
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yasuhiko Kondo | + 1624 word(s) | 1624 | 2021-08-11 08:41:09 | | | |
| 2 | Enzi Gong | Meta information modification | 1624 | 2021-08-12 07:40:23 | | |
Video Upload Options
The rodent nasal cavity has mainly two chemosensory sensory organs, the vomeronasal organ (VNO) and the olfactory epithelium (OE). However, recently, another chemosensory receptor in the end of the mouse nasal cavity, the Grueneberg ganglion, has been reported. It detects alarm pheromones and predator odors. The VNO hitherto had been thought to be a chemical detector specialized for pheromones, and the OE a detector of airborne chemicals, handling the role of general olfaction. Furthermore, these receptors’ ligands have almost no overlap; olfactory neurons in the OE express a single type of G-protein-coupled receptor (GPCR), that is, detecting a single ligand, whereas some vomeronasal neurons in the VNO express multiple GPCR types, detecting multiple ligands.
1. Overview
In mammalian reproduction, sexually active males seek female conspecifics, while estrous females try to approach males. This sex-specific response tendency is called sexual preference. In small rodents, sexual preference cues are mainly chemosensory signals, including pheromones. In this article, we review the physiological mechanisms involved in sexual preference for opposite-sex chemosensory signals in well-studied laboratory rodents, mice, rats, and hamsters of both sexes, especially an overview of peripheral sensory receptors, and hormonal and central regulation. In the hormonal regulation section, we discuss potential rodent brain bisexuality, as it includes neural substrates controlling both masculine and feminine sexual preferences, i.e., masculine preference for female odors and the opposite. In the central regulation section, we show the substantial circuit regulating sexual preference and also the influence of sexual experience that innate attractants activate in the brain reward system to establish the learned attractant. Finally, we review the regulation of sexual preference by neuropeptides, oxytocin, vasopressin, and kisspeptin. Through this review, we clarified the contradictions and deficiencies in our current knowledge on the neuroendocrine regulation of sexual preference and sought to present problems requiring further study.
2. Opposite-Sex Selection
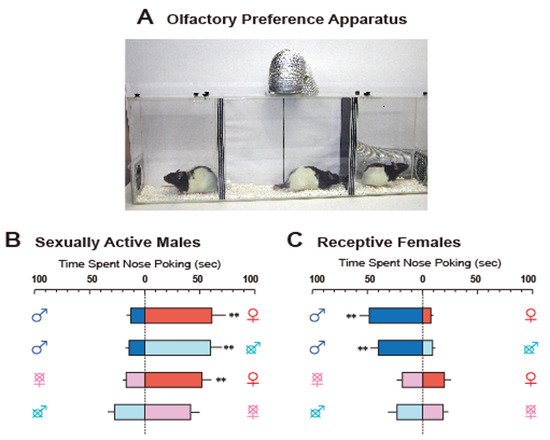
3. Detection of Sexual Chemicals
References
- Slob, A.; DeKlerk, L.; Brand, T. Homosexual and heterosexual partner preference in ovariectomized female rats: Effects of testosterone, estradiol and mating experience. Physiol. Behav. 1987, 41, 571–576.
- Broekman, M.; De Bruin, M.; Smeenk, J.; Slob, A.K.; Van Der Schoot, P. Partner preference behavior of estrous female rats affected by castration of tethered male incentives. Horm. Behav. 1988, 22, 324–337.
- Merkx, J.; Slob, A.; Bosch, J.V.D.W.T. Preference for an estrous female over a non-estrous female evinced by female rats requires dihydrotestosterone plus estradiol. Horm. Behav. 1989, 23, 466–472.
- Brand, T.; Kroonen, J.; Mos, J.; Slob, A. Adult partner preference and sexual behavior of male rats affected by perinatal endocrine manipulations. Horm. Behav. 1991, 25, 323–341.
- Bakker, J.; Van Ophemert, J.; Eijskoot, F.; Slob, A. A semiautomated test apparatus for studying partner preference behavior in the rat. Physiol. Behav. 1994, 56, 597–601.
- Xiao, K.; Kondo, Y.; Sakuma, Y. Sex-specific effects of gonadal steroids on conspecific odor preference in the rat. Horm. Behav. 2004, 46, 356–361.
- Brechbühl, J.; Klaey, M.; Broillet, M.-C. Grueneberg Ganglion Cells Mediate Alarm Pheromone Detection in Mice. Science 2008, 321, 1092–1095.
- Brechbühl, J.; Moine, F.; Klaey, M.; Nenniger-Tosato, M.; Hurni, N.; Sporkert, F.; Giroud, C.; Broillet, M.-C. Mouse alarm pheromone shares structural similarity with predator scents. Proc. Natl. Acad. Sci. USA 2013, 110, 4762–4767.
- Brechbuhl, J.; Klaey, M.; Moine, F.; Bovay, E.; Hurni, N.; Nenniger-Tosato, M.; Broillet, M.C. Morphological and physiological species-dependent characteristics of the rodent Grueneberg ganglion. Front. Neuroanat. 2014, 8, 87.
- Stowers, L.; Kuo, T.-H. Mammalian pheromones: Emerging properties and mechanisms of detection. Curr. Opin. Neurobiol. 2015, 34, 103–109.
- Halem, H.A.; Baum, M.J.; Cherry, J.A. Sex Difference and Steroid Modulation of Pheromone-Induced Immediate Early Genes in the Two Zones of the Mouse Accessory Olfactory System. J. Neurosci. 2001, 21, 2474–2480.
- Haga-Yamanaka, S.; Ma, L.; He, J.; Qiu, Q.; Lavis, L.D.; Looger, L.L.; Yu, C.R. Integrated action of pheromone signals in promoting courtship behavior in male mice. eLife 2014, 3, e03025.
- Kaur, A.W.; Ackels, T.; Kuo, T.-H.; Cichy, A.; Dey, S.; Hays, C.; Kateri, M.; Logan, D.; Marton, T.F.; Spehr, M.; et al. Murine Pheromone Proteins Constitute a Context-Dependent Combinatorial Code Governing Multiple Social Behaviors. Cell 2014, 157, 676–688.
- Chamero, P.; Marton, T.F.; Logan, D.; Flanagan, K.; Cruz, J.R.; Saghatelian, A.; Cravatt, B.F.; Stowers, L. Identification of protein pheromones that promote aggressive behaviour. Nature 2007, 450, 899–902.
- Roberts, S.A.; Simpson, D.M.; Armstrong, S.D.; Davidson, A.J.; Robertson, D.H.; McLean, L.; Beynon, R.J.; Hurst, J.L. Darcin: A male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010, 8, 75.
- Demir, E.; Li, K.; Bobrowski-Khoury, N.; Sanders, J.I.; Beynon, R.J.; Hurst, J.L.; Kepecs, A.; Axel, R. The pheromone darcin drives a circuit for innate and reinforced behaviours. Nature 2020, 578, 137–141.
- Haga, S.; Hattori, T.; Sato, T.; Sato, K.; Matsuda, S.; Kobayakawa, R.; Sakano, H.; Yoshihara, Y.; Kikusui, T.; Touhara, K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 2010, 466, 118–122.
- Stopka, P.; Kuntová, B.; Klempt, P.; Havrdová, L.; Černá, M.; Stopková, R. On the saliva proteome of the Eastern European house mouse (Mus musculus musculus) focusing on sexual signalling and immunity. Sci. Rep. 2016, 6, 32481.
- Niimura, Y. Olfactory Receptor Multigene Family in Vertebrates: From the Viewpoint of Evolutionary Genomics. Curr. Genom. 2012, 13, 103–114.
- Liberles, S.D. Trace Amine-associated Receptors Are Olfactory Receptors in Vertebrates. Ann. N. Y. Acad. Sci. 2009, 1170, 168–172.
- Li, Q.; Liberles, S.D. Aversion and Attraction through Olfaction. Curr. Biol. 2015, 25, R120–R129.
- Liberles, S.D. Trace amine-associated receptors: Ligands, neural circuits, and behaviors. Curr. Opin. Neurobiol. 2015, 34, 1–7.
- Harmeier, A.; Meyer, C.A.; Staempfli, A.; Casagrande, F.; Petrinovic, M.M.; Zhang, Y.-P.; Künnecke, B.; Iglesias, A.; Höner, O.P.; Hoener, M.C. How Female Mice Attract Males: A Urinary Volatile Amine Activates a Trace Amine-Associated Receptor That Induces Male Sexual Interest. Front. Pharmacol. 2018, 9.
- Li, Q.; Korzan, W.J.; Ferrero, D.M.; Chang, R.B.; Roy, D.S.; Buchi, M.; Lemon, J.K.; Kaur, A.W.; Stowers, L.; Fendt, M.; et al. Synchronous Evolution of an Odor Biosynthesis Pathway and Behavioral Response. Curr. Biol. 2013, 23, 11–20.
- Pacifico, R.; Dewan, A.; Cawley, D.; Guo, C.; Bozza, T. An Olfactory Subsystem that Mediates High-Sensitivity Detection of Volatile Amines. Cell Rep. 2012, 2, 76–88.
- Johnson, M.A.; Tsai, L.; Roy, D.S.; Valenzuela, D.H.; Mosley, C.; Magklara, A.; Lomvardas, S.; Liberles, S.D.; Barnea, G. Neurons expressing trace amine-associated receptors project to discrete glomeruli and constitute an olfactory subsystem. Proc. Natl. Acad. Sci. USA 2012, 109, 13410–13415.
- Kobayakawa, K.; Kobayakawa, R.; Matsumoto, H.; Oka, Y.; Imai, T.; Ikawa, M.; Okabe, M.; Ikeda, T.; Itohara, S.; Kikusui, T.; et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature 2007, 450, 503–508.
- Omura, M.; Mombaerts, P. Trpc2-Expressing Sensory Neurons in the Main Olfactory Epithelium of the Mouse. Cell Rep. 2014, 8, 583–595.




