanical techniques to this structure [34][37][45][46].
A biodegradable absorbable polymer implies a complete degradation of implants in the human body, which facilitates the adhesion of cells or tissues in regenerative medicine [23]. Additionally, this polymer can be used for drug delivery systems [41][47][48][49]. Therefore, biodegradable polymers are suitable for bioprinting when they comprise metabolites or harmless water-soluble polymer units and exhibit chemical structures that enable hydrolysis. It is propitious to utilize hydrophilic biodegradable polymers that can contain cells and bioactive materials in hydrogels [50][51]. Three-dimensional printing polymeric biomaterials include fibrin, collagen, gelatin, alginate, hyaluronic acid, polyethylene glycol (PEG), Matrigel®, agarose, ABS, polycaprolactone (PCL), PLA, and polylactic-co-glycolic acid (Figure 4) [37][47][50][52][53].
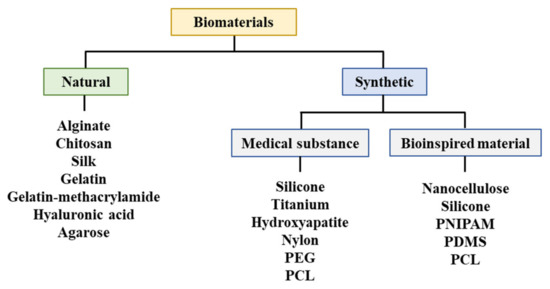
Figure 4. Materials utilized for bioprinting. PEG: Polyethylene glycol, PCL: Polycaprolactone, PNIPAM: Poly(N-isopropylacrylamide), PDMS: Polydimethylsiloxane.
According to the available market data for 2020, the price of each different material is as follows: ABS (USD 0.067/g), PLA (USD 0.067/g), resin (USD 50/L), polyadmide (USD 1.4/g), metal powder (USD 0.45/g), wax (USD 200/L), silk fibroin (USD 290/g), alginate (USD 1600/g), chitosan (USD 1.27/g), gelatin-methacrylamide (USD 206/g), hyaluronic acid (USD 60,200/g), agarose (USD 5.83/g), silicone (USD 670/L), titanium (USD 20.3/g), hydroxyapatite (USD 84,200/L), nylon (USD 1.87/g), PEG (USD 1230/g), PCL (USD 9.78/g), nanocellulose (USD 86.9/L), PNIPAM (USD 65.8/g), and PDMS (USD 70/g). Owing to the low filament cost, FDM remains the most popular type of 3D printing hardware.
References
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2019, 4, 370–380.
- Lin, K.; Sheikh, R.; Romanazzo, S.; Roohani, I. 3D printing of bioceramic scaffolds—barriers to the clinical translation: From promise to reality, and future perspectives. Materials 2019, 12, 2660.
- Ratinam, R.; Quayle, M.; Crock, J.; Lazarus, M.; Fogg, Q.; McMenamin, P. Challenges in creating dissectible anatomical 3D prints for surgical teaching. J. Anat. 2019, 234, 419–437.
- Kryou, C.; Leva, V.; Chatzipetrou, M.; Zergioti, I. Bioprinting for liver transplantation. Bioengineering 2019, 6, 95.
- Papaioannou, T.G.; Manolesou, D.; Dimakakos, E.; Tsoucalas, G.; Vavuranakis, M.; Tousoulis, D. 3D bioprinting methods and techniques: Applications on artificial blood vessel fabrication. Acta Cardiol. Sin. 2019, 35, 284.
- Pietrabissa, A.; Marconi, S.; Negrello, E.; Mauri, V.; Peri, A.; Pugliese, L.; Marone, E.M.; Auricchio, F. An overview on 3D printing for abdominal surgery. Surg. Endosc. 2020, 34, 1–13.
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115.
- Ruiters, S.; Mombaerts, I. Applications of three-dimensional printing in orbital diseases and disorders. Curr. Opin. Ophthalmol. 2019, 30, 372–379.
- Tong, Y.; Kaplan, D.J.; Spivak, J.M.; Bendo, J.A. Three-dimensional printing in spine surgery: A review of current applications. Spine J. 2019, 20, 833–846.
- Liaw, C.-Y.; Guvendiren, M. Current and emerging applications of 3D printing in medicine. Biofabrication 2017, 9, 024102.
- Galliger, Z.; Vogt, C.D.; Panoskaltsis-Mortari, A. 3D bioprinting for lungs and hollow organs. Transl. Res. 2019, 211, 19–34.
- Smith, B.; Dasgupta, P. 3D printing technology and its role in urological training. World J. Urol. 2019, 38, 2385–2391.
- Jiang, M.; Chen, G.; Coles-Black, J.; Chuen, J.; Hardidge, A. Three-dimensional printing in orthopaedic preoperative planning improves intraoperative metrics: A systematic review. ANZ J. Surg. 2020, 90, 243–250, doi:10.1111/ans.15549.
- Velázquez, J.S.; Cavas, F.; Bolarín, J.M.; Alió, J.L. 3D printed personalized corneal models as a tool for improving patient’s knowledge of an asymmetric disease. Symmetry 2020, 12, 151.
- Pugliese, L.; Marconi, S.; Negrello, E.; Mauri, V.; Peri, A.; Gallo, V.; Auricchio, F.; Pietrabissa, A. The clinical use of 3D printing in surgery. Updates Surg. 2018, 70, 381–388.
- Mitsouras, D.; Liacouras, P.; Imanzadeh, A.; Giannopoulos, A.A.; Cai, T.; Kumamaru, K.K.; George, E.; Wake, N.; Caterson, E.J.; Pomahac, B. Medical 3D printing for the radiologist. Radiographics 2015, 35, 1965–1988.
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3D printing in medical applications: A state of the art. J. Healthc. Eng. 2019, 5340616.
- Parthasarathy, J.; Krishnamurthy, R.; Ostendorf, A.; Shinoka, T.; Krishnamurthy, R. 3D printing with MRI in pediatric applications. J. Magn. Reson. Imaging 2020, 51, 1641–1658.
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D printing in medicine for preoperative surgical planning: A review. Ann. Biomed. Eng. 2020, 48, 536–555.
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118.
- Choi, H.S.; Gibbs, S.L.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Liu, F.; Hyun, H.; Park, G.; Xie, Y.; Bae, S. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat. Biotechnol. 2013, 31, 148–153.
- Zhang, S.; Wang, H. Current Progress in 3D Bioprinting of Tissue Analogs. Slas Technol 2019, 24, 70–78, doi:10.1177/2472630318799971.
- Mazzanti, V.; Malagutti, L.; Mollica, F. FDM 3D printing of polymers containing natural fillers: A review of their mechanical properties. Polymers 2019, 11, 1094, doi:10.3390/polym11071094.
- Chen, M.Y.; Skewes, J.; Desselle, M.; Wong, C.; Woodruff, M.A.; Dasgupta, P.; Rukin, N.J. Current applications of three-dimensional printing in urology. Bju Int. 2020, 125, 17–27, doi:10.1111/bju.14928.
- Zhang, J.M.; Ji, Q.; Duan, H. Three-dimensional printed devices in droplet microfluidics. Micromachines 2019, 10, 754, doi:10.3390/mi10110754.
- Han, T.; Kundu, S.; Nag, A.; Xu, Y. 3D printed sensors for biomedical applications: A Review. Sensors 2019, 19, 1706, doi:10.3390/s19071706.
- Jessop, Z.M.; Al-Sabah, A.; Gardiner, M.D.; Combellack, E.; Hawkins, K.; Whitaker, I.S. 3D bioprinting for reconstructive surgery: Principles, applications and challenges. J. Plast. Reconstr. Aesthet. Surg. 2017, 70, 1155–1170, doi:10.1016/j.bjps.2017.06.001.
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434, doi:10.1016/j.biotechadv.2015.12.011.
- Mussi, E.; Furferi, R.; Volpe, Y.; Facchini, F.; McGreevy, K.S.; Uccheddu, F. Ear reconstruction simulation: From handcrafting to 3D printing. Bioengineering 2019, 6, 14, doi:10.3390/bioengineering6010014.
- Virzi, A.; Muller, C.O.; Marret, J.B.; Mille, E.; Berteloot, L.; Grevent, D.; Boddaert, N.; Gori, P.; Sarnacki, S.; Bloch, I. Comprehensive review of 3D segmentation software tools for MRI usable for pelvic surgery planning. J. Digit. Imaging 2020, 33, 99–110, doi:10.1007/s10278-019-00239-7.
- Blake, C.; Birch, S.; Brandao, J. Medical three-dimensional printing in zoological medicine. Vet. Clin. N. Am. Exot. Anim. Pr. 2019, 22, 331–348, doi:10.1016/j.cvex.2019.05.004.
- Leberfinger, A.N.; Dinda, S.; Wu, Y.; Koduru, S.V.; Ozbolat, V.; Ravnic, D.J.; Ozbolat, I.T. Bioprinting functional tissues. Acta Biomater. 2019, 95, 32–49, doi:10.1016/j.actbio.2019.01.009.
- Araujo, M.R.P.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. The digital pharmacies era: How 3D printing technology using fused deposition modeling can become a reality. Pharmaceutics 2019, 11, 128, doi:10.3390/pharmaceutics11030128.
- Hoque, M.E.; Chuan, Y.L.; Pashby, I. Extrusion based rapid prototyping technique: An advanced platform for tissue engineering scaffold fabrication. Biopolymers 2012, 97, 83–93, doi:10.1002/bip.21701.
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341, doi:10.1007/s11548-010-0476-x.
- Kim, I.S. Augmentation rhinoplasty using silicone implants. Facial Plast. Surg. Clin. N. Am. 2018, 26, 285–293, doi:10.1016/j.fsc.2018.03.003.
- Huang, Y.; Zhang, X.F.; Gao, G.; Yonezawa, T.; Cui, X. 3D bioprinting and the current applications in tissue engineering. Biotechnol. J. 2017, 12, doi:10.1002/biot.201600734.
- Roh, S.; Parekh, D.P.; Bharti, B.; Stoyanov, S.D.; Velev, O.D. 3D Printing by multiphase silicone/water capillary inks. Adv. Mater. 2017, 29, doi:10.1002/adma.201701554.
- Mempin, M.; Hu, H.; Chowdhury, D.; Deva, A.; Vickery, K. The A, B and C's of silicone breast implants: Anaplastic large cell lymphoma, biofilm and capsular contracture. Materials 2018, 11, 2393, doi:10.3390/ma11122393.
- Angelopoulos, I.; Allenby, M.C.; Lim, M.; Zamorano, M. Engineering inkjet bioprinting processes toward translational therapies. Biotechnol. Bioeng. 2020, 117, 272–284, doi:10.1002/bit.27176.
- Patel, D.K.; Lim, K.T. Biomimetic polymer-based engineered scaffolds for improved stem cell function. Materials 2019, 12, 2950, doi:10.3390/ma12182950.
- Bauermeister, A.J.; Zuriarrain, A.; Newman, M.I. Three-dimensional printing in plastic and reconstructive surgery: A systematic review. Ann. Plast. Surg. 2016, 77, 569–576, doi:10.1097/SAP.0000000000000671.
- Parak, A.; Pradeep, P.; du Toit, L.C.; Kumar, P.; Choonara, Y.E.; Pillay, V. Functionalizing bioinks for 3D bioprinting applications. Drug Discov. Today 2019, 24, 198–205, doi:10.1016/j.drudis.2018.09.012.
- Ostrovidov, S.; Salehi, S.; Costantini, M.; Suthiwanich, K.; Ebrahimi, M.; Sadeghian, R.B.; Fujie, T.; Shi, X.; Cannata, S.; Gargioli, C.; et al. 3D bioprinting in skeletal muscle tissue engineering. Small 2019, 15, e1805530, doi:10.1002/smll.201805530.
- Salerno, A.; Cesarelli, G.; Pedram, P.; Netti, P.A. Modular strategies to build cell-free and cell-laden scaffolds towards bioengineered tissues and organs. J. Clin. Med. 2019, 8, 1816, doi:10.3390/jcm8111816.
- Hippler, M.; Lemma, E.D.; Bertels, S.; Blasco, E.; Barner-Kowollik, C.; Wegener, M.; Bastmeyer, M. 3D Scaffolds to Study Basic Cell Biology. Adv. Mater. 2019, 31, e1808110, doi:10.1002/adma.201808110.
- Dzobo, K.; Motaung, K.; Adesida, A. Recent trends in decellularized extracellular matrix bioinks for 3D printing: An Updated Review. Int. J. Mol. Sci. 2019, 20, 4628, doi:10.3390/ijms20184628.
- Prendergast, M.E.; Burdick, J.A. Recent advances in enabling technologies in 3D printing for precision medicine. Adv. Mater. 2020, 32, e1902516, doi:10.1002/adma.201902516.
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J. Extrusion-based 3D printing for pharmaceuticals: Contemporary research and applications. Curr. Pharm. Des. 2018, 24, 4991–5008, doi:10.2174/1381612825666190110155931.
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278, doi:10.3390/polym10111278.
- Xu, C.; Dai, G.; Hong, Y. Recent advances in high-strength and elastic hydrogels for 3D printing in biomedical applications. Acta Biomater. 2019, 95, 50–59, doi:10.1016/j.actbio.2019.05.032.
- Marques, C.F.; Diogo, G.S.; Pina, S.; Oliveira, J.M.; Silva, T.H.; Reis, R.L. Collagen-based bioinks for hard tissue engineering applications: A comprehensive review. J. Mater. Sci. Mater. Med. 2019, 30, 32, doi:10.1007/s10856-019-6234-x.
- Li, S.; Tian, X.; Fan, J.; Tong, H.; Ao, Q.; Wang, X. Chitosans for tissue repair and organ three-dimensional (3D) bioprinting. Micromachines 2019, 10, 765, doi:10.3390/mi10110765.




